Abstract
Purpose
To investigate the association of serum levels of 25(OH)D and 1,25(OH)2D3 in healthy and non-healthy controls with Crohn’s disease (CD) and ulcerative colitis (UC).
Methods
Three electronic databases: PubMed, EMbase and EBSCOhost CINAHL, were searched for observational studies to measure the relationship between serum levels of vitamin D (VitD) and CD (or UC).
Results
Fifty-five studies were included in the meta-analysis. We found that mean serum 25(OH)D levels in patients with CD were significantly lower than those in healthy controls (MD: − 3.17 ng/mL; 95% CI − 4.42 to − 1.93). Results from the meta-analysis examining 1,25(OH)2D3 levels in Crohn’s patients revealed higher levels in the CD group than in healthy (MD: 3.47 pg/mL; 95% CI − 7.72 to 14.66) and UC group (MD: 5.05 pg/mL; 95% CI − 2.42 to 12.52). Serum 25(OH)D levels were lower in the UC group than in the healthy control group (MD: − 2.52 ng/mL; 95% CI − 4.02 to − 1.02). In studies investigating the level of 1,25(OH)2D3 in UC and healthy control groups, the level of 1,25(OH)2D3 in the UC groups were found to be higher than that in the control groups (MD: 3.76 pg/mL; 95% CI − 8.36 to 15.57). However, the 1,25(OH)2D3 level in patients with UC was lower than that in CD groups (MD: − 6.71 pg/mL; 95% CI − 15.30 to 1.88). No significant difference was noted between CD patients and UC patients in terms of average serum 25(OH)D levels.
Conclusions
This study found that VitD levels were inversely related to CD and UC. Serum levels of 25(OH)D were lower in patients with CD and UC than in healthy people, and more than half of the patients had insufficient vitamin D levels. The serum level of 1,25(OH)2D3 in both the CD and UC groups was higher than that in healthy people.
Similar content being viewed by others
Introduction
Inflammatory bowel disease (IBD), including the two major forms: Crohn’s disease (CD) and ulcerative colitis (UC), is a chronic, relapsing–remitting systemic disease that typically begins in young adulthood and lasts throughout life. Although progress has been made in understanding these diseases, their etiology is unknown [1]. CD is a chronic inflammatory disease characterized by discontinuously affected areas with transmural, granulomatous inflammation and/or fistula, and can affect any region in the digestive tract, from the mouth to the anus, but is more likely to involve the small and large intestines (especially the ileocecum) and the perianal region. UC is a diffuse, non-specific inflammatory disease of unknown cause that continuously affects the proximal colonic mucosa from the rectum and often forms erosions and/or ulcers [2]. Since there is currently no cure for IBD, medical therapy remains the primary treatment for achieving and maintaining remission [3].
Currently, there is general agreement that variations in a patient’s genetic make-up, broad changes in the surrounding environment, alterations in the composition of gut microbiota, and the reactivity of the intestinal mucosal immune response are at the foundation of IBD pathogenesis [4]. Vitamin D (VitD) is known to induce and maintain the alleviation of IBD through anti-bacterial and anti-inflammatory actions and repair of the intestinal mucosal barrier [5, 6]. VitD belongs to a family of fat-soluble secosteroid hormones and comprises two major forms: VitD2 (ergocalciferol) and VitD3 (cholecalciferol) [7]. VitD3 is hydroxylated in the liver into 25(OH)D and subsequently in the kidney into 1,25(OH)2D3 [8]. VitD has been shown to target the three major components of the gastrointestinal epithelial barrier, intestinal immunity and intestinal microflora and has multiple effects on intestinal health [9]. Through active intestinal signaling, which has immunomodulatory and immunosuppressive effects on inflammatory and inhibitory markers of IBD, VitD interferes with the immune response to bacterial activity, antigen presentation and adaptive and innate immune regulation. Therefore, VitD may affect the incidence and progression of UC and CD [10,11,12]. While attempting to rule out VitD deficiency in patients with IBD due to reduced physical activity, sunlight exposure, malnutrition, inadequate dietary intake of VitD, or lower bioavailability, some studies [3, 13, 14] have found that VitD deficiency is also common in newly diagnosed IBD patients. Thus, VitD deficiency may play a role in the development of IBD and its severity. Other studies, however, have taken the opposite view of the relationship [15] between VitD and IBD and have left the controversy unresolved for patients with CD [16] and UC [17, 18]. Therefore, to explore this controversy we performed a pooled meta-analysis to investigate and determine the status of VitD in the serum of healthy and non-healthy controls and to study the association between serum 25(OH)D and 1,25(OH)2D3 concentrations and an IBD diagnosis (both UC and CD).
Materials and methods
Search strategy
All studies were obtained by searching PubMed, EMbase and EBSCOhost CINAHL for articles that were published through April 8, 2019. Detailed search strategies are shown in Additional file 1: Method S1.
Inclusion and exclusion criteria
Studies were eligible for analysis if they met the following criteria: (1) all included studies were limited to observational investigations in English; (2) serum VitD levels were detected in CD or UC patients; (3) when several trials from the same authors were identified as duplicates, we only included the most recent trial with the largest number of patients or with a longer follow-up period. The healthy control group was defined as those without CD or UC, and the non-healthy control was defined as patients diagnosed with CD or UC, but it was different from the exposed group.
Exclusion criteria included: (1) studies conducted exclusively on patients with IBD diseases, but not CD or UC; (2) studies that did not present any distinct serum levels of VitD; (3) studies that did not include the standard deviation of mean serum levels of VitD, and attempts to get these values by contacting the authors through email were unsuccessful; (4) non-full-text English articles.
Data extraction
For each included study, two investigators independently extracted the following essential information: name of the first author, publication year, study design, disease type, country, age, sex, use of any matching or adjustment approach, maturity, VitD assessment tool, VitD deficiency definition, and VitD supplementation. Disagreements were resolved through discussion or from a third party.
Study quality assessment
The quality of each study from case–control and cohort study in the meta-analysis was assessed using the Newcastle–Ottawa Scale [19, 20], which ranges from 1 to 9 stars and judges each study according to three aspects: selection of the study groups; the comparability of the groups; and, the ascertainment of the outcome of interest. For the cross-sectional study, the quality assessment method from were employed by The Joanna Briggs Institute Critical Appraisal tools for use in JBI Systematic Reviews [21].
Data analysis
For continuous data, the mean difference (MD) and 95% confidence interval (CI) were calculated [22]. If different measurement indices adopted different tools in the various studies, the standardized mean difference (SMD) was used [22]. A fixed-effects model was used when there was no significant heterogeneity (P > 0.1, I2 < 40%), otherwise, a random-effect model was employed [23]. To further explore sources of heterogeneity, subgroup analyses were performed according to age, VitD measurement tools, VitD supplementation, and study design based on both healthy and non-healthy populations using 25(OH)D and 1,25(OH)2D3. Publication bias was assessed by visual inspection of funnel plots [24]. Sensitivity analysis was used to explore the extent to which extrapolation might depend on a particular study or group of studies, excluding small sample studies (both groups < 30) and studies with low study scores (< 5) to discuss the sources of heterogeneity. R 3.4.4 software was performed for all statistical analyses.
Results
Study characteristics
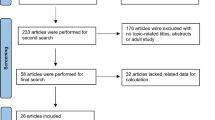
The literature search identified 1385 individual studies. After removing 298 duplicates, 1087 potentially relevant studies were selected on the basis of the abstract, and of these, 119 full texts were assessed for eligibility. In total, 55 publications [16, 18, 25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77] were included in the meta-analysis (Fig. 1).
A total of 19 cohort studies [18, 34, 38, 41, 50,51,52,53,54,55,56, 64, 67, 68, 71, 73, 74, 76, 77], 22 case–control studies [16, 25,26,27,28,29, 31,32,33, 35, 42, 43, 46, 49, 59,60,61,62,63, 66, 69, 70] and 14 cross-sectional studies [30, 36, 37, 39, 40, 44, 45, 47, 48, 57, 58, 65, 72, 75] were included in the analysis. The total number of participants was 5123 patients and 3033 healthy controls. Different studies investigated a range of VitD deficiency values: some used 20 ng/mL [16, 18, 35, 36, 40, 42, 48, 51, 54, 55, 64, 65, 67, 68, 72,73,74,75] (50 nmol/L) (n = 18); Other studies used 15 ng/mL [31, 37, 46, 49, 57] (n = 5), 10 ng/mL [32, 41, 50, 62] (n = 4), 12 ng/mL [59,60,61] (n = 3) or 30 ng/mL [56, 65] (n = 2). The mean difference in 25(OH)D concentrations among patients with CD compared with healthy controls ranged between − 16.58 and 8.19 ng/mL and between − 8.98 and 7.50 ng/mL for non-healthy controls. The values for 1,25(OH)2D ranged between − 11.50 and 34.79 pg/mL for healthy controls and between − 5.70 and 22.80 pg/mL for non-healthy controls. The mean difference between 25(OH)D levels among patients with UC compared with healthy controls ranged between − 18.07 and 2.90 ng/mL and between − 4.25 and 8.98 ng/mL for non-healthy controls. The values for 1,25(OH)2 D3 ranged between − 8.24 and 25.25 pg/mL for healthy controls and between − 22.80 and 5.70 pg/mL for non-healthy controls. Most of the studies matched cases and controls for age and gender. A few studies used race, body mass index, weight and smoking as additional matching variables and most did not include VitD supplements.
Table 1 shows that the quality scores of the included studies ranged from 2 to 7, with a median of 5. Thirty-two studies [16, 18, 29,30,31, 33,34,35, 37,38,39,40, 44, 45, 47,48,49, 51, 52, 54, 55, 61, 62, 64,65,66, 70, 73,74,75,76,77] were considered high quality and the others [25,26,27,28, 31, 36, 41,42,43, 46, 50, 53, 56,57,58,59,60, 63, 67,68,69, 71, 72] were low quality.
Findings of the meta-analysis for serum 25(OH)D levels in Crohn’s patients
A total of 31 studies [16, 25, 29, 31,32,33,34,35,36, 43, 44, 46, 49, 53,54,55, 57, 60,61,62,63,64,65,66, 68,69,70,71,72, 76, 77] were conducted on serum 25(OH)D levels in CD and healthy controls, and we conducted a meta-analysis of 29 effect values. We found mean serum 25(OH)D levels in patients with CD were significantly lower than in healthy controls (MD: − 3.17 ng/mL; 95% CI − 4.42 to − 1.93) (Fig. 2). There was significant heterogeneity among the studies (I2 = 88%, P < 0.01). Subgroup analysis (Table 2) showed that the mean serum 25(HO)D levels in adult CD patients was statistically significant compared to the control group (MD: − 3.22 ng/mL; 95% CI − 4.75 to − 1.70) and children (MD: − 3.16 ng/mL; 95% CI − 5.54 to − 0.77). Compared with the control group, CLIA (MD: − 1.32 ng/mL; 95% CI − 8.89 to 6.26), ELISA (MD: − 8.29 ng/mL; 95% CI − 13.83 to − 2.76) and RIA (MD: − 3.22 ng/mL; 95% CI − 4.46 to − 0.13) were statistically significant, while CPBA, HPLC and LC–MS showed no statistical significance. Both the presence and absence of VitD supplementation was statistically significant (MD: − 1.49 ng/mL; 95% CI − 4.40 to 1.42) and (MD: − 3.46 ng/mL; 95% CI − 4.90 to − 2.03), respectively. In regards to study design, case–control studies (MD: − 4.95 ng/mL; 95% CI − 7.18 to − 2.72) and cohort studies (MD: − 2.11 ng/mL; 95% CI − 3.69 to − 0.53) reported statistically significant results to the control group, but the cross-sectional studies did not find statistically significant differences. In sensitivity results, the residual results were unchanged after excluding small sample studies (MD: − 3.48 ng/mL; 95% CI − 4.78 to − 2.17) or excluding studies with lower quality score (MD: − 2.12 ng/mL; 95% CI − 3.34 to − 0.90).
The discussion between CD and UC about serum 25(OH)D levels were identified in thirty-seven studies [16, 18, 27,28,29,30, 32, 34, 36,37,38,39,40,41,42, 44,45,46,47,48, 50,51,52, 54, 56, 58, 61, 62, 64, 66,67,68, 71, 73, 75,76,77], which included a total of 2494 CD patients and 2017 non-healthy controls. The analysis revealed no significant difference in average serum 25(OH)D levels between the two groups (MD: − 0.58 ng/mL; 95% CI − 1.74 to 0.59) (Fig. 3). There was significant heterogeneity among the studies (I2 = 84%, P < 0.01). Subgroup analysis showed that only ECLIA (MD: 1.34 ng/mL; 95% CI 0.17–2.52) and the use of VitD supplementation (MD: 2.36 ng/mL; 95% CI 1.46–3.25) were statistically significant (Table 2). In sensitivity results, the residual results were unchanged after excluding small sample studies (MD: − 0.51 ng/mL; 95% CI − 1.69 to 0.66) or excluding studies with lower quality score (MD: − 0.90 ng/mL; 95% CI − 2.12 to 0.31).
Findings from the meta-analysis of 1,25(OH)2D3 levels in Crohn’s patients
Eight studies [26, 29, 32, 34, 46, 55, 59, 70] reported average serum 1,25(OH)2D3 concentrations in Crohn’s patients, and these were higher in the CD group in comparison with the healthy control group (MD: 3.47 pg/mL; 95% CI − 7.72 to 14.66) (Fig. 4). There was significant heterogeneity among the studies (I2 = 98%, P < 0.01). Subgroup analysis showed that the CPBA (MD: 15.70 ng/mL; 95% CI 15.20–16.20) was the only statistically significant variable (Table 2).
In sensitivity results, the residual results were unchanged after excluding small sample studies (MD: 5.02 ng/mL; 95% CI − 6.86 to 16.90) or excluding studies with lower quality score (MD: 3.46 ng/mL; 95% CI − 9.58 to 16.49).
In 9 included studies [26, 28,29,30, 32, 34, 38, 46, 59], the combined effect of the 1,25(OH)2D3 concentration on the comparison between CD patients and UC group was 5.05 pg/mL (95% CI − 2.42 to 12.52) (Fig. 5). There was significant heterogeneity among the studies (I2 = 97%, P < 0.01). Subgroup analysis showed that only the cohort study design (MD: 16.57 ng/mL; 95% CI 15.47–17.66) was statistically significant (Table 2). Sensitivity analysis results remained unchanged after the removing studies of lower quality score (MD: 3.56 ng/mL; 95% CI − 4.78 to 11.91).
Findings from a meta-analysis of serum 25(OH)D levels in UC patients
A meta-analysis of 15 studies [16, 29, 34, 36, 46, 54, 61, 62, 64, 66, 68, 71, 74, 76, 77] on serum 25(OH)D levels in both UC and healthy controls showed that patients with UC had lower levels of serum 25(OH)D than did the controls (MD: − 2.52 ng/mL; 95% CI − 4.02 to − 1.02) (Fig. 6). These studies had high heterogeneity (I2 = 83%, P < 0.01). Subgroup analysis showed that the following variables were statistically significant: adults (MD: − 2.38 ng/mL; 95% CI − 4.20 to − 0.56), HPLC (MD: − 7.00 ng/mL; 95% CI − 11.58 to − 2.42), lack of VitD supplementation (MD: − 3.29 ng/mL; 95% CI − 4.99 to − 1.60), and cross-sectional study design (MD: − 18.07 ng/mL; 95% CI − 26.50 to − 9.64) (Table 2). Sensitivity analysis results was stabilization after small sample studies were removed (MD: − 2.94 ng/mL; 95% CI − 4.55 to 1.33).
There was almost no difference between UC and CD in 34 studies [16, 18, 27, 29,30,31, 34, 36,37,38,39,40,41, 46,47,48, 50,51,52, 54, 56, 58, 61, 62, 64, 66,67,68, 71, 73, 75,76,77] investigating VitD levels (MD: 0.75 ng/mL; 95% CI − 0.44 to 1.94) (Fig. 7). These studies had high heterogeneity (I2 = 84%, P < 0.01). Subgroup analysis showed that ECLIA (MD: − 1.34 ng/mL; 95% CI − 2.52 to − 0.17), HPLC (MD: 3.69 ng/mL; 95% CI 0.34–7.04), lack of VitD supplementation (MD: − 2.11 ng/mL; 95% CI − 3.69 to − 0.53), and the use of VitD supplementation (MD: 0.71 ng/mL; 95% CI − 0.63 to 2.05) were statistically significant (Table 2). Sensitivity analysis results remained stable after the removal of small samples (MD: − 0.88 ng/mL; 95% CI − 0.34 to 2.10) or lower quality score (MD: 0.72 ng/mL; 95% CI − 0.52 to 1.96).
Findings from the meta-analysis of 1,25(OH)2D3 levels in UC patients
Five studies [26, 29, 34, 46, 59] reporting on levels of 1,25(OH)2D3 in UC and healthy control groups found higher levels of 1,25(OH)2 D3 in the UC group than in the control group (MD: 3.76 pg/mL; 95% CI − 8.36 to 15.57) (Fig. 8). There was significant heterogeneity among the studies (I2 = 96%, P < 0.01). None of the results of the subgroup analyses from these studies were statistically significant (Table 2). Sensitivity analysis results remained unchanged after small samples were removed (MD: 3.40 ng/mL; 95% CI − 10.26 to 17.06).
Overall, when all seven eligible studies [26, 29, 30, 34, 38, 46, 59] were analyzed using a random-effects model, the results showed that VitD levels were lower in patients with UC than in CD (MD: − 6.71 pg/mL; 95% CI − 15.30 to 1.88) (Fig. 9). There was significant heterogeneity among the studies (I2 = 94%, P < 0.01). Subgroup analysis showed that only the cohort studies (MD: − 16.57 ng/mL; 95% CI − 17.66 to − 15.47) were statistically significant (Table 2). Sensitivity analysis results remained unchanged after small samples were removed (MD: − 5.09 ng/mL; 95% CI − 15.28 to 5.10).
Publication bias
For the meta-analyses, publication bias was not assumed, as all funnel plots were essentially symmetrical.
Discussion
There are several competing views on the link between VitD deficiency and IBD in the literature. For UC, Ulitsky et al. [17] reported that VitD deficiency is not associated with UC, but another study [78] reported a correlation. With regard to CD, Khalili et al. [79] reported that VitD deficiency was associated with CD, but the Grunbaum’s [16] study did not. To explore this controversy, we performed a pooled meta-analysis to determine the status of VitD in the serum of healthy and non-healthy controls.
Vitamin D is the only fat-soluble vitamin that may provide potential effects in treating IBD [7]. From our meta-analysis, we have concluded that VitD levels are strongly associated with IBD. Our meta-analysis found that patients with CD and UC had mean lower levels of 25(OH)D than did healthy populations; however, there was no significant difference in serum 25(OH)D levels between CD and UC patients. So VitD levels may be independent of disease type. This can be explained by insufficient intake, insufficient absorption or excessive loss of VitD in patients with IBD [13]. When comparing the mean levels of 1,25(OH)2D3, we found that patients with CD and UC did not lack 1,25(OH)2D3, and, in fact, patients with CD and UC had higher levels of VitD than healthy populations. Moreover, the average concentration of 1,25(OH)2D3 in CD patients was significantly higher than in patients with UC.
Current studies [80,81,82] have suggested that VitD plays a role in IBD-specific complications. The best indicator of VitD status is serum 25(OH)D because it closely reflects both dietary intake and the amount of sunlight exposure [83], and 25(OH)D has a half-life of 12 to 19 days [5, 13], however, 1,25(OH)2D3 has a short half-life of 4 to 20 h and is not a reliable indicator of the total amount of vitamin D in the body [84]. Although the serum 1,25(OH)2D3 content of IBD patients was higher than that of healthy populations, we cannot ignore the importance of 1,25(OH)2D3. In accordance with our findings, Abreu’s study [34] also demonstrated that IBD patients have high levels of 1,25(OH)2D3, especially in CD patients. It has been suggested that elevated 1,25(OH)2D3 may be a direct cause of bone loss or act as a surrogate marker for the type of intestinal inflammation that results in osteoporosis. In addition, in the presence of intestinal inflammation, an increase in the number of lamina propria monocytes, combined with the availability of 25(OH)D as a 1a-hydroxylase substrate, resulted in increased levels of 1,25(OH)2D3 [34, 85]. In our study, we also found that the level of 1,25(OH)2D3 in patients with CD was significantly higher than that in patients with UC. However, in some studies, we also found that the serum level of 1,25(OH)2D3 was lower in IBD patients than in healthy control groups. This may be due to improved BMD after remission of IBD, making 1,25(OH)2D3 normal.
Based on the subgroup analysis of age, VitD deficiency was more common in adults and children with IBD. Although, there was no significant difference in VitD levels between adults and children, whether they were in an IBD or a healthy control group. In children, El-Matary et al. [44] found that VitD levels were lower (though not statistically significant) in UC patients than in a CD group. However, in Veit’s study, 25(OH)D was significantly higher in children with CD than in children with UC [65]. In our subgroup analysis, we found no significant differences in vitamin D levels between CD and UC pediatric patients; and, we found the same results in adults. An association between IBD risk and pre-diagnosis predicted VitD status has been established in adult populations. There may be differences in genetic susceptibility and immunopathogenic pathways between childhood and adult onset IBD, because children with IBD seem to be a unique group with special characteristics that require highly skilled and specialized methods for diagnosis and treatment [76, 86, 87].
With VitD intake and foods meeting only 20% of total daily needs, it is important to educate people about the importance of introducing foods rich in vitamin D into their daily diet [88]. The RDA is 400 international units (IU) or 10 ng for male and female infants (i.e., less than 1 year old), 600 IU or 15 ng for all male and female individuals from 1 to 70 years old, and 800 IU or 20 ng for those over 70 years old [89]. Dietary supplements are generally considered to be a rapid form of VitD supplementation, and the total intake of VitD always reflects the combined contribution of the food source and the supplement to the diet. VitD can be found in VitD2 or VitD3; however, the former is rarely used as a fortifier in dietary supplements [90, 91]. Increasing VitD in foods may be the best way to increase intake, but it does not significantly increase serum 25(OH)D levels. We believe that VitD supplements should be used to increase serum VitD levels more quickly and directly. Of course, dietary supplements with high VitD content may help improve the low VitD levels in patients with IBD.
VitD supplementation has been shown to reduce the recurrence of some immune-mediated diseases [92, 93], and adverse events associated with VitD supplementation is relatively low. VitD supplementation reduced clinical recurrence from 29 to 13% (P = 0.06) [94]. We measured VitD supplementation in the analysis, which was found in 12 studies. Jorgensen [57] found that CD patients reported taking VitD supplements in winter, and their levels of 25(OH)D were significantly higher than non-users. This further confirms the views of Pappa [47] and Grunbaum [16] who suggested that higher doses may yield better results. Other studies have shown that VitD is more necessary in winter and that large amounts of it are more effective (even up to 10,000 IU/day) [95,96,97]. High doses of VitD3 supplements (10,000 IU/day) may significantly reduce clinical recurrence and significantly improve quality of life [94, 98,99,100]. VitD3 is formed by exposure of the skin to sunlight [101]. In winter, when sunlight is scarce, VitD should be taken. Notably, in several studies more IBD patients were found to be taking VitD supplements, and subsequently tended to have higher total daily oral intake of vitamin D [43, 54, 77]. Since there is not enough trial data investigating different doses of vitamin D supplements, large, well-designed randomized controlled trials using different doses of vitamin D supplements are needed to help better understand the therapeutic significance of vitamin D in IBD.
In addition, we found that different VitD measurement tools may affect the final results. After our analysis, VitD deficiency in IBD patients measured by ELISA and HPLC was found to be more severe (though not statistically significant) in comparison to control groups. Therefore, different VitD measurements may affect the results. There are different methods for the determination of 25(OH)D, including competitive binding protein assays, immunoassays (such as chemiluminescence immunoassays [CLIA]), high performance liquid chromatography (HPLC), and liquid chromatography-tandem mass spectrometry (LC-MS/MS) that are currently considered more accurate and accurate [102, 103]. A studies have shown that different methods of vitamin D measurement can affect the results of vitamin D measurement [104,105,106,107]. Therefore, I believe that the standardization of vitamin D measurement is helpful for the diagnosis and treatment of IBD. In addition, free 25(OH)D may reflect the status of biologically active vitamin D better than total 25(OH)D [108]. Recent studies have shown that patients with IBD have normal or even higher levels of free 25(OH)D, despite a total deficiency of 25(OH)D [76]. Measuring free 25(OH)D may establish a relationship between IBD and vitamin D.
In terms of study design, a significant difference was found in the cohort studies for 1,25(OH)2D3 between the diseased patients and non-healthy controls, but this result may have been caused by small sample sizes. There was no significant difference between study designs among the other groups. Therefore, different research designs did not affect the final results.
It is unclear whether VitD deficiency is a consequence of IBD or a contributing factor to its pathogenesis. However, VitD may be an important mediator in the pathogenesis of CD and possibly UC [109]. Though our research found a relationship between the VitD deficiency and IBD, the relationship with UC was not obvious in some respects. It is possible that VitD deficiency is more closely related to celiac disease, and that the disease activity of celiac disease promotes the process of UC.
One advantage of this meta-analysis was that it included a large number of subjects, including CD and UC subjects, which examined the associations between 25(OH)D and 1,25(OH)2D3 levels, and considered healthy and non-healthy controls in their analyses. Furthermore, it was possible to perform subgroup analyses according to age group, VitD assessment tools, VitD supplementation and study design. In our sensitivity analysis, we excluded small samples and low-scoring studies to see if the results were altered. However, this meta-analysis has some limitations. First, there was no subgroup analysis based on gender, season, race, or disease activity, as there was not enough data. Second, although funnel plots showed no significant publication bias, there may still be publication biases in the retrieved articles. Third, there was no unified diagnostic standard for IBD in the included studies, which may have greatly increased the false positive rate and affected the results of the included studies. Fourth, the relevant parties of RDA cannot do in-depth analysis due to various objective reasons.
Conclusions
In summary, we found that VitD levels were inversely related to CD and UC. Serum levels of 25(OH)D3 were lower in these patients than in healthy controls, and more than half of the patients had insufficient vitamin D levels; however, the serum level of 1,25(OH)2 D3 was higher than that of healthy controls. Our analysis indicates that attention should be paid to VitD levels to prevent the occurrence of IBD. In clinical practice, IBD patients should supplement their diets with VitD and be aware of the effects different seasons have on VitD content. In follow-up studies, vitamin D may be used as a treatment for IBD, or as an adjunctive therapy. We believe our research can provide a reference point for other scholars; however, our results cannot clarify the pathogenesis or suggest a cure for IBD. Rather, these results should provide directions for future research, as more exploration is needed.
Availability of data and materials
Not applicable.
References
Cosnes J, Gower-Rousseau C, Seksik P, Cortot A. Epidemiology and natural history of inflammatory bowel diseases. Gastroenterology. 2011;140(6):1785–94.
Matsuoka K, Kobayashi T, Ueno F, Matsui T, Hirai F, Inoue N, et al. Evidence-based clinical practice guidelines for inflammatory bowel disease. J Gastroenterol. 2018;53(3):305–53.
Garg M, Lubel JS, Sparrow MP, Holt SG, Gibson PR. Review article: vitamin D and inflammatory bowel disease—established concepts and future directions. Aliment Pharmacol Ther. 2012;36(4):324–44.
Fiocchi C. Inflammatory bowel disease pathogenesis: where are we? J Gastroenterol Hepatol. 2015;30(Suppl 1):12–8.
Zhao H, Zhang H, Wu H, Li H, Liu L, Guo J, et al. Protective role of 1,25(OH)2 vitamin D3 in the mucosal injury and epithelial barrier disruption in DSS-induced acute colitis in mice. BMC Gastroenterol. 2012;12:57.
Gombart AF, Borregaard N, Koeffler HP. Human cathelicidin antimicrobial peptide (CAMP) gene is a direct target of the vitamin D receptor and is strongly up-regulated in myeloid cells by 1,25-dihydroxyvitamin D3. FASEB J. 2005;19(9):1067–77.
Limketkai BN, Mullin GE, Limsui D, Parian AM. Role of vitamin D in inflammatory bowel disease. Nutr Clin Pract. 2017;32(3):337–45.
Lips P. Vitamin D physiology. Prog Biophys Mol Biol. 2006;92(1):4–8.
Gubatan J, Moss AC. Vitamin D in inflammatory bowel disease: more than just a supplement. Curr Opin Gastroenterol. 2018;34(4):217–25.
Bora S, Cantorna MT. The role of UVR and vitamin D on T cells and inflammatory bowel disease. Photochem Photobiol Sci. 2017;16(3):347–53.
Barbalho SM, Goulart RA, Gasparini RG. Associations between inflammatory bowel diseases and vitamin D. Crit Rev Food Sci Nutr. 2017;59:1–10.
Ananthakrishnan AN. Epidemiology and risk factors for IBD. Nat Rev Gastroenterol Hepatol. 2015;12(4):205–17.
Lim WC, Hanauer SB, Li YC. Mechanisms of disease: vitamin D and inflammatory bowel disease. Nat Clin Pract Gastroenterol Hepatol. 2005;2(7):308–15.
Farraye FA, Nimitphong H, Stucchi A, Dendrinos K, Boulanger AB, Vijjeswarapu A, et al. Use of a novel vitamin D bioavailability test demonstrates that vitamin D absorption is decreased in patients with quiescent Crohn’s disease. Inflamm Bowel Dis. 2011;17(10):2116–21.
Castro FD, Magalhaes J, Carvalho PB, Moreira MJ, Mota P, Cotter J. Lower levels of vitamin d correlate with clinical disease activity and quality of life in inflammatory bowel disease. Arq Gastroenterol. 2015;52(4):260–5.
Grunbaum A, Holcroft C, Heilpern D, Gladman S, Burstein B, Menard M, et al. Dynamics of vitamin D in patients with mild or inactive inflammatory bowel disease and their families. Nutr J. 2013;12(1):145.
Ulitsky A, Ananthakrishnan AN, Naik A, Skaros S, Zadvornova Y, Binion DG, et al. Vitamin D deficiency in patients with inflammatory bowel disease: association with disease activity and quality of life. JPEN J Parenter Enteral Nutr. 2011;35(3):308–16.
Ko KH, Kim YS, Lee BK, Choi JH, Woo YM, Kim JY, et al. Vitamin D deficiency is associated with disease activity in patients with Crohn’s disease. Intest Res. 2018;17(1):70–7.
Margulis AV, Pladevall M, Riera-Guardia N, Varas-Lorenzo C, Hazell L, Berkman ND, et al. Quality assessment of observational studies in a drug-safety systematic review, comparison of two tools: the Newcastle–Ottawa scale and the RTI item bank. Clin Epidemiol. 2014;6:359–68.
Stang A. Critical evaluation of the Newcastle–Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25(9):603–5.
The Joanna Briggs Institute. Checklist for analytical cross sectional studies from The Joanna Briggs Institute Critical Appraisal tools for use in JBI systematic reviews. http://joannabriggs.org/research/critical-appraisal-tools.html. Joanna Briggs Institute; 2017.
Higgins JP, Whitehead A, Turner RM, Omar RZ, Thompson SG. Meta-analysis of continuous outcome data from individual patients. Stat Med. 2001;20(15):2219–41.
Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–60.
Mavridis D, Salanti G. Exploring and accounting for publication bias in mental health: a brief overview of methods. Evid Based Ment Health. 2014;17(1):11–5.
Driscoll RH Jr, Meredith SC, Sitrin M, Rosenberg IH. Vitamin D deficiency and bone disease in patients with Crohn’s disease. Gastroenterology. 1982;83(6):1252–8.
Harries AD, Brown R, Heatley RV, Williams LA, Woodhead S, Rhodes J. Vitamin D status in Crohn’s disease: association with nutrition and disease activity. Gut. 1985;26(11):1197–203.
Pollak RD, Karmeli F, Eliakim R, Ackerman Z, Tabb K, Rachmilewitz D. Femoral neck osteopenia in patients with inflammatory bowel disease. Am J Gastroenterol. 1998;93(9):1483–90.
Gokhale R, Favus MJ, Karrison T, Sutton MM, Rich B, Kirschner BS. Bone mineral density assessment in children with inflammatory bowel disease. Gastroenterology. 1998;114(5):902–11.
Ardizzone S, Bollani S, Bettica P, Bevilacqua M, Molteni P, Bianchi Porro G. Altered bone metabolism in inflammatory bowel disease: there is a difference between Crohn’s disease and ulcerative colitis. J Intern Med. 2000;247(1):63–70.
Jahnsen J, Falch JA, Mowinckel P, Aadland E. Vitamin D status, parathyroid hormone and bone mineral density in patients with inflammatory bowel disease. Scand J Gastroenterol. 2002;37(2):192–9.
Haderslev KV, Jeppesen PB, Sorensen HA, Mortensen PB, Staun M. Vitamin D status and measurements of markers of bone metabolism in patients with small intestinal resection. Gut. 2003;52(5):653–8.
Tajika M, Matsuura A, Nakamura T, Suzuki T, Sawaki A, Kato T, et al. Risk factors for vitamin D deficiency in patients with Crohn’s disease. J Gastroenterol. 2004;39(6):527–33.
Duggan P, O’Brien M, Kiely M, McCarthy J, Shanahan F, Cashman KD. Vitamin K status in patients with Crohn’s disease and relationship to bone turnover. Am J Gastroenterol. 2004;99(11):2178–85.
Abreu MT, Kantorovich V, Vasiliauskas EA, Gruntmanis U, Matuk R, Daigle K, et al. Measurement of vitamin D levels in inflammatory bowel disease patients reveals a subset of Crohn’s disease patients with elevated 1,25-dihydroxyvitamin D and low bone mineral density. Gut. 2004;53(8):1129–36.
McCarthy D, Duggan P, O’Brien M, Kiely M, McCarthy J, Shanahan F, et al. Seasonality of vitamin D status and bone turnover in patients with Crohn’s disease. Aliment Pharmacol Ther. 2005;21(9):1073–83.
Gilman J, Shanahan F, Cashman KD. Altered levels of biochemical indices of bone turnover and bone-related vitamins in patients with Crohn’s disease and ulcerative colitis. Aliment Pharmacol Ther. 2006;23(7):1007–16.
Pappa HM, Gordon CM, Saslowsky TM, Zholudev A, Horr B, Shih MC, et al. Vitamin D status in children and young adults with inflammatory bowel disease. Pediatrics. 2006;118(5):1950–61.
Sinnott BP, Licata AA. Assessment of bone and mineral metabolism in inflammatory bowel disease: case series and review. Endocr Pract. 2006;12(6):622–9.
Vagianos K, Bector S, McConnell J, Bernstein CN. Nutrition assessment of patients with inflammatory bowel disease. JPEN J Parenter Enteral Nutr. 2007;31(4):311–9.
Kuwabara A, Tanaka K, Tsugawa N, Nakase H, Tsuji H, Shide K, et al. High prevalence of vitamin K and D deficiency and decreased BMD in inflammatory bowel disease. Osteoporos Int. 2009;20(6):935–42.
Leslie WD, Miller N, Rogala L, Bernstein CN. Vitamin D status and bone density in recently diagnosed inflammatory bowel disease: the Manitoba IBD Cohort Study. Am J Gastroenterol. 2008;103(6):1451–9.
Joseph AJ, George B, Pulimood AB, Seshadri MS, Chacko A. 25(OH) vitamin D level in Crohn’s disease: association with sun exposure & disease activity. Indian J Med Res. 2009;130(2):133–7.
Kumari M, Khazai NB, Ziegler TR, Nanes MS, Abrams SA, Tangpricha V. Vitamin D-mediated calcium absorption in patients with clinically stable Crohn’s disease: a pilot study. Mol Nutr Food Res. 2010;54(8):1085–91.
El-Matary W, Sikora S, Spady D. Bone mineral density, vitamin D, and disease activity in children newly diagnosed with inflammatory bowel disease. Dig Dis Sci. 2011;56(3):825–9.
Levin AD, Wadhera V, Leach ST, Woodhead HJ, Lemberg DA, Mendoza-Cruz AC, et al. Vitamin D deficiency in children with inflammatory bowel disease. Dig Dis Sci. 2011;56(3):830–6.
El-Hodhod MA, Hamdy AM, Abbas AA, Moftah SG, Ramadan AA. Fibroblast growth factor 23 contributes to diminished bone mineral density in childhood inflammatory bowel disease. BMC Gastroenterol. 2012;12:44.
Pappa HM, Langereis EJ, Grand RJ, Gordon CM. Prevalence and risk factors for hypovitaminosis D in young patients with inflammatory bowel disease. J Pediatr Gastroenterol Nutr. 2011;53(4):361–4.
Atia A, Murthy R, Bailey BA, Manning T, Garrett LL, Youssef D, et al. Vitamin D status in veterans with inflammatory bowel disease: relationship to health care costs and services. Mil Med. 2011;176(6):711–4.
Suibhne TN, Cox G, Healy M, O’Morain C, O’Sullivan M. Vitamin D deficiency in Crohn’s disease: prevalence, risk factors and supplement use in an outpatient setting. J Crohn’s Colitis. 2012;6(2):182–8.
Hassan V, Hassan S, Seyed-Javad P, Ahmad K, Asieh H, Maryam S, et al. Association between serum 25(OH) vitamin D concentrations and inflammatory bowel diseases (IBDs) activity. Med J Malays. 2013;68(1):34–8.
Chatu S, Chhaya V, Holmes R, Neild P, Kang JY, Pollok RC, et al. Factors associated with vitamin D deficiency in a multicultural inflammatory bowel disease cohort. Frontline Gastroenterol. 2013;4(1):51–6.
Fu YT, Chatur N, Cheong-Lee C, Salh B. Hypovitaminosis D in adults with inflammatory bowel disease: potential role of ethnicity. Dig Dis Sci. 2012;57(8):2144–8.
Salacinski AJ, Regueiro MD, Broeder CE, McCrory JL. Decreased neuromuscular function in Crohn’s disease patients is not associated with low serum vitamin D levels. Dig Dis Sci. 2013;58(2):526–33.
Garg M, Rosella O, Lubel JS, Gibson PR. Association of circulating vitamin D concentrations with intestinal but not systemic inflammation in inflammatory bowel disease. Inflamm Bowel Dis. 2013;19(12):2634–43.
Prosnitz AR, Leonard MB, Shults J, Zemel BS, Hollis BW, Denson LA, et al. Changes in vitamin D and parathyroid hormone metabolism in incident pediatric Crohn’s disease. Inflamm Bowel Dis. 2013;19(1):45–53.
Miznerova E, Hlavaty T, Koller T, Toth J, Holociova K, Huorka M, et al. The prevalence and risk factors for osteoporosis in patients with inflammatory bowel disease. Bratisl Lek Listy. 2013;114(8):439–45.
Middleton JP, Bhagavathula AP, Gaye B, Alvarez JA, Huang CS, Sauer CG, et al. Vitamin D status and bone mineral density in African American children with Crohn disease. J Pediatr Gastroenterol Nutr. 2013;57(5):587–93.
Lorinczy K, Lakatos PL, Toth M, Salamon A, Nemes A, Csontos AA, et al. Vitamin D level in Hungarian patients with inflammatory bowel diseases. Orv Hetil. 2013;154(46):1821–8.
Alkhouri RH, Hashmi H, Baker RD, Gelfond D, Baker SS. Vitamin and mineral status in patients with inflammatory bowel disease. J Pediatr Gastroenterol Nutr. 2013;56(1):89–92.
de Bruyn JR, van Heeckeren R, Ponsioen CY, van den Brink GR, Lowenberg M, Bredenoord AJ, et al. Vitamin D deficiency in Crohn’s disease and healthy controls: a prospective case-control study in the Netherlands. J Crohn’s Colitis. 2014;8(10):1267–73.
Dumitrescu G, Mihai C, Dranga M, Prelipcean CC. Serum 25-hydroxyvitamin D concentration and inflammatory bowel disease characteristics in Romania. World J Gastroenterol. 2014;20(9):2392–6.
Tan B, Li P, Lv H, Li Y, Wang O, Xing XP, et al. Vitamin D levels and bone metabolism in Chinese adult patients with inflammatory bowel disease. J Dig Dis. 2014;15(3):116–23.
Oikonomou KA, Orfanidou TI, Vlychou MK, Kapsoritakis AN, Tsezou A, Malizos KN, et al. Lower fibroblast growth factor 23 levels in young adults with Crohn disease as a possible secondary compensatory effect on the disturbance of bone and mineral metabolism. J Clin Densitom. 2014;17(1):177–84.
Veit LE, Maranda L, Fong J, Nwosu BU. The vitamin D status in inflammatory bowel disease. PLoS ONE. 2014;9(7):e101583.
Raffner Basson A, Swart R, Jordaan E, Mazinu M, Watermeyer G. Vitamin D deficiency increases the risk for moderate to severe disease activity in Crohn’s disease patients in South Africa, measured by the Harvey Bradshaw index. J Am Coll Nutr. 2016;35(2):163–74.
Thorsen SU, Jakobsen C, Cohen A, Lundqvist M, Thygesen LC, Pipper C, et al. Perinatal vitamin D levels are not associated with later risk of developing pediatric-onset inflammatory bowel disease: a Danish case-cohort study. Scand J Gastroenterol. 2016;51(8):927–33.
Schaffler H, Schmidt M, Huth A, Reiner J, Glass A, Lamprecht G. Clinical factors are associated with vitamin D levels in IBD patients: a retrospective analysis. J Dig Dis. 2018;19(1):24–32.
Opstelten JL, Chan SSM, Hart AR, van Schaik FDM, Siersema PD, Lentjes E, et al. Prediagnostic serum vitamin D levels and the risk of Crohn’s disease and ulcerative colitis in European populations: a nested case–control study. Inflamm Bowel Dis. 2018;24(3):633–40.
Westarp CV, Thomson A, Overton T, Rogers R, Hodges P, Fornasier V, et al. Disorders of mineral and bone metabolism in patients with Crohn’s disease. Can J Gastroenterol Hepatol. 1987;1(1):11–7.
Martin A, Fries W, Luisetto G, Mussolin L, Plebani M, Giacomin D, et al. Bone density and calcium metabolism in patients with long-standing, quiescent Crohn’s disease. Eur J Gastroenterol Hepatol. 1994;6(7):611–6.
Souza HN, Lora FL, Kulak CA, Manas NC, Amarante HM, Borba VZ. Low levels of 25-hydroxyvitamin D (25OHD) in patients with inflammatory bowel disease and its correlation with bone mineral density. Arq Bras Endocrinol Metabol. 2008;52(4):684–91.
Jorgensen SP, Hvas CL, Agnholt J, Christensen LA, Heickendorff L, Dahlerup JF. Active Crohn’s disease is associated with low vitamin D levels. J Crohn’s Colitis. 2013;7(10):e407–13.
Burrelli Scotti G, Afferri MT, De Carolis A, Vaiarello V, Fassino V, Ferrone F, et al. Factors affecting vitamin D deficiency in active inflammatory bowel diseases. Dig Liver Dis. 2018;51(5):657–62.
Garg M, Hendy P, Ding JN, Shaw S, Hold G, Hart A. The effect of vitamin D on intestinal inflammation and faecal microbiota in patients with ulcerative colitis. J Crohn’s Colitis. 2018;12(8):963–72.
Caviezel D, Maissen S, Niess JH, Kiss C, Hruz P. High prevalence of vitamin D deficiency among patients with inflammatory bowel disease. Inflamm Intest Dis. 2018;2(4):200–10.
Strisciuglio C, Cenni S, Giugliano FP, Miele E, Cirillo G, Martinelli M, et al. The role of inflammation on vitamin D levels in a cohort of pediatric patients with inflammatory bowel disease. J Pediatr Gastroenterol Nutr. 2018;67(4):501–6.
Garg M, Royce SG, Tikellis C, Shallue C, Sluka P, Wardan H, et al. The intestinal vitamin D receptor in inflammatory bowel disease: inverse correlation with inflammation but no relationship with circulating vitamin D status. Ther Adv Gastroenterol. 2019;12:1756284818822566.
Stio M, Retico L, Annese V, Bonanomi AG. Vitamin D regulates the tight-junction protein expression in active ulcerative colitis. Scand J Gastroenterol. 2016;51(10):1193–9.
Kabbani TA, Koutroubakis IE, Schoen RE, Ramos-Rivers C, Shah N, Swoger J, et al. Association of vitamin D level with clinical status in inflammatory bowel disease: a 5-year longitudinal study. Am J Gastroenterol. 2016;111(5):712–9.
Sambrook P, Birmingham J, Kelly P, Kempler S, Nguyen T, Pocock N, et al. Prevention of corticosteroid osteoporosis. A comparison of calcium, calcitriol, and calcitonin. N Engl J Med. 1993;328(24):1747–52.
Gorham ED, Garland CF, Garland FC, Grant WB, Mohr SB, Lipkin M, et al. Vitamin D and prevention of colorectal cancer. J Steroid Biochem Mol Biol. 2005;97(1–2):179–94.
Wilkins CH, Sheline YI, Roe CM, Birge SJ, Morris JC. Vitamin D deficiency is associated with low mood and worse cognitive performance in older adults. Am J Geriatr Psychiatry. 2006;14(12):1032–40.
Hollis BW. Assessment and interpretation of circulating 25-hydroxyvitamin D and 1,25-dihydroxyvitamin D in the clinical environment. Rheum Dis Clin North Am. 2012;38(1):29–44.
Jones G. Pharmacokinetics of vitamin D toxicity. Am J Clin Nutr. 2008;88(2):582s–6s.
DeLuca HF. The vitamin D story: a collaborative effort of basic science and clinical medicine. FASEB J. 1988;2(3):224–36.
Guariso G, Gasparetto M, Dalla Pozza LV, D’Inca R, Zancan L, Sturniolo G, et al. Inflammatory bowel disease developing in paediatric and adult age. J Pediatr Gastroenterol Nutr. 2010;51(6):698–707.
Biank V, Broeckel U, Kugathasan S. Pediatric inflammatory bowel disease: clinical and molecular genetics. Inflamm Bowel Dis. 2007;13(11):1430–8.
Vranesic Bender D, Giljevic Z, Kusec V, Laktasic Zerjavic N, Bosnjak Pasic M, Vrdoljak E, et al. Guidelines for the prevention, detection and therapy of vitamin D deficiency in adults. Lijec Vjesn. 2016;138(5–6):121–32.
Institute of Medicine Committee to review dietary reference intakes for vitamin D, calcium. The national academies collection: reports funded by National Institutes of Health. In: Ross AC, Taylor CL, Yaktine AL, Del Valle HB, editors. Dietary reference intakes for calcium and vitamin D. Washington (DC): National Academies Press (US) National Academy of Sciences; 2011.
Rockell JE, Skeaff CM, Venn BJ, Williams SM, Green TJ. Vitamin D insufficiency in New Zealanders during the winter is associated with higher parathyroid hormone concentrations: implications for bone health? N Z Med J. 2008;121(1286):75–84.
Leskovar D, Mestrovic T, Baresic A, Kraljevic I, Panek M, Cipcic Paljetak H, et al. The role of vitamin D in inflammatory bowel disease—assessing therapeutic and preventive potential of supplementation and food fortification. Food Technol Biotechnol. 2018;56(4):455–63.
Burton JM, Kimball S, Vieth R, Bar-Or A, Dosch HM, Cheung R, et al. A phase I/II dose-escalation trial of vitamin D3 and calcium in multiple sclerosis. Neurology. 2010;74(23):1852–9.
Goldberg P, Fleming MC, Picard EH. Multiple sclerosis: decreased relapse rate through dietary supplementation with calcium, magnesium and vitamin D. Med Hypotheses. 1986;21(2):193–200.
Jorgensen SP, Agnholt J, Glerup H, Lyhne S, Villadsen GE, Hvas CL, et al. Clinical trial: vitamin D3 treatment in Crohn’s disease—a randomized double-blind placebo-controlled study. Aliment Pharmacol Ther. 2010;32(3):377–83.
Alrefai D, Jones J, El-Matary W, Whiting SJ, Aljebreen A, Mirhosseini N, et al. The association of vitamin D status with disease activity in a cohort of Crohn’s disease patients in Canada. Nutrients. 2017;9(10):1112.
Bischoff-Ferrari HA, Shao A, Dawson-Hughes B, Hathcock J, Giovannucci E, Willett WC. Benefit-risk assessment of vitamin D supplementation. Osteoporos Int. 2010;21(7):1121–32.
Leventis P, Kiely PD. The tolerability and biochemical effects of high-dose bolus vitamin D2 and D3 supplementation in patients with vitamin D insufficiency. Scand J Rheumatol. 2009;38(2):149–53.
Narula N, Cooray M, Anglin R, Muqtadir Z, Narula A, Marshall JK. Impact of high-dose vitamin D3 supplementation in patients with Crohn’s disease in remission: a pilot randomized double-blind controlled study. Dig Dis Sci. 2017;62(2):448–55.
Mathur J, Naing S, Mills P, Limsui D. A randomized clinical trial of vitamin D3 (cholecalciferol) in ulcerative colitis patients with hypovitaminosis D3. PeerJ. 2017;5:e3654.
Garg M, Rosella O, Rosella G, Wu Y, Lubel JS, Gibson PR. Evaluation of a 12-week targeted vitamin D supplementation regimen in patients with active inflammatory bowel disease. Clin Nutr (Edinburgh, Scotland). 2018;37(4):1375–82.
Stepman HC, Vanderroost A, Van Uytfanghe K, Thienpont LM. Candidate reference measurement procedures for serum 25-hydroxyvitamin D3 and 25-hydroxyvitamin D2 by using isotope-dilution liquid chromatography-tandem mass spectrometry. Clin Chem. 2011;57(3):441–8.
Tai SS, Bedner M, Phinney KW. Development of a candidate reference measurement procedure for the determination of 25-hydroxyvitamin D3 and 25-hydroxyvitamin D2 in human serum using isotope-dilution liquid chromatography-tandem mass spectrometry. Anal Chem. 2010;82(5):1942–8.
Wallace AM, Gibson S, de la Hunty A, Lamberg-Allardt C, Ashwell M. Measurement of 25-hydroxyvitamin D in the clinical laboratory: current procedures, performance characteristics and limitations. Steroids. 2010;75(7):477–88.
Rabenberg M, Scheidt-Nave C, Busch MA, Thamm M, Rieckmann N, Durazo-Arvizu RA, et al. Implications of standardization of serum 25-hydroxyvitamin D data for the evaluation of vitamin D status in Germany, including a temporal analysis. BMC Public Health. 2018;18(1):845.
Binkley N, Sempos CT. Standardizing vitamin D assays: the way forward. J Bone Miner Res. 2014;29(8):1709–14.
Snellman G, Melhus H, Gedeborg R, Byberg L, Berglund L, Wernroth L, et al. Determining vitamin D status: a comparison between commercially available assays. PLoS ONE. 2010;5(7):e11555.
Carter GD, Carter R, Jones J, Berry J. How accurate are assays for 25-hydroxyvitamin D? Data from the international vitamin D external quality assessment scheme. Clin Chem. 2004;50(11):2195–7.
Powe CE, Evans MK, Wenger J, Zonderman AB, Berg AH, Nalls M, et al. Vitamin D-binding protein and vitamin D status of black Americans and white Americans. N Engl J Med. 2013;369(21):1991–2000.
Ananthakrishnan AN, Khalili H, Higuchi LM, Bao Y, Korzenik JR, Giovannucci EL, et al. Higher predicted vitamin D status is associated with reduced risk of Crohn’s disease. Gastroenterology. 2012;142(3):482–9.
Acknowledgements
Not applicable.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
CZ and YF had full access to all of the data in the study, and took responsibility for the integrity of the data and the accuracy of the data analysis. XXL, JL and YL designed the study. XXL, JL and YL developed and tested the data collection forms. ZDH and XXL acquired the data. CZ and ZDH conducted the analysis and interpreted the data. XXL and YF drafted the manuscript. All authors critically revised the manuscript. CZ and YF had guarantor. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Additional file 1: Method S1
. Search strategy.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Li, XX., Liu, Y., Luo, J. et al. Vitamin D deficiency associated with Crohn’s disease and ulcerative colitis: a meta-analysis of 55 observational studies. J Transl Med 17, 323 (2019). https://doi.org/10.1186/s12967-019-2070-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12967-019-2070-5