Abstract
Background
This review addresses the strengths and weaknesses of 6 different prognostic indices, published since the Radiation Therapy Oncology Group (RTOG) developed and validated the widely used 3-tiered prognostic index known as recursive partitioning analysis (RPA) classes, i.e. between 1997 and 2008. In addition, other analyses of prognostic factors in groups of patients, which typically are underrepresented in large trials or databases, published in the same time period are reviewed.
Methods
Based on a systematic literature search, studies with more than 20 patients were included. The methods and results of prognostic factor analyses were extracted and compared. The authors discuss why current data suggest a need for a more refined index than RPA.
Results
So far, none of the indices has been derived from analyses of all potential prognostic factors. The 3 most recently published indices, including the RTOG's graded prognostic assessment (GPA), all expanded from the primary 3-tiered RPA system to a 4-tiered system. The authors' own data confirm the results of the RTOG GPA analysis and support further evaluation of this tool.
Conclusion
This review provides a basis for further refinement of the current prognostic indices by identifying open questions regarding, e.g., performance of the ideal index, evaluation of new candidate parameters, and separate analyses for different cancer types. Unusual primary tumors and their potential differences in biology or unique treatment approaches are not well represented in large pooled analyses.
Similar content being viewed by others
Background
Prognostic indices might represent a useful tool in palliative cancer treatment. Estimation of a patient's prognosis in terms of overall survival might allow for tailored treatment, i.e. more aggressive approaches when these are likely to impact on survival and focus on disease stabilisation, symptom control and toxicity minimization when the disease is more advanced, or comorbidity limits the tolerability of aggressive therapy. In addition, prognostic indices might also be used as inclusion/exclusion criteria for clinical trials and for comparison of results across different studies in relatively homogeneous patient groups.
Brain metastases continue to represent a formidable challenge in oncology [1–3]. With increasing numbers of local and systemic treatment options, the issue of patient selection gains importance. While surgery and stereotactic radiosurgery (SRS) provide long-term local control of macroscopic disease and in combination with whole-brain radiotherapy (WBRT) the best available overall brain control for the remaining life time [4–10], they represent overtreatment in patients with short survival, which typically is caused by uncontrollable systemic disease. This review will address the strengths and weaknesses of 6 different prognostic indices, published since the Radiation Therapy Oncology Group (RTOG) developed and validated the widely used 3-tiered prognostic index known as recursive partitioning analysis (RPA) classes [11, 12], i.e. between 1997 and 2008. In addition, other analyses of prognostic factors in groups of patients, which typically are underrepresented in large trials or databases, published in the same time period are reviewed. These include patients with primary tumors that do not commonly metastasize to the brain, and the elderly, who are often either excluded or under-represented in clinical trials.
Methods
The present review compares different prognostic indices and analyses of prognostic factors based on a systematic literature search by use of Medline (Pub Med by the National Library of Medicine, National Institutes of Health, Bethesda, Maryland, USA). It is limited to adult patients having received first-line treatment for parenchymal brain metastases in the absence of leptomeningeal disease. The key words used were "brain metastases", "metastatic brain tumor" and "cerebral metastases". The final search was performed on June 30, 2008. It also included the reference lists of all articles and the appropriate chapters in textbooks on brain metastases, neuro-oncology and radiation oncology. Case reports and review articles were not assessed. Only studies with more than 20 patients were included. If several subsequent reports were published from the same institution, the most recent publication was evaluated. The methods and results of prognostic factor analyses were extracted and compared.
Results
The search identified 6 different prognostic indices, which are shown in Table 1. Comparison of the patients' characteristics is shown in Table 2. Unfortunately, a considerable amount of information can not be extracted from the publications. The most widely used index over the last decade is the RPA index originally described by Gaspar et al. on behalf of the RTOG [11], which is based on 4 parameters (age, Karnofsky performance status (KPS), presence or absence of extracranial metastases, and the control status of the primary tumor), separating patients into 3 different classes. Lutterbach et al. suggested expansion of the classification by further dividing class III into 3 separate classes [13]. This was based on their multivariate analysis of 916 patients from a single institution, but was not adopted by other authors in subsequent publications. Their definition yielded class IIIa defined as age <65 years, controlled primary tumor and single brain metastasis, class IIIc defined as age ≥ 65 years, uncontrolled primary tumor and multiple brain metastases, while other patients would make up class IIIb. The original RPA classification has been validated by several authors, both in selected and unselected patient groups, e.g., patients with breast primary, lung primary (small cell and non-small cell), malignant melanoma, unknown primary, or surgical resection and SRS as main local treatment modalities [14–35].
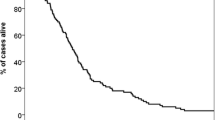
Probably, the surgically treated patients represent the most homogeneous cohorts assessed with the RPA system, as these were patients with rather favourable prognosis, fit to undergo surgery and with limited brain disease. Nevertheless, the differences in median survival between the individual studies were large. In RPA class I, median survival ranged from 15–29 months [31–35]. In class II, a survival range of 5.5–11 months has been reported. In class III, these figures reached 1.4–9 months. As illustrated here, survival within the same RPA class might vary by a factor of 2 or more between different studies (identical treatment approach). In series where the majority of patients were treated with WBRT, less variation between studies can be found (Figure 1). As shown in Table 1, both RPA class II and III contain quite heterogeneous groups of patients. The factor determining class III is KPS<70, which might result from many different causes including the brain metastases themselves, advanced and treatment-refractory extracranial metastases, severe pain or pathological fracture in patients with bone metastases, atelectasis or pneumonia from primary lung cancer, anemia induced by chemotherapy, recovery from recent surgery, and non-cancer-related comorbidity. In all the reports reviewed variable proportions of patients in the most favourable RPA class I unexpectedly died within 2 months, while some patients in class III survived for more than 6 months. For these reasons, there obviously is a need for a more refined index than RPA.
The first attempt in 1999 resulted in the Rotterdam Score, which did not gain wider acceptance [36]. Similar to RPA, performance status and extent of systemic disease were included, while the third parameter was response to steroids before WBRT. It can be assumed that the unavailability of this latter parameter in most databases or patient records prevented other groups from using the score. In addition, the definition of systemic tumor activity is not straight forward. The next attempt (Score Index for Radiosurgery (SIR)) was derived from a limited number of patients treated with this particular focal approach, which might have resulted in overfitting of the data [37]. However, several groups confirmed the performance of the SIR in patients treated with SRS, surgery, and WBRT with or without SRS, some of them with large numbers of patients (Figure 2) [35, 38–44]. To accurately define systemic disease activity, comprehensive diagnostic work-up is needed.
When evaluating the SIR and RPA indices in their SRS database, the group from Brussels, Belgium, arrived at a new score, which they called Basic Score for Brain Metastases (BSBM) [43]. Based on its greater convenience and simplicity, they advocated the use of this score, which uses the same definition of extracranial disease activity as the RTOG. Recent data indicate that BSBM can be applied to patients managed with WBRT with or without SRS and surgery plus WBRT [35, 42, 44], however its performance is not better than that of the other scores (Figure 3).
The RTOG has recently proposed a new index, which was compared to RPA, SIR, and BSBM (but not to the Rotterdam score) [44]. The new score (Graded Prognostic Assessment (GPA)) is different from RTOG's RPA, e.g., with regard to the number of prognostic classes, which increased from 3 to 4, and the larger number of patients. The analysis also includes patients managed with WBRT plus SRS from RTOG study 9508 [5]. In the GPA system, 3 different values (0, 0.5 or 1) are assigned for each of these 4 parameters: age (≥ 60; 50–59; <50), KPS (<70; 70–80; 90–100), number of brain metastases (>3; 2–3; 1), and extracranial metastases (present; not applicable; none). Assessment of primary tumor activity or control is no longer mandated. It was concluded by the authors that "GPA is the least subjective, most quantitative and easiest to use of the 4 indices" and that future trials should compare these scores and validate the GPA. One of the authors' group has embarked on this comparison in 2 different patient populations, i.e. those managed with WBRT with or without SRS (comparable to the RTOG study population) [42] and those managed with surgery and WBRT [35]. Both studies basically relied on the methods used by the RTOG in their analysis, though with patients treated in clinical routine outside of randomized trials. Compared to RTOG's patients treated with WBRT with or without SRS, the median age, KPS, number of lesions and lesion volume were similar. Obvious differences existed, however, regarding controlled primary tumor (47 vs. 67%) and extracranial metastases (56 vs. 36%). Thus, the cohort is expected to have inferior survival. Figure 4 shows the survival results.
Last but not least, Rades et al. developed a new prognostic index based on 4 parameters (age, KPS, extracranial metastases at the time of WBRT, interval from tumor diagnosis to WBRT) [45]. The major difference from the RPA classes is the replacement of primary tumor control by interval from tumor diagnosis to WBRT (not by number of brain metastases as in the GPA). This index separated patients into 4 subgroups with significantly different prognosis and was also validated in one of the authors' database (unpublished results, Figure 5).
Comparison of median survival in 2 studies using the index proposed by Rades et al. [45](treatment was WBRT with or without local measures, studies not limited to one particular cancer type, median survival estimated from the Kaplan-Meier curves in the publication).
Discussion
As stated on the website of the National Cancer Institute http://www.cancer.gov/templates/bd_alpha.aspx?CdrID=44246, a prognostic factor is regarded as a situation or condition, or a characteristic of a patient, that can be used to estimate the chance of recovery from a disease or the chance of the disease recurring. Based on such prognostic factors, 6 different prognostic indices for adult patients with brain metastases from solid tumors have been developed over the last decade. As demonstrated in Table 1, the 3 most recently published indices all expanded from the primary 3-tiered RPA system to a 4-tiered system. The 6 indices are based on a different number of prognostic factors, i.e. 3–6. Of course, increasing numbers of parameters will lead to less convenience and ease of administration. None of the groups that developed these indices included all potential prognostic factors in their analysis. This is most likely due to the unavailability of all the information in the databases and the difficulty in collecting missing data in 1,000 or more patients treated over many years. As can be seen in Figures 3, 4, 5, the performance of the 4-tiered indices is not tremendously different, although further data are needed to confirm this finding.
There is agreement in all indices on the importance of performance status and extracranial disease activity. However, whether both primary tumor and extracranial metastases should be considered is less clear (2 indices would not include primary tumor control). Assessment of extracranial disease status is not trivial. It might require considerable resources in patients with very limited life expectancy and therapeutic options. When collecting data over long time periods, one must expect a shift in diagnostic modalities, i.e. increasing use of magnetic resonance imaging of the brain as compared to computed tomography (CT) or increasing use of chest CT or even positron emission tomography (PET). Such a shift will likely result in no longer assigning patients to the most favourable prognostic class (stage migration). This might compromise the comparison of the different studies.
Two of the 6 indices did not include age and the ones that did, used slightly different cut-off values. A minority of studies (n = 2) included number of brain metastases and only one each included response to steroids, volume of the largest lesion in the brain, and time interval to development of brain metastases, respectively. Other previous reports lend credence to the examination of each of these factors. In their multivariate analysis of 334 patients, DiLuna et al. reported significantly better survival in patients with 1–3 vs 4 or more brain metastases and in those patients with both limited number and volume of brain metastases (<5 cc total volume) [46]. Bhatnagar et al. also reported on the impact of treatment volume as independent prognostic factor in patients treated with SRS [47]. In a randomised trial with 544 patients, Priestman et al. found that dose of steroids was independently associated with survival [48]. Interval to development of brain metastases appears particularly important in patients with primary NSCLC and malignant melanoma. The multivariate analyses of 3 studies with 292–686 patients support this observation [23, 49, 50].
The latter findings lead to the general question on the usefulness of lumping together patients with different primary tumors in these models. Breast cancer poses an interesting dilemma here, because although tumor type and histology were not prognostically significant in the RPA, recent data, especially since the advent of trastuzumab and lapatinib, suggest that, receptor status and her-2-neu expression might have prognostic impact, even if this issue is not without controversy (Table 3). The recently suggested prognostic factor lymphopenia falls into the same category [19, 51]. Unusual primary tumors and their potential differences in biology or unique treatment approaches are not well represented in large pooled analyses. Table 4 provides examples on analyses of prognostic factors in such groups.
Surrogate markers of disease activity that are easy to measure and inexpensive, such as lactate dehydrogenase and other laboratory parameters have repeatedly been shown to be independent prognostic factors for survival [71–74]. Studies that were not limited to patients with brain metastases suggest that the anorexia-cachexia syndrome, dyspnea, pain, and co-morbidity are further candidates for prospective evaluation [74]. The same holds true for neurofunction class [75, 76] and mini mental status examination results, which was an independent prognostic factor for survival in a multivariate model that also included KPS [77]. The current prognostic indices unfortunately do not incorporate these features.
One of the purposes of prognostic indices is to guide the choice of treatment in individual patients. In this context, a prognostic index should be accurate enough to avoid overtreatment in patients that actually have very short survival. Even more important, one should not withhold treatment because the index erroneously predicts an unfavorable outcome. These aspects of the indices have not been thoroughly evaluated, even in the recent GPA analysis [44]. In our analysis of 239 patients, which confirms that RPA, SIR, BSBM and GPA each split the dataset into groups with significantly different prognosis, this issue was addressed [42]. With regard to the outcome of patients with unfavorable survival, defined as ≤ 2 months (n = 93), no significant difference between the indices was observed. Regarding patients with favorable survival, defined as ≥ 6 months (n = 66), again no significant difference was observed, although RPA performed worse than the other indices. Overall, GPA misassigned 6% of the patients (9 out of 159), compared to 11% with RPA. Therefore, the available validation data certainly do not discourage further evaluation of the new GPA. However, such evaluation should also include comparison with the 2 other scores (Rotterdam and Rades et al.). It is just the stark reality of the disease process that in all of the scoring systems, the most favorable prognostic group is very small (e.g., GPA ≥ 3.5: 9% of RTOG and 7% of our own patients; RPA class I: 16% of RTOG and 11% of our own patients).
The open questions after publication of 6 prognostic indices include:
-
how should the ideal index perform?
-
how many parameters should form the basis of the ideal index?
-
can we lump together patients with breast cancer, small-cell lung cancer, malignant melanoma etc. or do we lose potentially important information?
-
do we need candidate parameters beyond the ones examined so far (lactate dehydrogenase, anemia, weight loss, pain etc.)?
-
is it justifiable to assign the same point value to different degrees of extracranial disease, e.g., 2 small asymptomatic lung metastases, 8 large liver metastases with increased bilirubin, skin metastases already treated by radiotherapy etc.?
-
can international groups collaborate to develop a consensus score, or maybe even an online tool?
Other aspects of predicting the outcome in patients with brain metastases that many clinicians might appreciate, relate to the important issue of neurologic function and quality of life. In many instances, radiotherapy aims more on improving deficits and preventing neurologic decline than prolonging survival, but no attempts have been made to develop scores that address endpoints other than overall survival. It appears therefore worthwhile to collect data on such endpoints, as done, e.g., in the recently completed randomized trial of radiotherapy with or without motexafin gadolinium [78], which used time to neurologic progression as primary endpoint. Other opportunities for future research include examination of prognostic models that provide estimates on both risk of systemic cancer progression with death from non-neurologic causes and risk of death from uncontrolled brain metastases.
References
Kunthia D, Brown P, Li J, Mehta MP: Whole-brain radiotherapy in the management of brain metastasis. J Clin Oncol 2006, 24: 1295-1304. 10.1200/JCO.2005.04.6185
Langer CJ, Mehta MP: Current management of brain metastases, with a focus on systemic options. J Clin Oncol 2005, 23: 6207-6219. 10.1200/JCO.2005.03.145
Nieder C, Grosu AL, Astner ST, Thamm R, Molls M: Integration of chemotherapy into current treatment strategies for brain metastases from solid tumors. Radiat Oncol 2006, 1: 19. 10.1186/1748-717X-1-19
Aoyama H, Shirato H, Tago M, Nakagawa K, Toyoda T, Hatano K, Kenjyo M, Oya N, Hirota S, Shioura H, Kunieda E, Inomata T, Hayakawa K, Katoh N, Kobashi G: Stereotactic radiosurgery plus whole-brain radiation therapy vs stereotactic radiosurgery alone for treatment of brain metastases. A randomized controlled trial. JAMA 2006, 295: 2483-2491. 10.1001/jama.295.21.2483
Andrews DW, Scott CB, Sperduto PW, Flanders AE, Gaspar LE, Schell MC, Werner-Wasik M, Demas W, Ryu J, Bahary JP, Souhami L, Rotman M, Mehta MP, Curran WJ Jr: Whole brain radiation therapy with or without stereotactic radiosurgery boost for patients with one to three brain metastases: phase III results of the RTOG 9508 randomised trial. Lancet 2004, 363: 1665-1672. 10.1016/S0140-6736(04)16250-8
Kondziolka D, Patel A, Lunsford LD, Kassam A, Flickinger JC: Stereotactic radiosurgery plus whole brain radiotherapy versus radiotherapy alone for patients with multiple brain metastases. Int J Radiat Oncol Biol Phys 1999, 45: 427-434.
Patchell RA, Tibbs PA, Regine WF, Dempsey RJ, Mohiuddin M, Kryscio RJ, Markesbery WR, Foon KA, Young B: Postoperative radiotherapy in the treatment of single metastases to the brain: a randomized trial. JAMA 1998, 80: 1485-1489. 10.1001/jama.280.17.1485
Noordijk EM, Vecht CJ, Haaxma-Reiche H, Padberg GW, Voormolen JH, Hoekstra FH, Tans JT, Lambooij N, Metsaars JA, Wattendorf AR: The choice of treatment of single brain metastasis should be based on extracranial tumor activity. Int J Radiat Oncol Biol Phys 1994, 29: 711-717.
Patchell RA, Tibbs PA, Walsh JW, Dempsey RJ, Maruyama Y, Kryscio RJ, Markesbery WR, Macdonald JS, Young B: A randomized trial of surgery in the treatment of single metastases to the brain. N Engl J Med 1990, 322: 494-500.
Nieder C, Astner ST, Grosu AL, Andratschke NH, Molls M: The role of postoperative radiotherapy after resection of a single brain metastasis: combined analysis of 643 patients. Strahlenther Onkol 2007, 183: 576-580. 10.1007/s00066-007-1756-4
Gaspar L, Scott C, Rotman M, Asbell S, Phillips T, Wasserman T, McKenna WG, Byhardt R: Recursive partitioning analysis (RPA) of prognostic factors in three Radiation Therapy Oncology Group (RTOG) brain metastases trials. Int J Radiat Oncol Biol Phys 1997, 37: 745-751.
Gaspar LE, Scott C, Murray K, Curran W: Validation of the RTOG recursive partitioning analysis (RPA) classification for brain metastases. Int J Radiat Oncol Biol Phys 2000, 47: 1001-1006.
Lutterbach J, Bartelt S, Stancu E, Guttenberger R: Patients with brain metastases: hope for recursive partitioning analysis (RPA) class 3. Radiother Oncol 2002, 63: 339-345. 10.1016/S0167-8140(02)00119-6
Nieder C, Nestle U, Motaref B, Walter K, Niewald M, Schnabel K: Prognostic factors in brain metastases: should patients be selected for aggressive treatment according to recursive partitioning analysis (RPA) classes? Int J Radiat Oncol Biol Phys 2000, 46: 297-302. 10.1016/S0360-3016(99)00416-2
Fleckenstein K, Hof H, Lohr F, Wenz F, Wannenmacher M: Prognostic factors for brain metastases after whole brain radiotherapy. Data from a single institution. Strahlenther Onkol 2004, 180: 268-273. 10.1007/s00066-004-1234-1
Saito EY, Viani GA, Ferrigno R, Nakamura RA, Novaes PE, Pellizzon CA, Fogaroli RC, Conte MA, Salvajoli JV: Whole brain radiation therapy in management of brain metastasis: results and prognostic factors. Radiat Oncol 2006, 1: 20. 10.1186/1748-717X-1-20
Mahmoud-Ahmed AS, Suh JH, Lee SY, Crownover RL, Barnett GH: Results of whole brain radiotherapy in patients with brain metastases from breast cancer: a retrospective study. Int J Radiat Oncol Biol Phys 2002, 54: 810-817.
Viani GA, Castilho MS, Salvajoli JV, Pellizzon AC, Novaes PE, Guimarães FS, Conte MA, Fogaroli RC: Whole brain radiotherapy for brain metastases from breast cancer: estimation of survival using two stratification systems. BMC Cancer 2007, 7: 53. 10.1186/1471-2407-7-53
Le Scodan R, Massard C, Mouret-Fourme E, Guinebretierre JM, Cohen-Solal C, De Lalande B, Moisson P, Breton-Callu C, Gardner M, Goupil A, Renody N, Floiras JL, Labib A: Brain metastases from breast carcinoma: validation of the Radiation Therapy Oncology Group recursive partitioning analysis classification and proposition of a new prognostic score. Int J Radiat Oncol Biol Phys 2007, 69: 839-845.
Kepka L, Cieslak E, Bujko K, Fijuth H, Wierzchowski M: Results of the whole-brain radiotherapy for patients with brain metastases from lung cancer: the RTOG RPA intra-classes analysis. Acta Oncol 2005, 44: 389-398. 10.1080/02841860510029699
Videtic GM, Adelstein DJ, Mekhail TM, Rice TW, Stevens GH, Lee SY, Suh JH: Validation of the RTOG recursive partitioning analysis (RPA) classification for small-cell lung cancer-only brain metastases. Int J Radiat Oncol Biol Phys 2007, 67: 240-243.
Gülbas H, Erkal HS, Serin M: The use of recursive partitioning analysis grouping in patients with brain metastases from non-small-cell lung cancer. Jpn J Clin Oncol 2006, 36: 193-196. 10.1093/jjco/hyl007
Rades D, Schild SE, Lohynska R, Veninga T, Stalpers LJ, Dunst J: Two radiation regimens and prognostic factors for brain metastases in nonsmall cell lung cancer patients. Cancer 2007, 110: 1077-1082. 10.1002/cncr.22877
Rades D, Bohlen G, Lohynska R, Veninga T, Stalpers LJ, Schild SE, Dunst J: Whole-brain radiotherapy with 20 Gy in 5 fractions for brain metastases in patients with cancer of unknown primary (CUP). Strahlenther Onkol 2007, 183: 631-636. 10.1007/s00066-007-1763-5
Kanner AA, Suh JH, Siomin VE, Lee SY, Barnett GH, Vogelbaum MA: Posterior fossa metastases: aggressive treatment improves survival. Stereotact Funct Neurosurg 2003, 81: 18-23. 10.1159/000075099
Nam TK, Lee JI, Jung YJ, Im YS, An HY, Nam DH, Park K, Kim JH: Gamma knife surgery for brain metastases in patients harboring four or more lesions: survival and prognostic factors. J Neurosurg 2005,102(suppl):147-150.
Buchsbaum JC, Suh JH, Lee SY, Chidel MA, Greskovich JF, Barnett GH: Survival by Radiation Therapy Oncology Group recursive partitioning analysis class and treatment modality in patients with brain metastases from malignant melanoma. Cancer 2002, 94: 2265-2272. 10.1002/cncr.10426
Harrison BE, Johnson JL, Clough RW, Halperin EC: Selection of patients with melanoma brain metastases for aggressive treatment. Am J Clin Oncol 2003, 26: 354-357. 10.1097/00000421-200308000-00009
Morris SL, Low SH, A'Hern RP, Eisen TG, Gore ME, Nutting CM, Harrington KJ: A prognostic index that predicts outcome following palliative whole brain radiotherapy for patients with metastatic malignant melanoma. Br J Cancer 2004, 91: 829-833.
Chidel MA, Suh JH, Reddy CA, Chao ST, Lundbeck MF, Barnett GH: Application of recursive partitioning analysis and evaluation of the use of whole brain radiation among patients treated with stereotactic radiosurgery for newly diagnosed brain metastases. Int J Radiat Oncol Biol Phys 2000, 47: 993-999.
Agboola O, Benoit B, Cross P, Da Silva V, Esche B, Lesiuk H, Gonsalves C: Prognostic factors derived from recursive partitioning analysis (RPA) of Radiation Therapy Oncology Group (RTOG) brain metastases trials applied to surgically resected and irradiated brain metastatic cases. Int J Radiat Oncol Biol Phys 1998, 42: 155-159.
Paek SH, Audu PB, Sperling MR, Cho J, Andrews DW: Reevaluation of surgery for the treatment of brain metastases: review of 208 patients with single or multiple brain metastases treated at one institution with modern neurosurgical techniques. Neurosurgery 2005, 56: 1021-1034.
Tendulkar RD, Liu SW, Barnett GH, Vogelbaum MA, Toms SA, Jin T, Suh JH: RPA classification has prognostic significance for surgically resected single brain metastasis. Int J Radiat Oncol Biol Phys 2006, 66: 810-817.
Rades D, Pluemer A, Veninga T, Dunst J, Schild SE: A boost in addition to whole-brain radiotherapy improves patient outcome after resection of 1 or 2 brain metastases in recursive partitioning analysis class 1 and 2 patients. Cancer 2007, 110: 1551-1559. 10.1002/cncr.22960
Nieder C, Geinitz H, Molls M: Validation of the graded prognostic assessment index for surgically treated patients with brain metastases. Anticancer Res 2008, 28: 3015-3017.
Lagerwaard FJ, Levendag PC, Nowak PJ, Eijkenboom WM, Hanssens PE, Schmitz PI: Identification of prognostic factors in patients with brain metastases: a review of 1292 patients. Int J Radiat Oncol Biol Phys 1999, 43: 795-803.
Weltman E, Salvajoli JV, Brandt RA, de Morais Hanriot R, Prisco FE, Cruz JC, de Oliveira Borges SR, Wajsbrot DB: Radiosurgery for brain metastases: A score index for predicting prognosis. Int J Radiat Oncol Biol Phys 2000, 46: 1155-1161.
Selek U, Chang EL, Hassenbusch SJ 3rd, Shiu AS, Lang FF, Allen P, Weinberg J, Sawaya R, Maor MH: Stereotactic radiosurgical treatment in 103 patients for 153 cerebral melanoma metastases. Int J Radiat Oncol Biol Phys 2004, 59: 1097-1106. 10.1016/j.ijrobp.2003.12.037
Goyal S, Prasad D, Harrell F Jr, Matsumoto J, Rich T, Steiner L: Gamma knife surgery for the treatment of intracranial metastases from breast cancer. J Neurosurg 2005, 103: 218-223.
Gaudy-Marqueste C, Regis JM, Muracciole X, Laurans R, Richard MA, Bonerandi JJ, Grob JJ: Gamma-Knife radiosurgery in the management of melanoma patients with brain metastases: a series of 106 patients without whole-brain radiotherapy. Int J Radiat Oncol Biol Phys 2006, 65: 809-816.
Akyurek S, Chang EL, Mahajan A, Hassenbusch SJ, Allen PK, Mathews LA, Shiu AS, Maor MH, Woo SY: Stereotactic radiosurgical treatment of cerebral metastases arising from breast cancer. Am J Clin Oncol 2007, 30: 310-314. 10.1097/01.coc.0000258365.50975.f6
Nieder C, Molls M: Validation of the graded prognostic assessment index for patients with brain metastases: in regards to Sperduto et al. (Int J Radiat Oncol Biol Phys 2008;70:510–514). Int J Radiat Oncol Biol Phys 2008, 72: 1619.
Lorenzoni J, Devriendt D, Massager N, David P, Ruiz S, Vanderlinden B, Van Houtte P, Brotchi J, Levivier M: Radiosurgery for treatment of brain metastases: Estimation of patient eligibility using three stratification systems. Int J Radiat Oncol Biol Phys 2004, 60: 218-224. 10.1016/j.ijrobp.2004.06.171
Sperduto PW, Berkey B, Gaspar LE, Mehta M, Curran W: A new prognostic index and comparison to three other indices for patients with brain metastases: an analysis of 1,960 patients in the RTOG database. Int J Radiat Oncol Biol Phys 2008, 70: 510-514.
Rades D, Dunst J, Schild SE: A new scoring system to predicting the survival of patients treated with whole-brain radiotherapy for brain metastases. Strahlenther Onkol 2008, 184: 251-255. 10.1007/s00066-008-1831-5
DiLuna ML, King JT Jr, Knisely JP, Chiang VL: Prognostic factors for survival after stereotactic radiosurgery vary with the number of cerebral metastases. Cancer 2007, 109: 135-145. 10.1002/cncr.22367
Bhatnagar AK, Kondziolka D, Lunsford LD, Flickinger JC: Recursive partitioning analysis of prognostic factors for patients with four or more intracranial metastases treated with radiosurgery. Technol Cancer Res Treat 2007, 6: 153-160.
Priestman TJ, Dunn J, Brada M, Rampling R, Baker PG: Final results of the Royal College of Radiologists' trial comparing two different radiotherapy schedules in the treatment of cerebral metastases. Clin Oncol (R Coll Radiol) 1996, 8: 308-315.
Tang SG, Tseng CK, Tsay PK, Chen CH, Chang JW, Pai PC, Hong JH: Predictors for patterns of brain relapse and overall survival in patients with non-small cell lung cancer. J Neurooncol 2005, 73: 153-161. 10.1007/s11060-004-3725-4
Fife KM, Colman MH, Stevens GN, Firth IC, Moon D, Shannon KF, Harman R, Petersen-Schaefer K, Zacest AC, Besser M, Milton GW, McCarthy WH, Thompson JF: Determinants of outcome in melanoma patients with cerebral metastases. J Clin Oncol 2004, 22: 1293-1300. 10.1200/JCO.2004.08.140
Claude L, Perol D, Ray-Coquard I, Petit T, Blay JY, Carrie C, Bachelot T: Lymphopenia: A new independent prognostic factor for survival in patients treated with whole brain radiotherapy for brain metastases from breast carcinoma. Radiother Oncol 2005, 76: 334-339. 10.1016/j.radonc.2005.06.004
Bartsch R, Fromm S, Rudas M, Wenzel C, Harbauer S, Roessler K, Kitz K, Steger GG, Weitmann HD, Poetter R, Zielinski CC, Dieckmann K: Intensified local treatment and systemic therapy significantly increase survival in patients with brain metastases from advanced breast cancer – A retrospective analysis. Radiother Oncol 2006, 80: 313-317. 10.1016/j.radonc.2006.08.001
Nam BH, Kim SY, Han HS, Kwon Y, Lee KS, Kim TH, Ro J: Breast cancer subtypes and survival in patients with brain metastases. Breast Cancer Res 2008, 10: R20. 10.1186/bcr1870
Kirsch DG, Ledezma CJ, Mathews CS, Bhan AK, Ancukiewicz M, Hochberg FH, Loeffler JS: Survival after brain metastases from breast cancer in the trastuzumab era. J Clin Oncol 2005, 23: 2114-2116. 10.1200/JCO.2005.05.249
Eichler AF, Kuter I, Ryan P, Schapira L, Younger J, Henson JW: Survival in patients with brain metastases from breast cancer. Cancer 2008, 112: 2359-2367. 10.1002/cncr.23468
Melisko ME, Moore DH, Sneed PK, De Franco J, Rugo HS: Brain metastases in breast cancer: clinical and pathologic characteristics associated with improvements in survival. J Neurooncol 2008, 88: 359-365. 10.1007/s11060-008-9578-5
Harputluoglu H, Dizdar O, Aksoy S, Kilickap S, Dede DS, Ozisik Y, Guler N, Barista I, Gullu I, Hayran M, Selek U, Cengiz M, Zorlu F, Tekuzman G, Altundag K: Characteristics of breast cancer patients with central nervous system metastases: a single-center experience. J Natl Med Assoc 2008, 100: 521-526.
Park BB, Uhm JE, Cho EY, et al.: Prognostic factor analysis in patients with brain metastases from breast cancer: how can we improve the treatment outcomes? Cancer Chemother Pharmacol 2008. epub.
Church DN, Modgil R, Guglani S, Bahl A, Hopkins K, Braybrooke JP, Blair P, Price CG: Extended survival in women with brain metastases from HER2 overexpressing breast cancer. Am J Clin Oncol 2008, 31: 250-254.
Ogawa K, Toita T, Sueyama H, Fuwa N, Kakinohana Y, Kamata M, Adachi G, Saito A, Yoshii Y, Murayama S: Brain metastases from esophageal carcinoma: natural history, prognostic factors, and outcome. Cancer 2002, 94: 759-764. 10.1002/cncr.10271
Weinberg JS, Suki D, Hanbali F, Cohen ZR, Lenzi R, Sawaya R: Metastasis of esophageal carcinoma to the brain. Cancer 2003, 98: 1925-1933. 10.1002/cncr.11737
Khuntia D, Sajja R, Chidel MA, Lee SY, Rice TW, Adelstein DJ, Carlson TP, Saxton JP, Barnett GH, Suh JH: Factors associated with improved survival in patients with brain metastases from esophageal cancer: a retrospective review. Technol Cancer Res Treat 2003, 2: 267-272.
Cohen ZR, Suki D, Weinberg JS, Marmor E, Lang FF, Gershenson DM, Sawaya R: Brain metastases in patients with ovarian carcinoma: prognostic factors and outcome. J Neurooncol 2004, 66: 313-325. 10.1023/B:NEON.0000014516.04943.38
Cormio G, Maneo A, Colamaria A, Loverro G, Lissoni A, Selvaggi L: Surgical resection of solitary brain metastasis from ovarian carcinoma: an analysis of 22 cases. Gynecol Oncol 2003, 89: 116-119. 10.1016/S0090-8258(03)00060-X
Growdon WB, Lopez-Varela E, Littell R, Oliva E, Seiden M, Krasner C, Lee H, Fuller A: Extent of extracranial disease is a powerful predictor of survival in patients with brain metastases from gynecological cancer. Int J Gynecol Cancer 2008, 18: 262-268. 10.1111/j.1525-1438.2007.01011.x
Tremont-Lukats IW, Bobustuc G, Lagos GK, Lolas K, Kyritsis AP, Puduvalli VK: Brain metastasis from prostate carcinoma: The M. D. Anderson Cancer Center experience. Cancer 2003, 98: 363-368. 10.1002/cncr.11522
Bartelt S, Lutterbach J: Brain metastases in patients with cancer of unknown primary. J Neurooncol 2003, 64: 249-253. 10.1023/A:1025621819250
Rudà R, Borgognone M, Benech F, Vasario E, Soffietti R: Brain metastases from unknown primary tumour: a prospective study. J Neurol 2001, 248: 394-398. 10.1007/s004150170180
Kim SH, Weil RJ, Chao ST, Toms SA, Angelov L, Vogelbaum MA, Suh JH, Barnett GH: Stereotactic radiosurgical treatment of brain metastases in older patients. Cancer 2008, 113: 834-840. 10.1002/cncr.23625
Noel G, Bollet MA, Noel S, Feuvret L, Boisserie G, Tep B, Delattre JY, Baillet F, Ambroise Valery C, Cornu P, Mazeron JJ: Linac stereotactic radiosurgery: an effective and safe treatment for elderly patients with brain metastases. Int J Radiat Oncol Biol Phys 2005, 63: 1555-1561. 10.1016/j.ijrobp.2005.04.037
Chatani M, Matayoshi Y, Masaki N, Inoue T: Radiation therapy for brain metastases from lung carcinoma. Prospective randomized trial according to the level of lactate dehydrogenase. Strahlenther Onkol 1994, 170: 155-161.
Jacot W, Quantin X, Boher JM, Andre F, Moreau L, Gainet M, Depierre A, Quoix E, Chevalier TL, Pujol JL: Brain metastases at the time of presentation of non-small cell lung cancer: a multi-centric AERIO analysis of prognostic factors. Br J Cancer 2001, 84: 903-909. 10.1054/bjoc.2000.1706
Gripp S, Moeller S, Bölke E, Schmitt G, Matuschek C, Asgari S, Asgharzadeh F, Roth S, Budach W, Franz M, Willers R: Survival prediction in terminally ill cancer patients by clinical estimates, laboratory tests, and self-rated anxiety and depression. J Clin Oncol 2007, 25: 3313-3320. 10.1200/JCO.2006.10.5411
Hauser CA, Stockler MR, Tattersall MH: Prognostic factors in patients with recently diagnosed incurable cancer: a systematic review. Support Care Cancer 2006, 14: 999-1011. 10.1007/s00520-006-0079-9
Rodrigus P, de Brouwer P, Raaymakers E: Brain metastases and non-small cell lung cancer. Prognostic factors and correlation with survival after irradiation. Lung Cancer 2001, 32: 129-136. 10.1016/S0169-5002(00)00227-0
Raizer JJ, Hwu WJ, Panageas KS, Wilton A, Baldwin DE, Bailey E, von Althann C, Lamb LA, Alvarado G, Bilsky MH, Gutin PH: Brain and leptomeningeal metastases from cutaneous melanoma: survival outcomes based on clinical features. Neuro Oncol 2008, 10: 199-207. 10.1215/15228517-2007-058
Murray KJ, Scott C, Zachariah B, Michalski JM, Demas W, Vora NL, Whitton A, Movsas B: Importance of the mini-mental status examination in the treatment of patients with brain metastases: a report from the Radiation Therapy Oncology Group protocol 91-04. Int J Radiat Oncol Biol Phys 2000, 48: 59-64. 10.1016/S0360-3016(00)00600-3
Mehta MP, Shapiro WR, Phan SC, Gervais R, Carrie C, Chabot P, Patchell RA, Glantz MJ, Recht L, Langer C, Sur RK, Roa WH, Mahe MA, Fortin A, Nieder C, Meyers CA, Smith JA, Miller RA, Renschler MF: Motexafin gadolinium combined with prompt whole brain radiotherapy prolongs time to neurologic progression in non-small-cell lung cancer patients with brain metastases: results of a phase III trial. Int J Radiat Oncol Biol Phys 2009, 73: 1069-1076.
Acknowledgements
None
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
CN and MM drafted the manuscript and participated in the design of the study. Both authors read and approved the final manuscript.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
This article is published under license to BioMed Central Ltd. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Nieder, C., Mehta, M.P. Prognostic indices for brain metastases – usefulness and challenges. Radiat Oncol 4, 10 (2009). https://doi.org/10.1186/1748-717X-4-10
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1748-717X-4-10