Abstract
Background
Malignant melanoma brain metastases (MBM) are the third most common cause for brain metastases (BM). Historically Whole-brain radiotherapy (WBRT) was considered the goldstandard of treatment even though melanoma cells are regarded as very radioresistant. Therapeutic possibilities have fundamentally changed since the availability of stereotactic radiotherapy (SRT), where it is possible to apply high ablative doses in a very precise manner. In this work we analyze prognostic factors of overall survival (OS) after SRT in patients with MBM and evaluate the applicability of popular prognostic indices that mainly stem from the WBRT-era.
Materials and methods
This work is a retrospective analysis of OS of 80 malignant melanoma (MM) patients who received SRT for intracranial melanoma metastases between 2004 and 2014 who had not received prior treatment for MBM in terms of surgery or WBRT. Potential prognostic factors were analyzed using univariable and multivariable analysis. Existing prognostic scores [Graded Prognostic Assessment (GPA), Diagnosis-Specific-GPA (DS-GPA), Golden Grading System (GGS) and RADES] were calculated and tested using log-rank analysis.
Results
Eighty patients, respectively 177 brain metastases, were irradiated. The median survival time from radiation was 7.06 months. Overall, GGS, GPA and DS-GPA were significant predictors of survival. The MM-specific index DS-GPA showed the best p-value but did not show adequate division when looking at the two intermediate risk subgroups. RADES did not show any statistically significant prognostic value. In univariable as well as in multivariable analyses a higher Karnofsky-Index, a single BM, and non nodular melanoma (NM) histology were positive predictors of survival.
Conclusion
The existing prognostic scores do not seem to ideally fit for this special group of patients. Our results indicate that the histologic subtype of MM could add to the prognostic value of specialized future indices.
Similar content being viewed by others
Background
Brain metastases (BM) are the most common intracranial malignant tumor [1] and 20-40% of cancer patients will develop BM [2]. Characterized by a high probability to metastasize to the brain, malignant melanoma (MM) is the third most common cause for brain metastases. Melanoma brain metastases (MBM) are also the most common cause of death in melanoma patients [3]. The incidence of MM has been increasing by 3–7% per year, which translates into a predicted doubling time of incidence of 10–20 years [4].
In general we have seen an increase in BM in the last decades mainly due to high life-expectancy, better imaging technology and the development of new systemic drugs that in many cases do not adequately penetrate the blood-brain barrier [5].
The median overall survival of a patient with BM is less than 12 months, but OS varies significantly interindividually depending on a plethora of factors like. Karnofsky performance status (KPS), extracranial metastasis (ECM), number of metastases, lesion size and volume, and histologic subtype [5].
Historically the goldstandard of treatment for patients with BM was whole brain radiotherapy (WBRT), a therapy that may improve OS and quality of life (QOL) [6]. However, the potential disadvantages of WBRT include treatment times of several weeks and a neurocognitive decline [7]. Therefore, some authors have tried to implement focal therapies like surgery and stereotactic radiosurgery (SRS) [8]. SRS has been shown to reduce neurocognitive decline while improving QOL without compromising OS [9]. However, it must be mentioned that some authors have shown higher intracranial relapse rates in patients treated with local therapies. Still these higher relapse-rates did not translate into worse OS rates [10]. In routine clinical practice, there is a tendency to use WBRT for patients with higher tumor burden, while focal approaches are offered to patients with higher potential survival rates.
As a consequence, many authors have tried to identify prognostic factors of survival in order to predict the individual patient’s potential survival time. Indices based on such prognostic factors include the Golden grading system (GGS), the graded prognostic index (GPA) the disease-specific graded prognostic index (DS-GPA) as well as the prognostic index published by Rades et al. in 2011 (RADES) [11,12,13,14]. While the GPA uses the four factors age, KPS, number of intracranial metastases and ECM, the GGS only uses the three factors age, KPS and presence of extracranial metastases, the MM-specific DS-GPA includes only the two factors KPS and the number of intracranial lesions. The RADES uses age, KPS, ECM and number of BM as well as the interval from primary tumor diagnosis to radiotherapy. These prognostic indices are widely used in clinical practice because most other indices developed to this date use components that are either time-consuming to quantify or subjective (e.g. control of extracranial disease or BM volume) [14].
In this study we evaluated the prognostic value of these four indices in MBM patients who received SRT as primary BM treatment.
Methods
Treatment decisions, patient selection and dose regimens
We performed a retrospective analysis of 80 patients (61.3% male, median age: 61 years) with MBMs who had undergone stereotactic radiotherapy as their primary treatment between 2004 and 2014 in our department. Treatment decisions had been based on a vote by an interdisciplinary medical team. Doses applied in multiple fractions were considered fractionated stereotactic radiotherapy (FSRT) and high single doses were considered SRS. Larger tumors in close proximity to critical structures were assigned to FSRT, while smaller tumors distant to critical structures were treated with SRS.
Stratification and variables
Patients were stratified according to age, gender, KPS, histologic subtype, Stage, number of BM, cumulative planning target volume (PTV), highest and lowest BED10, synchronous vs. metachronous diagnosis of BM (>1 month after non-small cell lung cancer (NSCLC) diagnosis was considered metachronous), tumor localization, presence of ECM and interval from BM diagnosis to radiotherapy. The prognostic scores, GPA, DS-GPA, GGS and RADES, were calculated and tested using the log-rank test. Prognostic parameters were identified using uni-and multivariable analyses.
Patients with tumor progress could undergo salvage SRS, WBRT or a resection of the BM. Follow-up examinations, including MRI as well as clinical and neurologic examinations were usually performed at 3 months intervals after radiotherapy or if clinically indicated.
Technical set-up
Patients were treated using Novalis® (BrainLab®) with beam shaping capability, built-in multi-leaf collimator (MLC) and image guidance. Novalis ExacTrac® image guided frameless system enabled us to image the patient at any couch position using a frameless positioning array. All patients received diagnostic contrast enhanced cranial magnetic resonance imaging (ceMRI) and cranial computed tomography (CT) imaging before CT/MRI-fusion planning was performed. The three-dimensional treatment planning system iplanRT® was used. Gross tumor volume (GTV) was defined as the area of contrast enhancement on T1-weighted MRI images, the PTV included a 2 mm isotropic safety margin. The dose was prescribed to the 80% isodose at the PTV margin.
Formulas and statistics
The biologically effective dose (BED) was calculated according to the following formula, where n is the number of fractions and d the dose per fraction. Following the Linear quadratic model, a value of 10 was used for the α/β-ratio.
OS was calculated starting with the first day of irradiation and estimated using Kaplan-Meier method. Subgroups were compared using the log-rank test for univariable analysis and the Cox proportional hazard model for multivariable analysis. A p-value of less than 0.05 was considered statistically significant. A p-value of less than 0.1 was considered a trend and was the criterion for the inclusion in multivariable analysis. All statistical analyses were performed using IBM SPSS Statistics 19 (New York, USA).
Results
Patients
Patient characteristics are summarized in detail in Table 1. Eighty patients with 177 brain metastases were irradiated. The majority of the patients were male (61.3%) and the median age was 61 years. Most Patients (86.4%) had a good KPS of 70% or higher. Twenty-five percent of patients were already diagnosed Union internationale contre le cancer (UICC) stage IV at the time of cancer diagnosis and 5% of the patients showed synchronous brain metastases. NM was the most common histologic subtype of melanoma (27.5%).
Almost half of the patients (43.8%) were treated for a single brain metastasis. Median cumulative lesion volume was 2.47 ccm (0.02-41.68 ccm). Median BED10 was 91.1 Gy (39-91.1 Gy).
Overall survival
Of patients alive at last follow-up, median follow-up time was 7.06 months;
OS rate was 58.2% after 6 months and 29.5% after 12 months.
Univariable and multivariable analysis of prognostic factors is shown in Table 2. Statistically significant factors of favourable OS in univariable analysis were higher KPS, single BM and non-NM histology.
In multivariable analysis KPS, single BM and non-NM histology were significant prognostic factors.
Prognostic indices
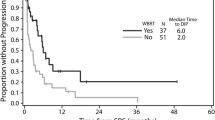
An overview of the prognostic value of the four examined indices is given in Fig. 1. In our patient cohort the GPA, DS-GPA and the GGS Index showed significant OS distribution in the Kaplan-Meyer analysis as predictors of survival. The best p-value was seen for the DS-GPA. while the RADES gave a poor result. However, none of the four indices gave a full set of non-intersecting survival curves for the respective subgroups (Fig. 1).
Discussion
The question which BM subpopulation benefits from WBRT or SRS has been a topic of debate in the neurooncologic community for years [15]. A plethora of prognostic indices has been proposed and published. With the help of these indices physicians are now trying to identify subpopulations that might benefit from more focal therapies. In this work we examined four well-known indices in a MM patient population treated with SRT. The indices used here are easy to calculate and do not rely on subjective variables.
All indices examined here (GPA, DS-GPA, GGS, and RADES) use the KPS. A significant predictor in univariable and multivariable analysis of survival in our cohort.
The number of BM was a significant predictor in our univariable and multivariable analysis and is used in the two indices with the best prognostic value (the GPA and the DS-GPA). The GGS does not use this factor but still gives significant prognostic values. The RADES uses the number of BM but fails to give significant prognostic values. Likhacheva et al. [16] were the first to publish a study on prognostic factors in patients with BM who had only received SRS. In their patient cohort the cumulative BM volume was a significant predictor of OS analysis, while the number of BM was not a significant factor. In their publication the authors advise to use BM volume in order to predict OS rather than the number of BM. This is contradictory to our data, where the number of BM was a predictor of OS but BM volume was not.
The factor age was not significant in univariable or multivariable analysis in our cohort. Of the examined indices all but the DS-GPA use the factor age for calculation. Another factor not significant in our cohort was ECM. This factor is used in the GGS, GPA and the RADES index.
The interval from diagnosis to radiotherapy is used in the RADES index. In our analysis we did not identify this factor as significant neither in univariable nor in multivariable analysis.
One factor we did find to be of significant value both in univariable and multivariable analysis was NM histologic subtype, a factor which has not been used before in any of the other examined indices. The primary tumor in NM tumors normally presents with greater thickness than other subtypes of melanoma and therefore metastasizes earlier than other histologic subtypes [17]. However, it should be discussed, that NM histology might actually be a surrogate marker of BRAF status. It is known that BRAF mutations are more common in NM [18] and that BRAF-positive patients survive longer than BRAF-negative patients [19], therefore BRAF status was also included in the new Melanoma-molGPA index published by Sperduto et al. [20].
Looking at the original publications by Sperduto, Rades and Golden, it must be remembered that the patient cohort analyzed here is much more homogeneous in terms of treatment than in the original works. We excluded all patients that had received WBRT or MBM resection as primary BM treatment [11,12,13,14].
The fact that the DS-GPA gave the best results can be explained by the fact, that it solely relies on the two most significant predictors of OS in our multivariable analysis: KPS and number of BM. The fact that patients are grouped in four classes in the DS-GPA instead of three classes like the GPA and the RADES makes the DS-GPA even more outstanding.
Kano et al. compared 4 prognostic indices in a cohort of 422 patients who had been treated with Gamma Knife SRS for melanoma BM. The patient cohort included patients who had received WBRT (n = 132) or craniotomy (n = 65) earlier. The authors compared the recursive partitioning analysis (RPA), the Score Index for Radiosurgery (SIR), the Basic Score for Brain Metastases (BSBM), and the DS-GPA. The authors found the DS-GPA to be the most proved most balanced and prognostic [21].
The limitations of this study should also be mentioned. Firstly, the retrospective nature of the analysis is prone to bias. Secondly, the number of patients may have been too low to find significance of some potential prognostic factors or even of the RADES index itself. Thirdly different regimes of systemic therapy may represent a potential bias.
Conclusion
Several authors have developed prognostic indices for BM and MBM patients, the ideal index has not been defined yet and further research into alternative approaches is needed. Of the indices examined here, the DS-GPA gave the best results in our analysis in this patient cohort with MBM treated with SRT. Apart from showing the best result in our analysis the DS-GPA is also the easiest index to calculate examined in this work. Our results indicate that the histologic subtype NM might improve the prognostic value of MBM-specific indices.
Abbreviations
- BED:
-
Biologically effective dose
- BM:
-
Brain metastases
- BSBM:
-
Basic Score for Brain Metastases
- CT:
-
Computed tomography
- DS-GPA:
-
Diagnosis-Specific-GPA
- ECM:
-
Extracranial metastasis
- FSRT:
-
Fractionated stereotactic radiotherapy
- GGS:
-
Golden Grading System
- GPA:
-
Graded Prognostic Assessment
- GTV:
-
Gross tumor volume
- KPS:
-
Karnofsky performance status
- MBM:
-
Malignant melanoma brain metastases
- MLC:
-
Multi-leaf collimator
- MM:
-
Malignant melanoma
- MRI:
-
Magnetic resonance imaging
- NM:
-
nodular melanoma
- NSCLC:
-
Non-small cell lung cancer
- OS:
-
Overall survival
- PTV:
-
Planning target volume
- QOL:
-
Quality of life
- RPA:
-
Recursive partitioning analysis
- SIR:
-
Score Index for Radiosurgery
- SRS:
-
Stereotactic radiosurgery
- SRT:
-
Stereotactic radiotherapy
- UICC:
-
Union internationale contre le cancer
- WBRT:
-
Whole-brain radiotherapy
References
Gavrilovic IT, Posner JB. Brain metastases: epidemiology and pathophysiology. J Neuro-Oncol. 2005;75:5–14.
Nussbaum ES, Djalilian HR, Cho KH, Hall WA. Brain metastases. Histology, multiplicity, surgery, and survival. Cancer. 1996;78:1781–8.
Sampson JH, Carter JH Jr, Friedman AH, Seigler HF. Demographics, prognosis, and therapy in 702 patients with brain metastases from malignant melanoma. J Neurosurg. 1998;88:11–20.
Garbe C, Leiter U. Melanoma epidemiology and trends. Clin Dermatol. 2009;27:3–9.
Jenkinson MD, Haylock B, Shenoy A, Husband D, Javadpour M. Management of cerebral metastasis: evidence-based approach for surgery, stereotactic radiosurgery and radiotherapy. Eur J Cancer. 2011;47:649–55.
Deutsch M, Parsons JA, Mercado R Jr. Radiotherapy for intracranial metastases. Cancer. 1974;34:1607–11.
Chang EL, Wefel JS, Hess KR, Allen PK, Lang FF, Kornguth DG, Arbuckle RB, Swint JM, Shiu AS, Maor MH, Meyers CA. Neurocognition in patients with brain metastases treated with radiosurgery or radiosurgery plus whole-brain irradiation: a randomised controlled trial. The Lancet Oncology. 2009;10:1037–44.
Linskey ME, Andrews DW, Asher AL, Burri SH, Kondziolka D, Robinson PD, Ammirati M, Cobbs CS, Gaspar LE, Loeffler JS, et al. The role of stereotactic radiosurgery in the management of patients with newly diagnosed brain metastases: a systematic review and evidence-based clinical practice guideline. J Neuro-Oncol. 2010;96:45–68.
Tsao M, Xu W, Sahgal A. A meta-analysis evaluating stereotactic radiosurgery, whole-brain radiotherapy, or both for patients presenting with a limited number of brain metastases. Cancer. 2012;118:2486–93.
Aoyama H, Shirato H, Tago M, Nakagawa K, Toyoda T, Hatano K, Kenjyo M, Oya N, Hirota S, Shioura H, et al. Stereotactic radiosurgery plus whole-brain radiation therapy vs stereotactic radiosurgery alone for treatment of brain metastases: a randomized controlled trial. JAMA. 2006;295:2483–91.
Sperduto PW, Chao ST, Sneed PK, Luo X, Suh J, Roberge D, Bhatt A, Jensen AW, Brown PD, Shih H, et al. Diagnosis-specific prognostic factors, indexes, and treatment outcomes for patients with newly diagnosed brain metastases: a multi-institutional analysis of 4,259 patients. Int J Radiat Oncol Biol Phys. 2010;77:655–61.
Rades D, Dziggel L, Haatanen T, Veninga T, Lohynska R, Dunst J, Schild SE. Scoring systems to estimate intracerebral control and survival rates of patients irradiated for brain metastases. Int J Radiat Oncol Biol Phys. 2011;80:1122–7.
Golden DW, Lamborn KR, McDermott MW, Kunwar S, Wara WM, Nakamura JL, Sneed PK. Prognostic factors and grading systems for overall survival in patients treated with radiosurgery for brain metastases: variation by primary site. J Neurosurg. 2008;109(Suppl):77–86.
Sperduto PW, Berkey B, Gaspar LE, Mehta M, Curran W. A new prognostic index and comparison to three other indices for patients with brain metastases: an analysis of 1,960 patients in the RTOG database. Int J Radiat Oncol Biol Phys. 2008;70:510–4.
Nieder C, Mehta MP. Prognostic indices for brain metastases--usefulness and challenges. Radiat Oncol. 2009;4:10.
Likhacheva A, Pinnix CC, Parikh N, Allen PK, Guha-Thakurta N, McAleer M, Sulman EP, Mahajan A, Shiu A, Luo D, et al. Validation of recursive partitioning analysis and diagnosis-specific graded prognostic assessment in patients treated initially with radiosurgery alone. J Neurosurg. 2012;117(Suppl):38–44.
Bergenmar M, Ringborg U, Mansson Brahme E, Brandberg Y. Nodular histogenetic type -- the most significant factor for thick melanoma: implications for prevention. Melanoma Res. 1998;8:403–11.
Akslen LA, Angelini S, Straume O, Bachmann IM, Molven A, Hemminki K, Kumar R. BRAF and NRAS mutations are frequent in nodular melanoma but are not associated with tumor cell proliferation or patient survival. The Journal of investigative dermatology. 2005;125:312–7.
Sperduto PW, Jiang W, Brown PD, Braunstein S, Sneed P, Wattson DA, Shih HA, Bangdiwala A, Shanley R, Lockney NA, et al. The prognostic value of BRAF, C-KIT, and NRAS mutations in melanoma patients with brain metastases. Int J Radiat Oncol Biol Phys. 2017;98:1069–77.
Sperduto PW, Jiang W, Brown PD, Braunstein S, Sneed P, Wattson DA, Shih HA, Bangdiwala A, Shanley R, Lockney NA, et al. Estimating survival in melanoma patients with brain metastases: an update of the graded prognostic assessment for melanoma using molecular markers (melanoma-molGPA). Int J Radiat Oncol Biol Phys. 2017;99:812–6.
Kano H, Morales-Restrepo A, Iyer A, Weiner GM, Mousavi SH, Kirkwood JM, Tarhini AA, Flickinger JC, Lunsford LD. Comparison of prognostic indices in patients who undergo melanoma brain metastasis radiosurgery. J Neurosurg. 2017;2017:1–9.
Acknowledgements
Not applicable.
Funding
Not applicable.
Availability of data and materials
Please contact author for data requests.
Author information
Authors and Affiliations
Contributions
HB planned the study, wrote part of the manuscript and took part in the discussion of the manuscript, FE acquired Data, performed statistical analysis and took part in the discussion of the manuscript, VB, SZ and PG took part in the discussion of the manuscript, DK planned the study, performed statistical analysis, wrote part of the manuscript, and supervised the discussion of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
For this type of study, formal consent is not required. All research has been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Badakhshi, H., Engeling, F., Budach, V. et al. Are prognostic indices for brain metastases of melanoma still valid in the stereotactic era?. Radiat Oncol 13, 3 (2018). https://doi.org/10.1186/s13014-017-0951-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13014-017-0951-4