Abstract
Background
Molecular assessment and treatment of metastatic colorectal cancer (mCRC) quickly evolved during the last decades, hampering longitudinal evaluation of prognostic markers. The aim of this study was to evaluate prognostic predictors of long-term survival in a retrospective series of mCRC, treated prior to the expanded RAS assessment era.
Methods
mCRC cases treated at the Città della Salute e della Scienza University Hospital (Turin, Italy) between January 2004 and December 2012 were evaluated, including cases with ≥ 5-year follow-up only. Long-term survival was defined as an overall survival (OS) ≥ 4 years based on the observed OS interquartile range values. Univariate/multivariate Cox proportional hazards regression models were performed to assess the prognostic significance of the clinical/biological features, while binary logistic regression models were used to verify their associations with long-term survival.
Results
Two hundred and forty-eight mCRC cases were included and analyzed. Sixty out of two hundred and forty-eight (24%) patients were long-term survivors. Univariate binary logistic regression analysis demonstrated a significant association between long-term survival and age at diagnosis < 65 (OR = 2.28, p = 0.007), single metastatic site (OR = 1.89, p = 0.039), surgical resection of metastases (OR = 5.30, p < 0.001), local non-surgical treatment of metastases (OR = 4.74, p < 0.001), and a bevacizumab-including first-line treatment schedule (OR = 2.19, p = 0.024). Multivariate binary logistic regression analysis confirmed the prognostic significance of surgical resection of metastases (OR = 3.96, p < 0.001), local non-surgical treatment of metastases (OR = 3.32, p = 0.001), and of bevacizumab-including first-line treatment schedule (OR = 2.49, p = 0.024).
Conclusion
Long-term survival could be achieved in a significant rate of patients with mCRC even in an era of limited molecular characterization. Local treatment of metastases proved to be a significant predictor of long-term survival.
Similar content being viewed by others
Background
Colorectal cancer (CRC) is a worldwide leading cause of cancer and cancer-related deaths [1]. Over the past decade, CRC incidence and mortality decreased due to multiple factors: screening protocols implementation, improved surgical techniques, and availability of novel systemic therapeutic options [2]. Nevertheless, metastatic disease is already present at presentation in about 20% of patients (stage IV), while about 30–40% of patients who receive surgical resection will ultimately experience disease recurrence requiring systemic treatments [3].
Although initial disease stage remains the main outcome predictor in CRC, in the precision medicine era, tumor molecular profiling has become of paramount importance [4]. The clinical and histopathological heterogeneity of CRC has been further supported by molecular profiling and specific mutational profiles resulted to be strongly correlated with clinical outcome and response to treatment [5]. Therefore, assessment of specific tumor characteristics and biomarkers [e.g., tumor site (right versus left colon), mismatch repair capability, KRAS, NRAS, and BRAF proto-oncogenes mutational statuses] is now deemed mandatory for treatment selection in patients with mCRC, and the main therapeutic options in this setting include chemotherapy drugs, targeted therapies against the EGFR (Epidermal Growth Factor Receptor), and VEGF (Vascular Endothelial Growth Factor) pathways and immunotherapy [6].
KRAS status was the first molecular predictive marker to be routinely assessed in mCRC since several studies proved that KRAS-mutant tumors do not benefit from treatment with EGFR inhibitors. Initially, the effect was thought to be restricted to patients with KRAS (exon 2) wild-type tumors, but extended RAS analyses demonstrated a lack of response also in patients with tumors harboring other KRAS (exons 3 and 4) or NRAS mutations [7,8,9]. Expanded RAS testing increased the RAS mutation rate from 40% to about 55% [10], thus avoiding a potentially ineffective or even harmful anti-EGFR therapy in a significant number of patients. At the same time, extended molecular testing identified the significance of other markers, such as the adverse prognostic effect of BRAF mutations at codon 600 [11].
In RAS-mutant metastatic CRC (mCRC) patients, first-line therapies usually consist of combined approaches like FOLFOX/CAPOX (5-fluorouracil plus oxaliplatin/capecitabine plus oxaliplatin) or FOLFIRI/CAPIRI (5-fluorouracil plus irinotecan/capecitabine plus irinotecan) plus an additional anti-angiogenetic biologic drug (bevacizumab, ramucirumab, ziv-aflibercept) [12, 13]. In BRAF-mutated tumors, a triple chemotherapeutic regimen is usually taken into consideration (FOLFOXIRI: 5-fluorouracil plus oxaliplatin and irinotecan). Conversely, in RAS wild-type mCRC, EGFR inhibitors, like cetuximab or panitumumab, are added to conventional chemotherapy drugs [7]. Tumor side also affects the choice of treatment, since left-sided neoplasms shown higher response rates to anti-EGFR treatments [14]. Most recently, novel options became available: regorafenib, a multi-kinase inhibitor, showed efficacy in previously treated mCRC [15,16,17], whereas programmed cell death protein 1 (PD-1) targeting by immunotherapy (e.g., nivolumab and pembrolizumab) proved to be effective in mCRC with high microsatellite instability (MSI-high) [18, 19].
Despite these efforts, the 5-year survival rate of mCRC remains largely unsatisfying, ranging around just 15% of patients [20]. An improved characterization of long-term survivors is thus warranted to optimize these patients' care, but the quickly evolving landscape hampers comparisons across long time periods.
The aim of this study was therefore to evaluate the long-term survivors’ characteristics in a retrospective series of mCRC treated prior to the expanded RAS assessment era and with extended follow up available, to define prognostic markers affecting progression free survival (PFS) and overall survival (OS).
Methods
Case series
This is a retrospective observational study on the mCRC cases treated at the Colorectal Cancer Unit of the Città della Salute e della Scienza University Hospital (Turin, Italy) between January 2004 and December 2012. Our study cohort included 248 mCRC (either at the initial diagnosis of CRC or diagnosed with metastatic disease during follow up) patients (≥ 18 year old) who underwent at least one cycle of systemic anti-neoplastic treatment (cytotoxic drug therapy with or without molecular targeted agents). Cases with a follow-up time < 5 years were excluded [median follow up was 7.58 years, IQR (interquartile range) 5.41–9.16 years]. Considered the “real-life”, observational nature of the study no other exclusion criteria were established. The start of the study period was chosen to collect a broadly homogenous cohort, accounting for the introduction of anti-angiogenetic and anti-EGFR agents. Conversely, the accrual was stopped in 2012 prior to the deployment of expanded RAS assessment protocols and to achieve a sufficient follow-up. To define long-term survivors (LTS), a survival time ≥ 4 years was selected based upon the observed overall survival IQR values (1.54–4.00 years): this cutoff value enabled to have a representative number of cases (about one quarter of the whole series) available for the subsequent analyses while also being a clinically meaningful time period.
Clinical information was gathered from patients’ charts, hospital discharge forms, imaging repositories, and contacts with general practitioners. Collected data included gender, age at diagnosis, tumor side, KRAS status (exon 2, codons 12, and 13), number and sites of metastases, details regarding systemic and locoregional (surgery and/or radiofrequency) treatments, and disease outcome (PFS and OS). Chemotherapy schedules comprised a combination of drugs including fluorouracil, capecitabine, oxaliplatin, irinotecan, mitomycin, bevacizumab, cetuximab, and panitumumab according to the disease setting and available clinical trials.
The study was conducted in accordance with The Code of Ethics of the World Medical Association (Declaration of Helsinki) for experiments involving humans and within the guidelines and regulations defined by the Research Ethics Committee of the University of Turin. This study was approved by the Research Ethics Committee of the University of Turin; considered the retrospective nature of the research protocol and that it had no impact at all on patients’ care, no specific written informed consent was required.
Statistical analyses
All analyses were performed using the Stata/MP 15.0 Statistical Software (StataCorp, College Station, TX, USA). Continuous variables were summarized by mean and standard deviation (SD); whereas for categorical variables, the frequency and percentage were provided. The characteristics at diagnosis were compared using the chi-square test for categorical variables and the T test or ANOVA test for continuous ones.
The follow-up time, calculated with the reverse Kaplan–Meier method, was summarized as median and interquartile range (IQR). The survival times were measured from the start of treatment at metastasis diagnosis until disease progression (PFS) and death from any cause (OS); patients lost at follow-up (after a minimum of 5 years as per inclusion criteria) were censored on the last follow-up date. Survival curves were estimated with the Kaplan–Meier method and compared by log-rank test. The impact of possible confounders was explored by univariate and multivariate Cox proportional hazards regression models, including clinical/biological features as covariates. The proportional hazards assumption was tested for all the endpoints. Hazard ratios (HR) and 95% confidence intervals (95% CI) were estimated. Univariate/multivariate binary logistic regression models were performed using long-term survival as the dependent variable (yes or no) and patient/tumor characteristics as covariates. Odds ratios and 95% CIs were estimated. The Hosmer–Lemeshow goodness-of-fit test was used to determine whether the model adequately described the data. Differences were considered significant when p < 0.05 for reported two-sided p values.
Results
Patients’ clinical and pathological characteristics
The clinical and pathological characteristics of patients are presented in Table 1. The median age was 65 years (range 32–82), and the male to female ratio was 1.32. Primary tumor was right-sided in 83/248 (33%) cases, left-sided in 97/248 (39%), and localized in the rectum in 68/248 (28%). A KRAS mutation was detected in 45.2% of patients (based on codon 2 assessment only, i.e., before expanded RAS protocols).
The overall number of treatment lines are reported in Table 2. A higher rate of patients with an OS < 4 years received only one line of treatment (18% versus 8%); conversely, more long-term survivors (≥ 4 years) received 4 lines (47% versus 27%).
Overall outcome analysis
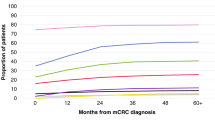
Median overall PFS after first-line treatment was 11.6 months (IQR 7.5–16.7 months), while median OS time was 2.62 years (IQR 1.54–4.00 years), and 60 out of 248 (24%) patients were long-term survivors (OS ≥ 4 years) (Fig. 1a).
a Kaplan–Meier curve of OS. Median OS time was 2.62 years (red arrow), but survival was longer than 4 years in 24% of patients (green arrow). b Kaplan–Meier curve of 1st-line treatment PFS according to LTS. c Kaplan–Meier curve of OS according to LTS-associated treatments (bevacizumab-including 1st-line regimen, surgical resection of metastases, local non-surgical treatment of metastases). d Kaplan–Meier curve of OS according to local treatment of metastases (either by surgery or other approaches)
At univariate analysis (Table 3), the variables significantly associated with first-line PFS were age at diagnosis, age at metastasis, surgical resection of primary tumor, surgical resection of metastasis, local non-surgical treatment of metastasis, and bevacizumab administration at first line. Conversely, no correlation was found between gender, tumor site (right sided versus other), site of metastases, KRAS mutational status (based on exon 2 evaluation), or adjuvant treatment.
Variables affecting OS were age at diagnosis, age at metastasis, right-sided tumor, KRAS exon 2 mutational status, surgical resection of primary tumor, surgical resection of metastasis, and local non-surgical treatment of metastasis. No correlation was found with gender, site of metastases, adjuvant treatment, or a bevacizumab-including first-line regimen.
Multivariable Cox regression analysis was stratified by local non-surgical treatment of metastases because this covariate violated the proportional hazards assumption. Surgical resection of metastasis was confirmed to be a significant predictor in terms of first line PFS and/or OS by multivariate analyses (Table 4), as well as first-line treatment including bevacizumab, age at metastasis diagnosis, and right sided tumor.
Characteristics of long-term survivors
No significant differences were observed in terms of gender, primary tumor site, histological grade, KRAS mutational status, or adjuvant treatment according to long-term survival status (Table 1). Conversely, long-term survivors (≥ 4 years) showed a lower median age at diagnosis (60 versus 66, p = 0.012) and at metastasis diagnosis (62 versus 66 years, p = 0.026), a lower rate of multiple metastatic sites (33.3 % versus 48.4%, p = 0.038), and higher rates of metastasis surgical treatment (63% versus 26%, p < 0.001) and/or local non-surgical metastasis treatment (57% versus 21%, p < 0.001).
Long-term survivors also showed a significant longer first-line treatment median PFS compared with other patients (15.7 versus 10.8 months, respectively, p = 0.0001) (Table 5 and Fig. 1b). This statistical difference was maintained in subsequent treatment lines as well (Table 5).
Outcome analysis of long-term survivors
Based on the previous results in terms of variables affecting outcome, univariate binary logistic regression analysis was performed (Table 6): a significant association was observed between long-term survival and age at diagnosis < 65, a single metastatic site, surgical resection of metastases, local non-surgical treatment of metastases, and a bevacizumab-including first-line treatment schedule.
Multivariate binary logistic regression analysis (Table 7) confirmed the significant association between long-term survival and surgical resection of metastases, local non-surgical treatment of metastases, and a bevacizumab-including first-line treatment schedule.
Based upon these results, we wanted to verify the association between these long-term survival (LTS) associated treatments and OS in the whole series. Considered that both surgical and non-surgical local treatment of metastases were aimed at the local control of a systemic disease, we considered these two treatments as equivalent. Thus, the identified LTS-associated treatments were (i) bevacizumab at first line and (ii) surgical resection of metastasis and/or local non-surgical treatment of metastasis. Kaplan–Meier analysis of the whole series (n = 248) showed a significantly longer OS in patients treated with LTS-associated treatments (log-rank test p < 0.001): 3.92 years median (IQR 0.69–11.7) versus 2.15 years median (IQR 0.30–9.17) (Fig. 1c). Considering local treatment of metastases exclusively (irrespectively if by surgery or other approaches), an association with longer OS was still confirmed (log-rank test < 0.001): 3.83 years median (IQR 2.23–5.26) versus 2.05 years median (IQR 1.37–3.26) (Fig. 1d).
Finally, considered the prognostic role of local metastasis treatment, we verified the characteristics of metastatic disease in the series (Table 8). Overall, a higher rate of single metastases was found within long-term survivors compared with other patients (67% versus 52%). Among patients with single site metastases, a higher rate of long-term survivors received local treatments (75% versus 22% in patients with < 4 years survival).
Discussion
In the present study, outcome analysis of a real-life series of mCRC treated prior to expanded KRAS assessment and with long-term follow-up was performed: a high rate (24%) of long-surviving patients (≥ 4 years) was observed, and their characteristics were evaluated. Local treatment of metastases (surgical or non-surgical) and a first-line treatment including bevacizumab resulted significantly associated with PFS/OS and long-term survival at univariate and multivariate analyses.
Overall characteristics of the series were as expected: tumor involved right colon in 1/3 of cases, while the neoplasm was left-sided (left colon or rectum) in the remaining 2/3 [21]. Molecular status (KRAS mutation rate of about 45%) was consistent with the results observed prior to expanded KRAS evaluation [10]. Most of the patients had synchronous metastases at diagnosis (64%), and the majority of them had single site involvement (55%).
mCRC is a leading cause of cancer-related death despite our fast evolving knowledge regarding the molecular inner-workings of this disease and the availability of novel therapeutic options. Thanks to the introduction of the FOLFOX/FOLFIRI schedules, median OS reached the 2 years milestone, while addition of anti-angiogenetic and anti-EGFR biological therapies led to a further improvement to about 30 months [22]. Despite the real-life retrospective nature of our series and the time period analyzed, we observed a median OS of more than 30 months which is in line or even superior to what could have been expected, considered the analyzed years and the now outdated molecular profiling based on KRAS exon 2 evaluation only. This is a first interesting result and a possible explanation of this good overall outcome could be the specific study setting: a tertiary university hospital with a dedicated colorectal cancer unit. Management of patients with colorectal cancer by multidisciplinary teams proved to be especially relevant for advanced disease [23] and was found to be associated with improved outcomes in multiple reports [24,25,26,27,28].
Lower age at metastasis (p = 0.009), surgical resection of metastases (p < 0.001), and a left-sided tumor (p = 0.048) were associated with longer OS by multivariate Cox regression analysis stratified by local treatment. Data accrued during the last few years showed an association between right sided primary tumors and poorer outcome even when accounting for the specific tumor molecular profile (like RAS status) or other variables [14, 29, 30]. This finding could be potentially due to the different embryologic origin of the right versus left colon and is now being accounted for when designing new clinical trials. Our results confirm these findings in a large series with extended follow-up.
Focusing on long-term survivors (≥ 4 years), this group differed in terms of (i) lower age at initial diagnosis (60 versus 66, p = 0.012) and at metastasis (62 versus 66 years, p = 0.026); (ii) lower rate of multiple metastatic sites (33.3 % versus 48.4%, p = 0.038); (iii) higher rates of surgical (63% versus 26%, p < 0.001) and/or local non-surgical metastasis treatment (57% versus 21%, p < 0.001); (iv) a significantly longer first-line treatment median PFS (15.7 versus 10.8 months respectively, p = 0.0001). Although this latter association could be expected and considered an example of responder bias, it is interesting to note that the statistically significant difference was confirmed in all subsequent treatment lines as well; thus, the sequential PFSs observed during the disease course seem to remain informative to estimate patients’ outcome.
Regarding outcome analysis, the variables which showed the strongest association with long-term survival were the local treatments of metastases (either surgically or not). This finding was confirmed by multivariate binary regression logistic analysis. The availability of a specific dedicated multidisciplinary colorectal team probably had a role in enabling this kind of treatment in such a significant group of patients (37%, 92/248), including 37% (41/111) of those with multiple sites involvement.
Our results encourage a focused, active treatment of metastases if clinically feasible, even when multiple sites involvement is present. Indeed, it has been long known that surgical resection of liver metastasis in mCRC can achieve long-term survival or cure in a high rate of patients (even > 50%), especially in oligometastatic patients with liver involvement only, but the present results support that a long-term benefit can be achieved also in case of metastatic spread to multiple sites [31, 32]. This observation is consistent with the recent trial results in patients with unresectable metastases which demonstrated a significantly longer OS thanks to a combined approach (systemic treatment plus aggressive local treatment by radiofrequency ablation ± resection) compared with systemic therapy alone [33]. Conversely, randomized trials comparing surgical and non-surgical approaches are lacking. In our cohort, both approaches were associated with an improved survival, but no conclusions can be inferred regarding the superiority of a specific option since many patients received both the treatments.
Bevacizumab at first line was the second variable which resulted associated with long-term survival and first line PFS despite a non-significant finding in terms of OS. This benefit is consistent with literature data, and the missing association with OS could be due to the limited sample size of this group of patients [34]. These results highlight the importance of long-term outcome analysis to comprehensively assess the efficacy of different prognostic factors and treatments, both in terms of their combinations and sequencing. The potential synergistic effect of a bevacizumab-including regimen followed by metastasis surgical resection should also be kept in mind considered the recent data suggesting the positive impact of this approach on OS of mCRC [35, 36].
Nevertheless, these findings must be interpreted cautiously due to the retrospective, real-life nature of the study and its consequent, intrinsic limitations. During the study period, some variations occurred both in terms of the routine treatment protocols and the available clinical trials. The limited sample size, in particular of the long-survivors group, is a second limitation. Although this drawback could have been tackled by broadening the time period selected to collect the cases, it would have significantly increased the heterogeneity in terms of molecular assessment, treatments, and patients’ management; thus, we tried to reach a compromise between numerosity and heterogeneity to enable a meaningful longitudinal analysis. Nevertheless, the characteristics of such a real-life retrospective cohort allowed to identify variables which were significantly associated with outcome despite these changes over time [37]. Another relevant perspective, which would have been interested to investigate, is quality of life and patient-reported outcomes measures which are especially relevant in long-term cancer survivors [38]. This shortcoming could be addressed by implementing a brief assessment of these parameters into the routine clinical evaluation, but these efforts are hindered by the ever-increasing demands on healthcare staff.
Further studies, based on more recent cohorts, will allow to verify the significance of the prognostic markers here identified within specific mCRC molecular subgroups and their correlations with the newly introduced drugs and regimens. As shown during the last few years in melanoma or lung adenocarcinoma, new treatments like immunotherapy and/or targeted therapies can dramatically reshape the therapeutic approaches, even in patients with advanced/metastatic disease [39, 40]. This is another promising perspective of tailored medicine which shall not be undervalued: precision oncology should not only rest on new molecularly driven drugs, but also on the optimization of the already validated treatments within the different clinical settings and for each single patient.
Conclusions
Our study shows that long-term survival could often be achieved in mCRC even in an era of limited molecular characterization. Although the clinical setting of the present study (a tertiary-level university hospital) has likely played a role in achieving these overall results, local treatment of metastases proved to be a clear predictor of long-term survival. These data will also help compare and interpret the long-term results of the new cohorts of patients which are now being treated according to more extensive molecular profiling and novel protocols.
Availability of data and materials
The datasets generated and/or analyzed during the current study are not publicly available to protect patients’ privacy, but are available from the corresponding author upon reasonable request.
Abbreviations
- mCRC:
-
Metastatic colorectal cancer
- CRC:
-
Colorectal cancer
- EGFR:
-
Epidermal Growth Factor Receptor
- VEGF:
-
Vascular Endothelial Growth Factor
- FOLFOX/CAPOX:
-
5-fluorouracil plus oxaliplatin/capecitabine plus oxaliplatin
- FOLFIRI/CAPIRI:
-
5-fluorouracil plus irinotecan/capecitabine plus irinotecan
- FOLFOXIRI:
-
5-fluorouracil plus oxaliplatin and irinotecan
- MSI-high:
-
High microsatellite instability
- PFS:
-
Progression free survival
- OS:
-
Overall survival
- SD:
-
Standard deviation
- IQR:
-
Interquartile range
- HR:
-
Hazard ratios
- 95% CI:
-
95% confidence intervals
- LTS:
-
Long-term survival
References
Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68(1):7–30.
Siegel RL, Miller KD, Fedewa SA, Ahnen DJ, Meester RGS, Barzi A, et al. Colorectal cancer statistics, 2017. CA Cancer J Clin. 2017;67(3):177–93.
Shah MA, Renfro LA, Allegra CJ, Andre T, de Gramont A, Schmoll HJ, et al. Impact of patient factors on recurrence risk and time dependency of oxaliplatin benefit in patients with colon cancer: analysis from Modern-Era Adjuvant Studies in the Adjuvant Colon Cancer End Points (ACCENT) Database. J Clin Oncol. 2016;34(8):843–53.
Guinney J, Dienstmann R, Wang X, de Reynies A, Schlicker A, Soneson C, et al. The consensus molecular subtypes of colorectal cancer. Nat Med. 2015;21(11):1350–6.
Fontana E, Eason K, Cervantes A, Salazar R, Sadanandam A. Context matters-consensus molecular subtypes of colorectal cancer as biomarkers for clinical trials. Ann Oncol. 2019;30(4):520–7.
Wu C. Systemic therapy for colon cancer. Surg Oncol Clin N Am. 2018;27(2):235–42.
Douillard JY, Oliner KS, Siena S, Tabernero J, Burkes R, Barugel M, et al. Panitumumab-FOLFOX4 treatment and RAS mutations in colorectal cancer. N Engl J Med. 2013;369(11):1023–34.
Bokemeyer C, Kohne CH, Ciardiello F, Lenz HJ, Heinemann V, Klinkhardt U, et al. FOLFOX4 plus cetuximab treatment and RAS mutations in colorectal cancer. Eur J Cancer. 2015;51(10):1243–52.
Van Cutsem E, Lenz HJ, Kohne CH, Heinemann V, Tejpar S, Melezinek I, et al. Fluorouracil, leucovorin, and irinotecan plus cetuximab treatment and RAS mutations in colorectal cancer. J Clin Oncol. 2015;33(7):692–700.
Peeters M, Kafatos G, Taylor A, Gastanaga VM, Oliner KS, Hechmati G, et al. Prevalence of RAS mutations and individual variation patterns among patients with metastatic colorectal cancer: a pooled analysis of randomised controlled trials. Eur J Cancer. 2015;51(13):1704–13.
Roth AD, Tejpar S, Delorenzi M, Yan P, Fiocca R, Klingbiel D, et al. Prognostic role of KRAS and BRAF in stage II and III resected colon cancer: results of the translational study on the PETACC-3, EORTC 40993, SAKK 60-00 trial. J Clin Oncol. 2010;28(3):466–74.
Hurwitz H, Fehrenbacher L, Novotny W, Cartwright T, Hainsworth J, Heim W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350(23):2335–42.
Tabernero J, Van Cutsem E, Lakomy R, Prausova J, Ruff P, van Hazel GA, et al. Aflibercept versus placebo in combination with fluorouracil, leucovorin and irinotecan in the treatment of previously treated metastatic colorectal cancer: prespecified subgroup analyses from the VELOUR trial. Eur J Cancer. 2014;50(2):320–31.
Arnold D, Lueza B, Douillard JY, Peeters M, Lenz HJ, Venook A, et al. Prognostic and predictive value of primary tumour side in patients with RAS wild-type metastatic colorectal cancer treated with chemotherapy and EGFR directed antibodies in six randomized trials. Ann Oncol. 2017;28(8):1713–29.
Grothey A, Van Cutsem E, Sobrero A, Siena S, Falcone A, Ychou M, et al. Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): an international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet. 2013;381(9863):303–12.
Van Cutsem E, Martinelli E, Cascinu S, Sobrero A, Banzi M, Seitz JF, et al. Regorafenib for patients with metastatic colorectal cancer who progressed after standard therapy: results of the large, single-arm, open-label phase IIIb CONSIGN study. Oncologist. 2019;24(2):185–92.
Li J, Qin S, Xu R, Yau TC, Ma B, Pan H, et al. Regorafenib plus best supportive care versus placebo plus best supportive care in Asian patients with previously treated metastatic colorectal cancer (CONCUR): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2015;16(6):619–29.
Le DT, Uram JN, Wang H, Bartlett BR, Kemberling H, Eyring AD, et al. PD-1 Blockade in tumors with mismatch-repair deficiency. N Engl J Med. 2015;372(26):2509–20.
Overman MJ, McDermott R, Leach JL, Lonardi S, Lenz HJ, Morse MA, et al. Nivolumab in patients with metastatic DNA mismatch repair-deficient or microsatellite instability-high colorectal cancer (CheckMate 142): an open-label, multicentre, phase 2 study. Lancet Oncol. 2017;18(9):1182–91.
Noone AM HN, Krapcho M, Miller D, Brest A, Yu M, Ruhl J, Tatalovich Z, Mariotto A, Lewis DR, Chen HS, Feuer EJ, Cronin KA. SEER Cancer Statistics Review, 1975-2015, National Cancer Institute. 2018 [Bethesda, MD, https://seer.cancer.gov/csr/1975_2015/, based on November 7 SEER data submission, posted to the SEER web site, April 8.].
Loree JM, Pereira AAL, Lam M, Willauer AN, Raghav K, Dasari A, et al. Classifying colorectal cancer by tumor location rather than sidedness highlights a continuum in mutation profiles and consensus molecular subtypes. Clin Cancer Res. 2018;24(5):1062–72.
Cremolini C, Schirripa M, Antoniotti C, Moretto R, Salvatore L, Masi G, et al. First-line chemotherapy for mCRC-a review and evidence-based algorithm. Nat Rev Clin Oncol. 2015;12(10):607–19.
Wesselmann S, Winter A, Ferencz J, Seufferlein T, Post S. Documented quality of care in certified colorectal cancer centers in Germany: German Cancer Society benchmarking report for 2013. Int J Colorectal Dis. 2014;29(4):511–8.
Morris E, Haward RA, Gilthorpe MS, Craigs C, Forman D. The impact of the Calman-Hine report on the processes and outcomes of care for Yorkshire's colorectal cancer patients. Br J Cancer. 2006;95(8):979–85.
Kilsdonk MJ, van Dijk BA, Otter R, Siesling S, van Harten WH. The impact of organisational external peer review on colorectal cancer treatment and survival in the Netherlands. Br J Cancer. 2014;110(4):850–8.
MacDermid E, Hooton G, MacDonald M, McKay G, Grose D, Mohammed N, et al. Improving patient survival with the colorectal cancer multi-disciplinary team. Colorectal Dis. 2009;11(3):291–5.
Lordan JT, Karanjia ND, Quiney N, Fawcett WJ, Worthington TR. A 10-year study of outcome following hepatic resection for colorectal liver metastases - The effect of evaluation in a multidisciplinary team setting. Eur J Surg Oncol. 2009;35(3):302–6.
Van Cutsem E, Cervantes A, Adam R, Sobrero A, Van Krieken JH, Aderka D, et al. ESMO consensus guidelines for the management of patients with metastatic colorectal cancer. Ann Oncol. 2016;27(8):1386–422.
Boeckx N, Koukakis R. Op de Beeck K, Rolfo C, Van Camp G, Siena S, et al. Primary tumor sidedness has an impact on prognosis and treatment outcome in metastatic colorectal cancer: results from two randomized first-line panitumumab studies. Ann Oncol. 2017;28(8):1862–8.
Holch JW, Ricard I, Stintzing S, Modest DP, Heinemann V. The relevance of primary tumour location in patients with metastatic colorectal cancer: a meta-analysis of first-line clinical trials. Eur J Cancer. 2017;70:87–98.
Cummings LC, Payes JD, Cooper GS. Survival after hepatic resection in metastatic colorectal cancer: a population-based study. Cancer. 2007;109(4):718–26.
House MG, Ito H, Gonen M, Fong Y, Allen PJ, DeMatteo RP, et al. Survival after hepatic resection for metastatic colorectal cancer: trends in outcomes for 1,600 patients during two decades at a single institution. J Am Coll Surg. 2010;210(5):744–52 52-5.
Ruers T, Van Coevorden F, Punt CJ, Pierie JE, Borel-Rinkes I, Ledermann JA, et al. Local treatment of unresectable colorectal liver metastases: results of a randomized phase II trial. J Natl Cancer Inst. 2017;109(9):djx015.
Mody K, Baldeo C, Bekaii-Saab T. Antiangiogenic therapy in colorectal cancer. Cancer J. 2018;24(4):165–70.
Uetake H, Yasuno M, Ishiguro M, Kameoka S, Shimada Y, Takahashi K, et al. A multicenter phase II trial of mFOLFOX6 plus bevacizumab to treat liver-only metastases of colorectal cancer that are unsuitable for upfront resection (TRICC0808). Ann Surg Oncol. 2015;22(3):908–15.
Yasuno M, Uetake H, Ishiguro M, Mizunuma N, Komori T, Miyata G, et al. mFOLFOX6 plus bevacizumab to treat liver-only metastases of colorectal cancer that are unsuitable for upfront resection (TRICC0808): a multicenter phase II trial comprising the final analysis for survival. Int J Clin Oncol. 2019;24(5):516–25.
Modest DP, Pant S, Sartore-Bianchi A. Treatment sequencing in metastatic colorectal cancer. Eur J Cancer. 2019;109:70–83.
Gordon BE, Chen RC. Patient-reported outcomes in cancer survivorship. Acta Oncol. 2017;56(2):166–73.
Luther C, Swami U, Zhang J, Milhem M, Zakharia Y. Advanced stage melanoma therapies: detailing the present and exploring the future. Crit Rev Oncol Hematol. 2019;133:99–111.
Herbst RS, Morgensztern D, Boshoff C. The biology and management of non-small cell lung cancer. Nature. 2018;553(7689):446–54.
Acknowledgements
We would like to thank Dr. Cristina Sonetto for assisting in clinical data retrieval and curation.
Funding
This research was funded by the Rete Oncologica del Piemonte e della Valle d’Aosta (grant to L.B. and P.C.; no specific grant number applicable) and received funding specifically dedicated to the Department of Medical Sciences, University of Turin from Italian Ministry for Education, University and Research (Ministero dell'Istruzione, dell'Università e della Ricerca - MIUR) under the program “Dipartimenti di Eccellenza 2018 – 2022,” Project no. D15D18000410001.
Author information
Authors and Affiliations
Contributions
L.B., R.S., S.O., S.M., I.C., and A.G. collected, analyzed, and interpreted data; L.B. and S.O. drafted the manuscript; P.R., M.M., and P.C. contributed to data interpretation and designed the study; all authors edited, reviewed, and approved the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study was conducted in accordance with The Code of Ethics of the World Medical Association (Declaration of Helsinki) for experiments involving humans and within the guidelines and regulations defined by the Research Ethics Committee of the University of Turin. This study was approved by the Research Ethics Committee of the University of Turin, considered the retrospective nature of the research protocol and that it had no impact at all on patients’ care, and no specific written informed consent was required.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Bertero, L., Spadi, R., Osella-Abate, S. et al. Metastatic colorectal cancer prior to expanded RAS assessment: evidence from long-term outcome analysis of a real-life cohort within a dedicated colorectal cancer unit. World J Surg Onc 18, 65 (2020). https://doi.org/10.1186/s12957-020-01844-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12957-020-01844-5