Abstract
Background
The relationship between serum vitamin D and atrial fibrillation (AF) or postoperative atrial fibrillation (POAF) in patients undergoing coronary artery bypass graft (CABG) is still debated. It is also unclear whether there is a dose-response relationship between circulating vitamin D and the risk of AF or POAF.
Methods
The Cochrane Library, PubMed, and Embase databases were searched for relevant studies. We used a “one-stage approach” with a restricted cubic spline model to summarize the dose-specific relationships between serum vitamin D and AF. Relative risk (RR) was used to measure the effects in this meta-analysis.
Results
In total, 13 studies were included with a total of 6519 cases of AF among 74,885 participants. Vitamin D deficiency (< 20 ng/ml) was associated with increased risks of AF (RR: 1.23, 95% CI: 1.05–1.43). In the dose-response analysis, the summary RR for a 10 ng/ml increased in vitamin D was 0.88 (95% CI: 0.78–0.98) and there was no evidence of a non-linear association, Pnon-linearity = 0.86. In the age subgroup, high vitamin D (per 10 ng/ml increase) reduced the risk of AF in the older group (> 65 years) (RR = 0.68, 95% CI = 0.52–0.89) but not among young individuals (< 65 years) (RR = 0.87, 95% CI = 0.72–1.06). In addition, a strong association was found between a 10 ng/ml increased in vitamin D and POAF incident in the patient after CABG (RR: 0.44, 95% CI: 0.24–0.82).
Conclusion
Our dose-response meta-analysis suggested serum vitamin D deficiency was associated with an increased risk of AF in the general population and POAF in patients after CABG. Further studies are needed to explore the age difference in the association between serum vitamin D level and the risk of AF and whether vitamin D supplements will prevent AF.
Trial registration
This study has been registered with PROSPERO (International prospective register of systematic reviews)-registration number-CRD42019119258.
Similar content being viewed by others
Introduction
Historically, vitamin D is known for its important role in skeletal disease, [1,2,3]. The focus in recent decades has been on the risks of vitamin D and non-skeletal diseases, such as cardiovascular disease or atrial fibrillation (AF) [4]. AF is the most common cardiac arrhythmia in clinical practice and is associated with increased risk of morbidity. Vitamin D deficiency is common in many countries [5], only 23% of people would reach serum vitamin D concentration above 30 ng/ml [6]. A cause-and-effect relation between low vitamin D status and AF incident would be of considerable benefit to public health. However, unlike for skeletal disease, the evidence for serum vitamin D deficiency (< 20 ng/ml) and the risk of AF has been inconclusive [7, 8]. To date, results from several observational studies have suggested that patients with vitamin D deficiency were approximately twice as likely to have AF than patients with normal levels (> 30 ng/ml) [8,9,10,11]. Conversely, several prospective studies did not find this association [7, 12,13,14]. Thereafter, several articles have reviewed published studies and yielded conflicting results [7, 15, 16]. Alonso et al. did not find a clinically relevant association of circulating vitamin D per 1 standard deviation (8.5 ng/ml) decreased with AF risk [7]. In contrast, a meta-analysis concluded a weak but positive association between vitamin D deficiency and AF [15]. However, there are several limitations in the previous meta-analyses. For example, vitamin D levels were analyzed as either a categorical or continuous variable in the individual studies, so they could not pool all of the studies together. In addition, the shape of the dose-response association between vitamin D and AF had been explored. Moreover, several new research articles reported higher serum 25(OH) D is associated with new-onset AF after coronary artery bypass grafting (CABG) surgery [17,18,19,20]. Therefore, we performed a comprehensive meta-analysis to evaluate the shape of the dose-response relation between circulating 25(OH) D concentration and the risk of AF and post-operation AF (POAF) after CABG.
Methods
This work has been performed according to PRISMA guidelines (http://www.prisma-statement.org; Additional file 1: Table S1) [21]. We systematically searched the PubMed, Embase databases and Cochrane Library up to March 10, 2019. Additional file 1: Table S2 provides a detailed description of the search strategy. Two researchers independently worked in the whole process of this meta-analysis from the literature search and selection to data analysis. Both randomized controlled trials and observational studies, reporting data about serum vitamin D level and AF were considered eligible for this meta-analysis. All discrepancies were resolved through discussion by the two authors. We used the robust error meta-regression method (REMR) for the dose-response analysis of the vitamin D level and AF [22, 23]. All statistical analyses were done by using Review Manager (RevMan) version 5.3 (The Cochrane Collaboration 2014; Nordic Cochrane Center Copenhagen, Denmark) and Stata software (Version 14.0, Stata Corp LP, College Station, Texas, USA). We used the Newcastle-Ottawa quality assessment scale (NOS) to evaluate the quality for all included studies [24], a NOS score of ≥6 stars was regarded as high-quality, otherwise, as low-quality studies [25, 26]. Full details of the literature search strategy, study selection criteria, quality assessment, and statistical analysis have been reported in the Supplement Methods (Additional file 1). This study has been registered with PROSPERO (International prospective register of systematic reviews)-registration number-CRD42019119258.
Results
Study selection
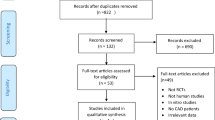
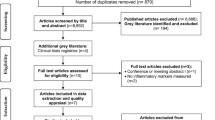
We identified 1484 studies in our initial database search. After removing duplicates and studies with inadequate information on vitamin D and AF, 20 studies were reviewed in more detail. Of these 20 studies, 5 were excluded for the following reasons: a) they were focused on recurrence AF (n = 1) [27]; b) they were reviews or case reports (n = 2) [15, 28]; or c) cross-section study (n = 1, 20]. Finally, 13 studies (14 reports) were included in this meta-analysis (Fig. 1).
Study characteristics and quality
Detailed characteristics of the included studies are presented in Table 1. Thirteen studies (14 reports) with 6519 AF cases and 74,885 participants were included in this meta-analysis [7,8,9,10,11,12,13,14, 16,17,18,19, 29]. Overall, these studies were published between 2011 and 2018. The sample sizes of the included studies varied from 48 to 47,062. The mean age ranged from 57 to 77 years. The duration of follow-up across the studies varied from 48 h to 18 years. Among the 13 articles, six were cohort studies [7, 12,13,14, 16], four were nest-case-control (prospective case-control) studies [17,18,19, 29], four [8,9,10,11] were case-control studies. Nine studies [7,8,9,10,11,12,13,14, 16] examine the serum vitamin D status and AF and 4 [17,18,19, 29] focused POAF in patients undergoing CABG. Only two studies [10, 16] were scored as low quality with a NOS of 5 stars. The rest eleven [7,8,9, 11,12,13,14, 17,18,19, 29] were high quality (≥6 stars) (Table 2).
Categorical analysis of serum vitamin D on AF
Six studies with 5503 cases/66,139 participants were included [7, 9, 12, 13, 16, 18]. As shown in Fig. 2, vitamin D deficiency (< 20 ng/ml) was associated with increased risks of AF (RR: 1.23, 95% CI: 1.05–1.43; I2 = 61%; P = 0.008). The results were consistent both in the cohort (RR: 1.09, 95% CI: 1.01–1.19; I2 = 0%; P = 0.03) and case-control studies (RR: 1.80, 95% CI: 1.38–2.35; I2 = 0%, P < 0.001).
Three [7, 9, 13] studies with 2291 cases and 15,920 individuals assessed the association between 25(OH) D insufficiency (21–29 ng/ml) and risk of AF. The pooled RR suggested that vitamin D insufficiency is associated with the occurrence of AF (RR: 1.14, 95% CI: 1.01–1.29; I2 = 15%, P = 0.03) with no evidence heterogeneity.
Dose-response association between circulating vitamin D and incident AF
Nine studies (10 reports) [7,8,9,10,11,12,13,14, 16] with 6364 cases/42,776 participants were included in the dose-response analysis of vitamin D and AF. The summary RR for a 10-unit increased of vitamin D was 0.88 (95% CI: 0.78–0.98, I2 = 85%, P = 0.03) (Fig. 3) and there was no evidence of a non-linear association, Pnon-linearity = 0.34. To address the main source of heterogeneity, we implemented subgroup analyses according to study design, and significant evidence of heterogeneity was shown between study design subgroups (Pheterogeneity < 0.001). The results were similar in both cohort and case-control studies (Table 3). Moreover, high vitamin D (per 10 ng/ml increase) reduced the risk of AF in the older group (≥65 years) (RR = 0.68, 95%CI = 0.52–0.89, P = 0.005) but not among young individuals (< 65 years) (RR = 0.87, 95%CI = 0.72–1.06, P = 0.17) although no significant heterogeneity was found in the age subgroup (P = 0.15) (Table 3).
Then, we conducted a non-linear dose-response by using restricted cubic model and found an inverse relationship between vitamin D and AF (Fig. 4).
In subgroup and sensitivity analysis, the positive association between vitamin D and risk AF persisted in almost all subgroup analyses defined by the duration of follow-up, geographic location, number of cases, study quality and adjustment for confounding and potential intermediate factors (Table 3).
Four studies [17,18,19, 29] with included 202 cases/520 patients were included in this analysis of vitamin D status and AF post-CABG. Serum vitamin D per 10 ng/ml increase were associated with decreased POAF incident (RR: 0.44, 95% CI: 0.24–0.82, I2 = 70%, P = 0.01) with modest heterogeneity (Fig. 5). The nonlinear dose-response analysis was not available because of limited information.
Publication bias
There was some indication of publication bias with Egger’s test, p = 0.07, or with Begg’s test, p = 0.03 or by inspection of the funnel plot (Additional file 1: Figure S1-S3). Thus, we used the “trim-and-fill” method for the adjustment of publication bias. However, the results showed “no trimming performed and data unchanged”, which demonstrated that our results were stable. The publication bias of POAF was not conducted as limited studies (N < 10) according to the guideline [30].
Discussion
To the best of our knowledge, this is the first meta-analysis to evaluate the dose-response association between vitamin D and AF. Our results by dose-response analysis suggested that vitamin D deficiency is a moderate predictor of AF. We found vitamin D deficiency (< 20 ng/ml) or vitamin inadequate (< 30 ng/ml) increased the risk of AF by 23% or 14%, respectively. Interestingly, the present dose-response analysis first showed vitamin D deficiency is also a moderate predictor for POAF, and novelty showed a 12% (AF) or 56% (AF post-CABG) increase in the RR per 10-unit increase in vitamin D with evidence of linear association.
Formerly, the role of circulating vitamin D in AF remains unclear. A meta-analysis found a vitamin D deficiency is associated with a 31% increased risk for AF [15]. However, another study which only pooled cohort studies did not support this association [7]. Our results showed there is an inverse association between vitamin D and risk of AF, both in cohort and case-control studies. However, significant heterogeneity between the subgroup of study design (cohort and case-control study) was observed. The association in cohorts was significantly weaker than that in case-control studies. Besides the recall bias, the heterogeneity in the study population appeared to be one of the potential reasons. The cohort studies were general population-based and the case-control studies mainly focused on patients with cardiovascular disease (CVDs). Recent studies also have shown that vitamin D was a potential connection with CVDs (eg. ischemic heart disease) and diabetes [15, 31]. Therefore, it might be reasonable to speculate that the association between vitamin D deficiency and AF may be amplified in patients with CVDs or at high risk of developing CVDs. However, considering the limited sample size, the role of vitamin D in patients with CVDs or at high CVDs risk need to be further studied.
The association between vitamin D deficiency and AF has several potential pathophysiological mechanisms. Inflammation has a crucial role in the pathogenesis of AF [32]. For example, C-reactive protein (CRP), the most robust and reproducible marker of vascular inflammation, could increase the risk of AF by up to two-fold [33]. Noticeable, low vitamin D status could directly or indirectly increase the synthesis of CRP [34]. Another important mechanism might be the activation of the renin-angiotensin-aldosterone system (RAAS). RASS plays an important role in both structural and electrical remodeling of the atrium. Studies in experimental animals showed vitamin D could inhibit the RAAS system [35]. Clinical researches showed that the use of ACEIs was associated with less atrial fibrosis, and the blockade of angiotensin II has been shown to have beneficial effects on electrical remodeling in human atrial tissue [36, 37]. Moreover, a previous meta-analysis also reported that inhibition of RASS might reduce the risk of developing new-onset atrial fibrillation [38]. Therefore, low vitamin D level might increase the AF risk secondary to its negative regulatory property of the RAAS.
It is not surprised that we found vitamin D deficiency is associated with increased POAF. Previous studies have suggested that deficiency of total vitamin D is associated with increased prevalence of electrocardiographic abnormalities (e.g. prolonging the duration of action potentials) [39]. Moreover, in a recent prospective cohort study of patients undergoing cardiac surgery, low total 25(OH) D levels were independently associated with the risk of major cardiac and cerebrovascular events [39]. Another study also showed an inverse relationship between serum 25(OH) D level and left atrial or AF recurrence in patients after undergoing catheter ablation [40]. However, considering the small sample size and short-term follow-up, the relationship between vitamin D and POAF need to be further confirmed in larger, well-designed studies.
We also studied the role of age in the present meta-analysis, we found low 25(OH) D level increased the risk of AF in the older individuals (age ≥ 65 years) but not young group (< 65 years). This result should be with caution in elder individuals. However, there was significant heterogeneity in the results, which might come from the study population or study design. When we excluded the case-control studies, both the older (RR: 0.96, 95%CI: 0.93–1.00) and young people (RR:0.94, 95%CI: 0.89–1.00) showed a weak association between vitamin D deficiency and risk of AF. Of note, these results were incosistent with the recent analysis of ARIC study [7], which showed that low vitamin D was a stronger indicator of AF in the youngest group (< 54 years) but not in the oldest (> 60 years), with an intermediate association in those aged 54–59 years. Therefore, based on current evidence, the age difference in the relationship between vitamin D and risk of AF is still unclear. Further prospective cohort studies are needed to clarify the age difference.
Study limitations
The present meta-analysis has several limitations. First, this was a meta-analysis of observational studies, which cannot chiefly prove causation, and the unmeasured and insufficiently measured variables (e.g. seasonal variation in vitamin D) would result in the possibility of residual confoundings. However, most of our studies were performed during the winter or spring months, which could reduce this confounding factor. Second, due to data restriction, the impact of vitamin D supplements on AF was not analyzed and need to be further investigated as we previously discussed. Third, some studies suggested that available vitamin D may be a more reliable marker of vitamin D status than total 25 (OH) D. However, none of the included studies measured available vitamin D.
Conclusion
Our dose-response suggested serum vitamin D deficiency was associated with an increased risk of AF in the general population and POAF in patients after CABG. Further studies are needed to explore if there is an age difference in the association between serum vitamin D level and the risk of AF and whether vitamin D supplements will prevent AF.
Availability of data and materials
All data generated or analyzed during this study are included in this published article [and its supplementary information files].
Abbreviations
- AF:
-
Atrial fibrillation
- CABG:
-
Coronary artery bypass graft
- CI:
-
Confidence interval
- NOS:
-
Newcastle-Ottawa Scale score
- POAF:
-
Postoperative atrial fibrillation
- RR:
-
Relative risk
References
Avenell A, Mak JC, O'Connell D. Vitamin D and Vitamin D analogues for preventing fractures in post-menopausal women and older men. Cochrane Database Syst Rev. 2014. https://doi.org/10.1002/14651858.CD000227.pub4
Halfon M, Phan O, Teta D. Vitamin D: a review on its effects on muscle strength, the risk of fall, and frailty. Biomed Res Int. 2015;2015:953241.
Lieben L, Carmeliet G. Vitamin D signaling in osteocytes: effects on bone and mineral homeostasis. Bone. 2013;54:237–43.
Wang TJ, Pencina MJ, Booth SL, Jacques PF, Ingelsson E, Lanier K, Benjamin EJ, D’Agostino RB, Wolf M, Vasan RS. Vitamin D deficiency and risk of cardiovascular disease. Circulation. 2008;117:503–11.
Lip GY, Tellomontoliu A. Management of atrial fibrillation. Int J Clin Pract. 2007;61:9–11.
Ginde AA, Liu MC, Camargo CA Jr. Demographic differences and trends of vitamin D insufficiency in the US population, 1988-2004. Arch Intern Med. 2009;169:626–32.
Alonso A, Misialek JR, Michos ED, Eckfeldt J, Selvin E, Soliman EZ, Chen LY, Gross MD, Lutsey PL. Serum 25-hydroxyvitamin D and the incidence of atrial fibrillation: the atherosclerosis risk in communities (ARIC) study. Europace. 2016;18:1143–9.
Belen E, Aykan A, Kalay E, Sungur M, Sungur A, Cetin M. Low-level Vitamin D is associated with atrial fibrillation in patients with chronic heart failure. Adv Clin Exp Med. 2016;25:51–7.
Chen WR, Liu ZY, Shi Y, Yin DW, Wang H, Sha Y, Chen YD. Relation of low vitamin D to nonvalvular persistent atrial fibrillation in Chinese patients. Ann Noninvasive Electrocardiol. 2014;19:166–73.
Demir M, Uyan U, Melek M. The effects of vitamin D deficiency on atrial fibrillation. Clin Appl Thromb Hemost. 2014;20:98–103.
Ozcan OU, Gurlek A, Gursoy E, Gerede DM, Erol C. Relation of vitamin D deficiency and new-onset atrial fibrillation among hypertensive patients. J Am Soc Hypertens. 2015;9:307–12.
Rienstra M, Cheng S, Larson MG, McCabe EL, Booth SL, Jacques PF, Lubitz SA, Yin X, Levy D, Magnani JW, et al. Vitamin D status is not related to development of atrial fibrillation in the community. Am Heart J. 2011;162:538–41.
Vitezova A, Cartolano NS, Heeringa J, Zillikens MC, Hofman A, Franco OH, Kiefte-de Jong JC. Vitamin D and the risk of atrial fibrillation--the Rotterdam study. PLoS One. 2015;10:e0125161.
Mathew JS, Sachs MC, Katz R, Patton KK, Heckbert SR, Hoofnagle AN, Alonso A, Chonchol M, Deo R, Ix JH, et al. Fibroblast growth factor-23 and incident atrial fibrillation: the multi-ethnic study of atherosclerosis (MESA) and the cardiovascular health study (CHS). Circulation. 2014;130:298–307.
Zhang Z, Yang Y, Ng CY, Wang D, Wang J, Li G, Liu T. Meta-analysis of Vitamin D deficiency and risk of atrial fibrillation. Clin Cardiol. 2016;39:537–43.
Turin A, Bax JJ, Doukas D, Joyce C, Lopez JJ, Mathew V, Pontone G, Shah F, Singh S, Wilber DJ, Rabbat MG. Interactions among Vitamin D, atrial fibrillation, and the renin-Angiotensin-aldosterone system. Am J Cardiol. 2018;122:780–4.
Emren SV, Aldemir M, Ada F. Does deficiency of Vitamin D increase new onset atrial fibrillation after coronary artery bypass grafting surgery? Heart Surg Forum. 2016;19:E180–4.
Ozsin KK, Sanri US, Toktas F, Kahraman N, Yavuz S. Effect of plasma level of Vitamin D on postoperative atrial fibrillation in patients undergoing isolated coronary artery bypass grafting. Braz J Cardiovasc Surg. 2018;33:217–23.
Gode S, Aksu T, Demirel A, Sunbul M, Gul M, Bakir I, Yeniterzi M. Effect of vitamin D deficiency on the development of postoperative atrial fibrillation in coronary artery bypass patients. J Cardiovasc Thorac Res. 2016;8:140–6.
Shadvar K, Ramezani F, Sanaie S, Maleki TE, Arbat BK, Nagipour B. Relationship between plasma level of vitamin D and post operative atrial fibrillation in patients undergoing CABG. Pak J Med Sci. 2016;32:900–4.
Moher D, Liberati A, Tetzlaff J, Altman DG. The PG: preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097.
Doi SAR, Sar D. The robust error meta-regression method for dose-response meta-analysis. Int J Evid Based Healthc. 2018;16(3):138.
Xu C, Liu Y, Jia PL, Li L, Liu TZ, Cheng LL, Deng K, Borhan ASM, Thabane L, Sun X. The methodological quality of dose-response meta-analyses needed substantial improvement: a cross-sectional survey and proposed recommendations. J Clin Epidemiol. 2019;107:1–11.
Mcpheeters ML. Newcastle-Ottawa Quality Assessment Scale; 2012.
Liu X, Ma J, Huang L, Zhu W, Yuan P, Wan R, Hong K. Fluoroquinolones increase the risk of serious arrhythmias: a systematic review and meta-analysis. Medicine (Baltimore). 2017;96:e8273.
Zhu W, Shen Y, Zhou Q, Xu Z, Huang L, Chen Q, Hong K. Association of Physical Fitness with the risk of atrial fibrillation: a systematic review and meta-analysis. Clin Cardiol. 2016;39:421–8.
Canpolat U, Aytemir K, Hazirolan T, Ozer N, Oto A. Relationship between vitamin D level and left atrial fibrosis in patients with lone paroxysmal atrial fibrillation undergoing cryoballoon-based catheter ablation. J Cardiol. 2017;69:16–23.
Thompson J, Nitiahpapand R, Bhatti P, Kourliouros A. Vitamin D deficiency and atrial fibrillation. Int J Cardiol. 2015;184:159–62.
Skuladottir GV, Cohen A, Arnar DO, Hougaard DM, Torfason B, Palsson R, Indridason OS. Plasma 25-hydroxyvitamin D2 and D3 levels and incidence of postoperative atrial fibrillation. J Nutr Sci. 2016;5:e10.
Higgins JP, Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]: The Cochrane Collaboration; 2011. Available from http://handbook.cochrane.org.chanpter: 10.4.3.1 Recommendations on testing for funnel plot asymmetry.2011
Seida JC, Mitri J, Colmers IN, Majumdar SR, Davidson MB, Edwards AL, Hanley DA, Pittas AG, Tjosvold L, Johnson JA. Clinical review: effect of vitamin D3 supplementation on improving glucose homeostasis and preventing diabetes: a systematic review and meta-analysis. J Clin Endocrinol Metab. 2014;99:3551–60.
Katritsis DG. Is atrial fibrillation an inflammatory disorder? Eur Heart J. 2006;27:136–49.
Asselbergs FW, van den Berg MP, Diercks GF, van Gilst WH, van Veldhuisen DJ. C-reactive protein and microalbuminuria are associated with atrial fibrillation. Int J Cardiol. 2005;98:73–7.
Shea MK, Booth SL, Massaro JM, Jacques PF, D'Agostino RB Sr, Dawson-Hughes B, Ordovas JM, O'Donnell CJ, Kathiresan S, Keaney JF Jr. Vitamin K and vitamin D status: associations with inflammatory markers in the Framingham offspring study. Am J Epidemiol. 2007;167:313–20.
Yi-Jen C, Yao-Chang C, Ching-Tai T, Hung-I Y, Cheng-I L, Shih-Ann C. Angiotensin II and angiotensin II receptor blocker modulate the arrhythmogenic activity of pulmonary veins. Br J Pharmacol. 2010;147:12–22.
Boldt A, Scholl A, Garbade J, Resetar ME, Mohr FW, Gummert JF, St D. ACE-inhibitor treatment attenuates atrial structural remodeling in patients with lone chronic atrial fibrillation. Basic Res Cardiol. 2006;101:261–7.
Forman JP, Williams JS, Fisher NDL. Plasma 25-hydroxyvitamin D and regulation of the renin-angiotensin system in humans. Hypertension. 2010;55:1283.
Schneider MP, Hua TA, Bohm M, Wachtell K, Kjeldsen SE, Schmieder RE. Prevention of atrial fibrillation by renin-Angiotensin system inhibition a meta-analysis. J Am Coll Cardiol. 2010;55:2299–307.
Armin Z, Joachim K, Ernst JB, Tobias B, Jens D, Cornelius K, Gummert JF, Jochen BR. 25-hydroxyvitamin D, 1,25-dihydroxyvitamin D and postoperative outcome in cardiac surgery. J Clin Endocrinol Metab. 2015;100:72–80.
Cerit L, Duygu H. Association of pre-ablation level of vitamin D with atrial fibrillation recurrence after catheter ablation. Europace. 2017;19:1586.
Acknowledgements
Not applicable
Funding
This work was supported by grants from the National Natural Science Foundation of China (81530013), the National Key Research and Development Program of China (2017YFC1307804).
Author information
Authors and Affiliations
Contributions
KH was responsible for the entire project and revised the draft. XL and ZCT, performed the systematic literature review and drafted the first version of the manuscript. All authors took part in the interpretation of the results and prepared the final version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable
Consent for publication
Not applicable
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Additional file 1.
Online Data Supplement.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Liu, X., Wang, W., Tan, Z. et al. The relationship between vitamin D and risk of atrial fibrillation: a dose-response analysis of observational studies. Nutr J 18, 73 (2019). https://doi.org/10.1186/s12937-019-0485-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12937-019-0485-8