Abstract
Many currently used vaccines are less immunogenic in the elderly compared to young adults. The impact of latent infection with Cytomegalovirus (CMV) on vaccine-induced antibody responses has been discussed controversially. We have demonstrated that recall responses to diphtheria vaccination are frequently insufficient in elderly persons and that antibody concentrations decline substantially within 5 years. In the current study we show that within a cohort of healthy elderly (n = 87; median age 71 years, range 66–92) antibody responses to a booster vaccination against diphtheria do not differ between CMV-negative and CMV-positive individuals 4 weeks after vaccination.. However, the goal of diphtheria-vaccination is long-term protection and this is achieved by circulating anti-toxin antibodies. Diphtheria-specific antibody concentrations decline faster in CMV-positive compared to CMV-negative older adults leading to an increased proportion of persons without protective antibody concentrations 5 years after booster vaccination and endangering long-term protection. This finding could be relevant for vaccination schedules.
Similar content being viewed by others
Aging is associated with characteristic changes of the immune system, collectively termed immunosenescence, which contribute to increased incidence and severity of infection [1] and to decreased immunogenicity and efficacy of vaccination [2, 3]. One hallmark of immunosenescence is the involution of the thymus, i.e. the gradual replacement of functional thymic tissue by fat [4], leading to a severely decreased output of newly generated naïve T cells and as a consequence to the loss of naïve T cells in lymphoid organs and in the periphery [5, 6]. Concomitantly, highly differentiated effector T cells accumulate resulting in alterations of the cytokine profile and in decreased diversity of the T cell repertoire [7]. Several studies have demonstrated that latent infection with the human β-herpesvirus Cytomegalovirus (CMV), which is prevalent without clinical symptoms in 60–100% of the adult population, aggravates age-related changes of the T cell compartment [6, 8, 9] and is part of the “immune risk phenotype”, which predicts 2-year mortality in the very elderly [10]. Epidemiological studies indicate that CMV-seropositivity is associated with a slight increase in overall mortality [11, 12]. It has been demonstrated recently that CMV also affects B cell function. CMV-seropositivity is associated with decreased switched memory B cells and in vitro activation of activation-induced cytidine deaminase (AID), which are predictors for successful influenza vaccination [13]. In addition, CMV drives the expansion of CD56dimCD57+NKG2C+ NK cells [14], skewing the NK cell repertoire to more cytotoxic responses at the expense of cytokine-driven functions. As a result, in vitro NK cell responses to influenza and pertussis vaccine antigens are impaired [15]. The impact of CMV-seropositivity or the level of CMV-specific antibodies on immune responses after vaccination is controversially discussed. Some studies report that antibody responses to vaccines, e.g. against influenza, are lower in CMV-seropositive older individuals, or in persons with high concentrations of CMV-specific antibodies [16, 17]. In contrast, other studies did not observe an impact of CMV-infection on vaccine-induced immune responses against influenza or Streptococcus pneumoniae [18, 19]. No data are available regarding the impact of latent infection with CMV on the long-term maintenance of vaccine-induced antibodies. We therefore addressed this question using data from one of our previously published studies on the maintenance of tetanus- and diphtheria-specific antibodies after vaccination of an elderly cohort [20, 21]. We have demonstrated that recall responses to diphtheria vaccination are frequently insufficient in elderly persons and that antibody concentrations decline substantially within 5 years. Two hundred two older adults (>60 years) received a single shot of tetanus and diphtheria containing vaccine and antibody concentrations were measured before and 4 weeks after vaccination [20]. Five years later 87 persons of the original cohort were willing to participate in a follow-up study and received a second dose of tetanus and diphtheria vaccine. Analysis of the long-term persistence of tetanus- and diphtheria-specific antibodies was performed for this sub-cohort [21]. We demonstrated that tetanus- and diphtheria-specific antibody concentrations had dropped to the level before the first vaccination within 5 years. As tetanus-specific antibody concentrations were generally higher, almost all participants were still protected. In contrast, 45% of our elderly cohort did not have protective levels of diphtheria-specific antibodies 5 years after re-vaccination (Table 1).
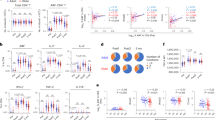
As protection against tetanus was generally high, we focused on diphtheria in the current study and aimed to evaluate the impact of latent infection with CMV on the long-term maintenance of diphtheria-specific antibodies. Antibody concentrations were similar for CMV-negative and CMV-positive participants before the first vaccination. The vaccination history was very variable at this time point probably masking a potential impact of latent CMV-infection. Antibody concentrations increased to the same extent in both groups after vaccination. Five years later antibody concentrations had dropped to the original levels in the CMV-positive group. In contrast, antibody concentrations were still higher than baseline for the CMV-negative group. The difference between CMV-negative and CMV-positive participants was statistically significant at this time point. After the second vaccination, antibody concentrations increased to similar levels in both groups (Fig. 1a).
Antibody concentrations and percentage of persons with protective antibody concentrations. Diphtheria-specific antibody concentrations were measured by ELISA prior to and 4 weeks after vaccination. Two doses of diphtheria toxoid containing vaccines were applied in a 5-year interval. a Boxes show 25th and 75th percentiles, the median value is indicated. Whiskers depict 5th and 95th percentiles. Only donors, for whom data points were available at all 4 time points are included in the analysis. Differences between CMV-negative and CMV-positive groups were calculated using Mann-Whitney U test. Differences between day 0 (1st vaccination) and day 0 (2nd vaccination) were calculated using Wilcoxon signed-rank test. *p < 0.05; **p < 0.01; n.s.: not significant. b Percentage of participants with antibody concentrations above (protected, solid bar) or below (unprotected, dashed bar) levels considered to be protective (0.1 IU/ml). The CMV-negative group is shown in white, the CMV-positive group in gray. Differences in the percentage of persons protected / unprotected were calculated using Pearson Chi-square test. *p < 0.05; **p < 0.01; n.s.: not significant
These differences in antibody concentrations were also reflected in the percentage of persons with antibody concentrations below the level considered to be protective (≤0.1 IU/ml). Five years after the first vaccination, 54% of the CMV-positive donors were not protected, whereas only 33% of the CMV-negative donors had antibody concentrations below the protective level at this time point. There were no differences in the levels of protection between CMV-positive and CMV-negative participants at the other time points (Fig. 1b). The difference in antibody concentrations observed prior to the second vaccination is due to a more pronounced decline of antibody concentrations over 5 years in the CMV-positive cohort (Fig. 2).
Decrease of diphtheria-specific antibody concentrations over 5 years. Depicted is the fold-reduction of diphtheria-specific antibodies from 4 weeks after the first vaccination until immediately before the second vaccination (5 years). Boxes show 25th and 75th percentiles, the median value is indicated. Whiskers depict 5th and 95th percentiles. The differences between CMV-negative and CMV-positive groups was calculated using Mann-Whitney U test. *p < 0.05
T and B cells subsets of 19 CMV-negative and 18 CMV-positive participants were analyzed by flow cytometry at the time of the second vaccination. CMV-related changes of T cell subsets, namely a decrease of naïve and an increase of effector T cell subsets in CMV-positive persons have been described [6, 8, 9] and could be confirmed in our cohort. In contrast, no differences of B cell subsets were observed (Table 2). Age-related changes in the B cell compartment include a loss of naïve B cells and an accumulation of highly differentiated double negative (IgD−CD27−) cells [22], which were also termed exhausted, and have been described to be associated with lower responses to influenza vaccination [23]. However, in accordance with our data, it has previously been reported that the influence of latent CMV infection on the composition of B cell subsets is only minimal [24], but that CMV infection might influence the B cell repertoire [25].
summary, our data show that diphtheria-specific antibody concentrations decline faster in CMV-positive compared to CMV-negative older adults leading to an increased proportion of persons without protective antibody concentrations 5 years after booster vaccination and endangering long-term protection. This finding could be relevant for vaccination schedules. One possible reason for the faster decline of antibody concentrations might be an impaired maintenance and/or survival of long-lived plasma cells in the bone marrow. We have previously reported a decrease of diphtheria-specific plasma cells in the bone marrow with age [26], but the CMV-status was not taken into consideration in this small cohort. Recent data in our laboratory showed an increase of inflammatory and oxidative stress parameters in the bone marrow of older patients and at the same time a decrease of IL-7 and a proliferation-inducing ligand (APRIL), which is a survival factor for plasma cells [27]. The impact of latent CMV-infection on the bone marrow microenvironment and the antigen-experienced lymphocytes residing there is not yet known.
Materials and methods
Study cohort
For this study the 87 persons, who completed the 5-year follow-up and received two vaccinations against tetanus and diphtheria were included. In accordance with the original study protocol persons with chronic viral infection (Human Immunodeficiency virus, Hepatitis B virus, Hepatitis C virus), transplant recipients and patients under immunosuppressive or chemotherapy were excluded. Routine laboratory parameters (liver and kidney function, blood count) were determined. All participants were shown to be in good health and there were no differences between CMV-negative and CMV-positive persons. Table 3 shows the patient characteristics for the CMV-negative and the CMV-positive sub-cohort.
Determination of IgG antibody concentrations
Microtiter plates were coated with 1 μg/ml diphtheria toxoid (Statens Serum Institute) and blocked with 0.01 M Glycin. Serum samples were tested in duplicates. Peroxidase-labeled rabbit anti-human IgG (Chemicon/Millipore) antibody was used as secondary antibody. IgG antibodies were quantified in IU/ml using standard human anti-diphtheria serum (NIBSC). The detection limit of the assays used was 0.01 IU/ml and values below the limit of detection were set to 0.005 IU/ml. Antibody concentrations above 0.1 IU/ml were considered as protective.
Antibodies against Cytomegalovirus (CMV) were determined using a commercially available ELISA Kit (Siemens). Reciprocal titers above 231 were considered positive.
Flow cytometry
PBMC were washed with PBS and stained with anti-CD3-PE-Cy7 (Biolegend), anti-CD4-PerCP (BD Pharmingen), anti-CD8-PE (BD Pharmingen), anti CD28-APC (Biolegend), anti CD45RO-FITC (BD Pharmingen), anti-CD20-PerCP (Biolegend), anti-CD27-APC-Cy7(Biolegend) and anti-IgD-FITC (BD Pharmingen) antibodies for 20 min, 4 °C in the dark. After washing with PBS, cells were analyzed using a FACS Canto II cytometer and FACSDiva software (BD).
Statistical analysis
Comparisons between two independent groups (CMV-negative vs. CMV-positive) were calculated using Mann-Whitney U test. Differences between paired samples (different time points) were calculated using Wilcoxon signed-rank test. The distribution of categorical data (e.g. protected/unprotected) was calculated using the Pearson Chi-square test. p < 0.05 was considered significant for all tests.
References
Gavazzi G, Krause KH. Ageing and infection. Lancet Infect Dis. 2002;2:659–66.
Weinberger B, Herndler-Brandstetter D, Schwanninger A, Weiskopf D, Grubeck-Loebenstein B. Biology of immune responses to vaccines in elderly persons. Clin Infect Dis. 2008;46:1078–84.
Chen WH, Kozlovsky BF, Effros RB, Grubeck-Loebenstein B, Edelman R, Sztein MB. Vaccination in the elderly: an immunological perspective. Trends Immunol. 2009;30:351–9.
Steinmann GG. Changes in the human thymus during aging. Curr Top Pathol. 1986;75:43–88.
Lazuardi L, Jenewein B, Wolf AM, Pfister G, Tzankov A, Grubeck-Loebenstein B. Age-related loss of naive T cells and dysregulation of T-cell/B-cell interactions in human lymph nodes. Immunology. 2005;114:37–43.
Almanzar G, Schwaiger S, Jenewein B, Keller M, Herndler-Brandstetter D, Wurzner R, et al. Long-term cytomegalovirus infection leads to significant changes in the composition of the CD8+ T-cell repertoire, which may be the basis for an imbalance in the cytokine production profile in elderly persons. J Virol. 2005;79:3675–83.
Arnold CR, Wolf, J, Brunner, S, Herndler-Brandstetter, D, Grubeck-Loebenstein, B. Gain and loss of T cell subsets in old age-age-related reshaping of the T cell repertoire. J Clin Immunol. 2011;in print:
Khan N, Shariff N, Cobbold M, Bruton R, Ainsworth JA, Sinclair AJ, et al. Cytomegalovirus seropositivity drives the CD8 T cell repertoire toward greater clonality in healthy elderly individuals. J Immunol. 2002;169:1984–92.
Weinberger B, Lazuardi L, Weiskirchner I, Keller M, Neuner C, Fischer KH, et al. Healthy aging and latent infection with CMV lead to distinct changes in CD8+ and CD4+ T-cell subsets in the elderly. Hum Immunol. 2007;68:86–90.
Olsson J, Wikby A, Johansson B, Lofgren S, Nilsson BO, Ferguson FG. Age-related change in peripheral blood T-lymphocyte subpopulations and cytomegalovirus infection in the very old: the Swedish longitudinal OCTO immune study. Mech Ageing Dev. 2000;121:187–201.
Simanek AM, Dowd JB, Pawelec G, MelzerD DA, Aiello AE. Seropositivity to cytomegalovirus, inflammation, all-cause and cardiovascular disease-related mortality in the United States. PLoS One. 2011;6:e16103.
Roberts ET, Haan MN, Dowd JB, Aiello AE. Cytomegalovirus antibody levels, inflammation, and mortality among elderly Latinos over 9 years of follow-up. Am J Epidemiol. 2010;172:363–71.
Frasca D, Diaz A, Romero M, Landin AM, Blomberg BB. Cytomegalovirus (CMV) seropositivity decreases B cell responses to the influenza vaccine. Vaccine. 2015;33:1433–9.
Guma M, Angulo A, Vilches C, Gomez-Lozano N, Malats N, Lopez-Botet M. Imprint of human cytomegalovirus infection on the NK cell receptor repertoire. Blood. 2004;104:3664–71.
Nielsen CM, White MJ, Bottomley C, Lusa C, Rodriguez-Galan A, Turner SE, et al. Impaired NK cell responses to Pertussis and H1N1 influenza vaccine antigens in human cytomegalovirus-infected individuals. J Immunol. 2015;194:4657–67.
Trzonkowski P, Mysliwska J, Szmit E, Wieckiewicz J, Lukaszuk K, Brydak LB, et al. Association between cytomegalovirus infection, enhanced proinflammatory response and low level of anti-hemagglutinins during the anti-influenza vaccination--an impact of immunosenescence. Vaccine. 2003;21:3826–36.
Derhovanessian E, Theeten H, Hahnel K, Van DP, Cools N, Pawelec G. Cytomegalovirus-associated accumulation of late-differentiated CD4 T-cells correlates with poor humoral response to influenza vaccination. Vaccine. 2013;31:685–90.
den Elzen WP, Vossen AC, Cools HJ, Westendorp RG, Kroes AC, Gussekloo J. Cytomegalovirus infection and responsiveness to influenza vaccination in elderly residents of long-term care facilities. Vaccine. 2011;29:4869–74.
O'Connor D, Truck J, Lazarus R, Clutterbuck EA, Voysey M, Jeffery K, et al. The effect of chronic cytomegalovirus infection on pneumococcal vaccine responses. J Infect Dis. 2014;209:1635–41.
Kaml M, Weiskirchner I, Keller M, Luft T, Hoster E, Hasford J, et al. Booster vaccination in the elderly: their success depends on the vaccine type applied earlier in life as well as on pre-vaccination antibody titers. Vaccine. 2006;24:6808–11.
Weinberger B, Schirmer M, Matteucci GR, Siebert U, Fuchs D, Grubeck-Loebenstein B. Recall responses to tetanus and diphtheria vaccination are frequently insufficient in elderly persons. PLoS One. 2013;8:e82967.
Bulati M, Caruso C, Colonna-Romano G. From lymphopoiesis to plasma cells differentiation, the age-related modifications of B cell compartment are influenced by "inflamm-ageing". Ageing Res Rev. 2017;36:125–36.
Frasca D, Diaz A, Romero M, Blomberg BB. Human peripheral late/exhausted memory B cells express a senescent-associated secretory phenotype and preferentially utilize metabolic signaling pathways. Exp Gerontol. 2017;87:113–20.
Goldeck D, Oettinger L, Janssen N, Demuth I, Steinhagen-Thiessen E, Pawelec G. Cytomegalovirus infection minimally affects the frequencies of B-cell phenotypes in peripheral blood of younger and older adults. Gerontology. 2016;62:323–9.
Wang C, Liu Y, Xu LT, Jackson KJ, Roskin KM, Pham TD, et al. Effects of aging, cytomegalovirus infection, and EBV infection on human B cell repertoires. J Immunol. 2014;192:603–11.
Pritz T, Lair J, Ban M, Keller M, Weinberger B, Krismer M, et al. Plasma cell numbers decrease in bone marrow of old patients. Eur J Immunol. 2015;45:738–46.
Pangrazzi L, Meryk A, Naismith E, Koziel R, Lair J, Krismer M, et al. "Inflamm-aging" influences immune cell survival factors in human bone marrow. Eur J Immunol. 2016;47(3):481–92.
Acknowledgements
Not applicable.
Funding
This work was supported by funds of the Oesterreichische Nationalbank (Anniversary Fund, project number 13524; www.oenb.at). The research leading to these results has received funding from the European Union’s Seventh Framework Programme [FP7/2007–2013] under Grant Agreement No: 280,873 ADITEC. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Availability of data and materials
The datasets supporting the conclusions of this article are included within the article and the Additional file 1. All authors read and approved the final manuscript.
Author information
Authors and Affiliations
Contributions
BW planned the study, performed experiments, analyzed data, recruited participants and wrote the manuscript. MK performed experiments and analyzed data. BGL planned the study and wrote the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study was approved by the local ethics committee (Medical University, Innsbruck, Austria) and in accordance with changes in the legal requirements the second vaccination was registered at the EU Clinical Trials Register (EU-CTR) as an open exploratory Phase 4 clinical trial with the EUDRACT number 2009–011742-26. All participants gave their written informed consent.
Consent for publication
Not applicable.
Competing interests
All authors declare that they do not have any competing interests.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Additional file
Additional file 1:
Data table. (XLSX 23 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Weinberger, B., Keller, M. & Grubeck-Loebenstein, B. Long-term maintenance of diphtheria-specific antibodies after booster vaccination is hampered by latent infection with Cytomegalovirus. Immun Ageing 14, 16 (2017). https://doi.org/10.1186/s12979-017-0099-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12979-017-0099-y