Abstract
Background
The existing evidence is limited and contradicting on the co-occurrence of anemia and stunting (CAS) at individual level, despite a great overlap in their risk factors. We aimed to determine the prevalence of CAS, and the dietary and non-dietary factors associated with it, among infants and young children in Ethiopia.
Method
We used a nationally representative sample of 2902 children aged 6–23 months from the Ethiopian demographic and health survey, conducted in 2016. The study was cross-sectional in design. Samples were selected by two-stage clustering sampling method. CAS prevalence was estimated by various sociodemographic factors. To identify the dietary and non-dietary factors associated with CAS, we conducted hierarchical logistic regression analyses.
Result
The overall prevalence of CAS was 23.9%. The dietary factors found significantly linked to lower odds of CAS were use of vitamin A supplement [adjusted odds ratio (AOR) = 1.19, 95%CI = 1.06–1.33, P = 0.003], consumption of vitamin A rich fruit and vegetables (AOR = 1.15, 95%CI = 1.04–1.27, P = 0.006), meat (AOR = 1.55, 95%CI = 1.17–2.05, P = 0.002), legumes (AOR = 1.38, 95%CI = 1.05–1.81, P = 0.021), and meal frequency > 3 (AOR = 1.22, 95%CI = 1.04–1.37, P = 0.020). The non-dietary household and child factors found significantly linked to higher odds of CAS were rural residence (AOR = 1.29, 95%CI = 1.18–1.41, P < 0.001), low household wealth (AOR = 1.91, 95%CI = 1.53–2.39, P < 0.001), low caregivers’ education level (AOR = 2.14, 95%CI = 1.33–3.44, P < 0.001), male sex (AOR = 1.25, 95%CI = 1.04–1.50, P = 0.015), age 12–23 months (AOR = 1.65, 95%CI = 1.57–1.73, P < 0.001), history of infection (AOR = 1.14, 95%CI = 1.00–1.30, P = 0.048), and small birth size (AOR = 1.99, 95%CI = 1.58–2.51, P < 0.001).
Conclusion
Among infants and young children in Ethiopia, there was a concerning high level of CAS, which was associated with various dietary and non-dietary factors. Enhanced public health/nutrition interventions, with due emphasis on the multifactorial nature of CAS, might stand an important consideration to reduce the burden of CAS in Ethiopia and beyond.
Similar content being viewed by others
Introduction
Malnutrition remains a major public health challenge in Ethiopia, with anemia and stunting being the top two prevalent nutritional problems among infants and young children [1, 2]. The recent period has seen a significant and unexpected increment in anemia prevalence among under-5 Ethiopian children, rising from 44% in 2011 to 57% in 2016 [1]. According to the World Health Organization (WHO), anemia prevalence above 40% is classified as a severe public health problem and requires designing comprehensive interventions [3]. Stunting also remains a major problem despite it has been declining. In 2016, 38% of under-5 Ethiopian children were stunted [1].
Clustering of nutrition problems could occur at country, household, or individual levels. For most of the under-nutrition problems, there is a considerable risk factor overlap, particularly in the basic and underlying determinants [4,5,6]. Poor socioeconomic status, suboptimal child feeding and hygiene practices, and childhood illness coupled with poor health service utilization are often associated with multiple negative health outcomes, including malnutrition [5,6,7,8]. Thus, individuals are likely to be affected by double or even multiple forms of malnutrition. Anemia and stunting are of multiple overlapping influences originating from various levels [9,10,11]. Thus, a considerable co-occurrence of anemia and stunting (CAS) would be expected in settings with poor child care practices.
As each of anemia and stunting poses a significant challenge to the health system as well as the survival of children [12, 13], their co-occurrence (CAS) would be even more detrimental. However, studies on CAS are limited despite the availability of a large body of literature on each of anemia and stunting. Furthermore, the existing few studies on the clustering of the major forms of undernutrition, including CAS, are inconsistent [14,15,16,17]. Studies from Latin American countries reported a low level of anemia and stunting clustering. These studies recommended programmers to consider anemia and stunting as independent of each other and to focus on tailored, rather than comprehensive, malnutrition prevention interventions [15,16,17]. However, provided anemia and stunting share many of their basic and underlying risk factors, one could presume a child at risk of anemia be also at risk of stunting or vice versa. The existing national and international guidelines, including the WHO guidelines, also recommend adopting comprehensive and multi-sectoral malnutrition prevention strategies [12].
To the best of our knowledge, there is no previous study on CAS in Ethiopia as the existing studies were focused on either of anemia or stunting, i.e. did not report on CAS. In Ethiopia, both anemia and stunting are high. Child care and complementary feeding practices also remain poorly practiced [1]. Thus, we presumed there would be a high level of CAS for the two conditions share many risk factors. This study was aimed to determine the prevalence of CAS among infants and young children in Ethiopia and also investigate the dietary and non-dietary factors associated with it.
Methods
Data source
The data used for this work were extracted from the Ethiopian Demographic and Health Survey (EDHS). The survey was conducted in 2016 by the United States Agency for International Development (USAID) in collaboration with the Ministry of Health of Ethiopia and other partner organizations [1].
Study design, sample size, and sampling procedures
The study was cross-sectional in design and representative at both national and regional levels, and urban and rural divisions [1]. The survey was done following a multistage sampling scheme. In the first stage of sampling, 645 enumeration areas (EAs) were randomly selected from all administrative divisions. The second sampling stage, selection of households within selected EAs, was done by systematic random sampling. In total, 18,060 households were selected. All children under-5 years of age in the selected households were eligible for the survey. More information about the EDHS sampling methodology and procedures is available elsewhere [1]. For this specific work, we extracted only the data of children below 24 months of age. Our interest in this age group was guided by two criteria: a) dietary and breastfeeding data were available only for the age group below 24 months, and b) most growth faltering (stunting) occurs during the first 2 years of life [11], during which the risk of anemia is also high. Infants below 6 months of age were also excluded because hemoglobin level was not measured for this age group during the conduction of the survey [1]. A total of 3105 children aged 6–23 months were found in the dataset. After data clearing and exclusion of cases with incomplete data on the variables of interest, 2675 children were included in the final dataset. Data incompleteness was only 13.8% (430 cases). The sample selection process is shown in Fig. 1.
Data collection procedure
Data were taken on various demographic, health and nutrition variables of public health importance using the standardized DHS questionnaire, that has been used in Ethiopia as well as other countries included in the International DHS Project. The data collectors received a four weeks training on the survey questionnaire administration, anthropometric measurement, and biological sample collection procedures. Pilot testing and field practice were also done in clusters not included in the actual survey. The closest caregiver of the child, mostly the biological mother, was the primary source of information on the child-related variables of interest. When the biological mother could not be accessed, a member of the family responsible for the caring of the child was interviewed. The household head, mostly the father or the mother, was the primary informant on household-related variables of interest. All data were collected through house-to-house visits [1].
Description of variables
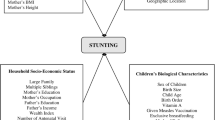
The main variable of interest was CAS, defined when a child was both anemic and stunted. Anemia status was defined by hemoglobin < 11 g/dL [3]. Hemoglobin level was measured by battery operated HemoCue®201 analyzers (Sweden) and adjusted for altitude [1]. The length of children was measured in flat position using a Shorr measuring board, produced under the United Nations International Children’s Emergency Fund (UNICEF) guidance [1]. Length-for-age (LFA) z-scores were calculated using the WHO 2006 child growth standards. Stunting was defined by LFA < − 2 z-scores [18]. CAS prevalence, in this work, refers to the proportion of children with both anemia and stunting. A list of the potential predictors of CAS was developed, guided by the literature, the UNICEF conceptual framework of malnutrition causation [12], and the availability of the variables in the dataset. Based on their level of influence and interrelationships, the variables were categorized into three groups: basic, underlying, and proximal factors. A detailed description of the explanatory variables is presented in Table 1.
Statistical analysis
All analyses were done taking into account the complex design of the survey, such that all the estimates were done after weighting the sample by the sample weighting factor and taking into account the cluster design of the study. While it ensures data representativeness at both national and subnational levels, the DHS sampling procedure over-represents small regions. Thus, sample weighting was applied to compensate for the unequal probability of sample selection and ensure the sample resembles the true population distribution. The procedure resulted in a final weighted sample size of 2902 children. Besides, as the study participants were selected by two-stage cluster sampling strategy, adjustment for effect of the cluster design was done by applying the STATA command ‘svyset:’. More information about the DHS sample weighting and adjustment for cluster design is found in the guide to DHS statistics [19].
CAS prevalence by various characteristics was estimated. Adjusted odds ratios (AOR) were estimated by running multiple hierarchical logistic regression analyses, which took into account the hierarchical interrelationship among the potential predictors of CAS. The selection of the variables was done taking into account both biological (theoretical) plausibility and statistical assumptions for multivariate analyses. Thus, bivariate analyses were done for all variables with the potential to influence CAS. Then, multivariate models were run using variables which demonstrated P < 0.20 during the bivariate analyses. The reasons for our use of a relaxed P-value (P < 0.20), instead of the usual P = 0.05, were: a) the purpose of the bivariate analyses was to identify potential predictor variables for the multivariate analyses rather than testing hypothesis, and b) it would minimize the risk of excluding variables with a biological (theoretical) plausibility from the multivariate analyses due to reasons, including confounding [20, 21]. However, the statistical significance of the results of the multivariate analyses was determined at P ≤ 0.05, because of its common usage in medical research.
After the bivariate analyses, three-level hierarchical regression models were run following the recommendation of Victoria et al. [22]. The first, second, and third models contained distal, underlying, and proximal factors, respectively. Variables with P < 0.20 value during the bivariate analyses were included in the multiple hierarchical logistic regression analyses. Thus, Model-1 included the distal factors which demonstrated P < 0.20 during the bivariate analyses. Model-2 included the underlying factors which demonstrated P < 0.20 during the bivariate analyses and factors from model-1 which demonstrated P < 0.20. Model-3 included the proximal factors and factors from Model-2 which demonstrated P < 0.20. Statistical significance (P ≤ 0.05) of a specific variable during the hierarchical regression analyses was determined at the corresponding model in which the variable was first entered, irrespective its performance in the next model(s). The approach was aimed to rule out the possibility that the intermediate factors weaken the relationship between the distal factors and the dependent variable of interest [22]. All statistical analyses were done using STATA-15.
Result
Sociodemographic characteristics of the sample
The main characteristics of the study participants are shown in Table 2. In totally, the study included 2902 (weighted) children aged 6 to 23 months, a third of whom were younger than 12 months. Mean age was 14.0 months (SD = 5.1). 1542 (53.1%) were girls and 1360 (46.9%) boys. Most of the participants were from rural areas (88.1%) and of less educated caregivers.
Prevalence of CAS
Overall, 72 and 32.6% of children were anemic and stunted, respectively. The overall prevalence of CAS was 23.9%. CAS prevalence among urban and rural children was 16.1 and 25.0%, respectively. Among boys and girls, the prevalence was 21.2 and 20.1%, respectively. The age-specific estimates were 12.1 and 25.4% in those aged under 12 months and above 12 months, respectively. The prevalence of CAS by other maternal and child characteristics is shown in Table 2.
Factors associated with CAS
Results of the bivariate analyses of the basic, underlying, and proximal variables with CAS are shown in Table 2. These estimates were, however, crude and less informative, i.e. not adjusted for any covariate. The co-variate adjusted estimates, i.e. based on the hierarchical regression analyses, are presented in Table 3. Rural residence was associated with a significantly higher odds of CAS (AOR = 1.29, 95%CI = 1.18–1.41, P < 0.001), compared to urban residence. The odds of CAS was significantly higher in children of low household wealth category, compared to children of high household wealth category (AOR = 1.91, 95%CI = 1.53–2.39, P < 0.01). Compared to children of caregivers with secondary+ education level, the odds of CAS was 2.14 times higher in children of caregivers with no education (95%CI = 1.33–3.44, P < 0.01) and 2.38 times higher in children of caregivers with only primary education (95%CI = 1.47–3.86, P < 0.01). In boys, the likelihood of CAS was 1.25 times that of girls (95%CI = 1.04–1.50, P = 0.015). Above 12 months of age was significantly associated with a higher odds of CAS, compared to below 12 months of age (AOR = 1.65, 95%CI = 1.57–1.73, P < 0.001). Small birth size was significantly associated with a higher odds of CAS, compared to large birth size (AOR = 1.99, 95%CI = 1.58–2.51, P < 0.001). Infection was linked to a marginally higher odds of CAS (AOR = 1.14, 95%CI = 1.00–1.30, P = 0.048).
Children who did not take vitamin A supplement within the previous six months had a higher odds of CAS, compared to those who took (AOR = 1.19, 95%CI = 1.06–1.1.33, P = 0.003). The odds of CAS was also significantly higher in children who did not receive VARFV, compared to those who received (AOR = 1.15, 95%CI = 1.04–1.27, P = 0.006). The odds of CAS related to no meat consumption was 1.55 times higher compared to its consumption (95%CI = 1.17–2.05, P = 0.002). The odds of CAS related with no legumes consumption was 1.38 times, higher compared to its consumption (95%CI = 1.05–1.81, P = 0.021). Meal frequency was also significantly associated with CAS, such that the odds of CAS was 1.22 times higher in those who didn’t meet MMF, compared to those who meet it (95%CI = 1.04–1.37, P = 0.020).
Discussion
This was the first study aimed to determine the prevalence of CAS, and its multilevel associated factors, among infants and young children in Ethiopia. The work provided evidence that there was a high level of anemia and stunting co-occurrence in Ethiopia, with almost a quarter of the children affected. We found CAS linked to various multiple-level influences. The distal factors associated with higher odds of CAS were living in rural areas, low household wealth, and low caregivers’ educational level. The proximal factors found associated with higher odds of CAS were male sex, age above 12 months, small birth size, no vitamin A supplement use, no consumption of vitamin A rich food items, meat and legumes, and low meal frequency.
We found a concerning high level of anemia, with only less than a third of the children being not anemic. The finding was, however, consistent with the recent national DHS report which showed 72 and 56% anemia prevalence among under-2 and under-5 children, respectively [1]. Stunting was also highly prevalent, though not as high as anemia. The same finding was reported in previous studies, which showed a two-fifth prevalence of stunting among under-5 Ethiopian children [1, 13, 23]. We found that almost a quarter of the children were concurrently anemic and stunted. There was no previous report on the magnitude of CAS, and factors associated with it, in Ethiopia as well as other African countries, limiting the comparison of our findings. However, our finding was within the range of reports from Asian and Latin American countries. Albalak et al. [17] reported a 15.2% CAS prevalence in Honduras. Castejon et al. [15] reported a 5.9% CAS prevalence in Venezuela. CAS prevalence was 21.5% in India and 30.4% in Peru [16].
We found various factors linked to CAS. Children of low household wealth or caregivers of low education level were more likely to be concurrently anemic and stunted. These findings could be easily acknowledged because child health-enhancing behaviors, like proper feeding, hygiene, and utilization of health services, are often sub-optimally practiced among communities of low wealth and educational status [8, 11, 24]. We also found a more clustering of CAS in those above 12 months of age. This could be due to the nature of stunting that it takes more time to manifest than anemia which takes a shorter time. The existing literature shows that most stunting occurs more during the period 12 to 23 months of age [11, 25]. In general, children under-2 years of age bear a higher burden of both anemia and stunting, particularly in developing countries [4, 9, 12]. Our finding of higher odds of CAS in boys than in girls was in agreement with previous reports which consistently demonstrated higher risks of anemia and stunting in boys [10, 25, 26]. Small birth size was associated with a significantly higher odds of CAS. This finding was also consistent with the existing literature which shows low birthweight linked to various poor health and nutritional outcomes [25, 26].
Vitamin A intake, in dietary as well as supplement form, was associated with a significantly lower CAS prevalence. This would be most likely due to the role of vitamin A in promoting optimal hematologic and linear growth status [24, 27,28,29]. Vitamin A also boosts humoral as well as cell-mediated immunity, thereby reducing the risk of anemia due to infection [29]. Vitamin A also plays an important role in promoting child growth, thereby reducing the risk of stunting [27, 28, 30]. Thus, it could be easily acknowledged that a vitamin A deficient child would be at a higher risk of being concurrently affected by anemia and stunting. Lack of meat and legumes consumption, as well as low meal frequency, were independently associated with higher odds of CAS. This could be due to the better amino acid and iron profiles in meat and legumes [31, 32]. Thus, suboptimal intake of legume or meat products might be expected to negatively impact both hemoglobin and growth statuses [23, 31].
Among the dietary factors, milk consumption was not significantly linked to CAS. This could be, in part, because most of the study participants were of less educated caregivers and from rural areas, where animal milk is consumed mainly raw. Previous reports showed opposing effects of raw animal milk on height and hemoglobin statuses [33, 34]. It promotes height gain, thus reduces the risk of stunting [34, 35], but predisposes to gastroenteritis and occult bleeding, thus increases the risk of anemia [33, 36]. Iron supplement use also did not demonstrate a significant link to CAS, though it would be expected to promote both hemoglobin and height [7]. Our finding could be likely due to factors like: a) the children taking iron supplement might be the already anemic ones, b) we did not account for dose, frequency, and adherence to the iron supplement use, or c) the sample lacked the power to detect the association, if any, because the number of children who took iron supplement was low. Notwithstanding the role of iron in red blood cells formation and body growth, previous randomized control and meta-analysis studies also reported as iron supplement use lacked a demonstrable effect on physical growth as well as hemoglobin level of children [37, 38].
The findings of this study have important policy and research implications. The high level of CAS was concerning given each of the two conditions are of significant consequences and their co-occurrence would be more threating to the health of children. It is also important to note the criticality of the first 1000 days of life, during which the body is more vulnerable to both nutritional and non-nutritional threats [4, 11]. Thus, the finding might be an indicator of the need to report on CAS and also investigate whether these children are being reached with a priority through the existing health/nutrition programs. Currently, there is confusion on how to address the various forms of malnutrition. To address stunting and anemia, WHO guidelines recommend a comprehensive and integrated approach, as it also has multiple benefits [12, 24]. Some authors, however, questioned the approach arguing that anemia and stunting are independent of each other and better be addressed by tailored interventions [15,16,17]. We are of the WHO recommendation as anemia and stunting share most of their risk factors. Thus, we recommend strengthening the existing public health and nutrition efforts, including improving infant and young child feeding practices, micronutrient supplementation, hygiene, and health care.
The main strengths of this study were it was based on a nationally representative data and took into account the multi-factorial nature of CAS, by incorporating not only the immediate dietary factors but also the non-dietary factors with the potential to influence CAS. Our analysis approach, hierarchical regression, took into account the interrelationships among the various explanatory variables and enabled building models according to the level of the variable influence. One of the main limitations of the study was that a cause-effect relationship could not be inferred as it was based on cross-sectional data. The collection of data on some variables, like birth size, infection history, dietary frequency, and diversity, based on the subjective memory of the caregiver might have introduced recall bias and miss-classification.
Conclusion
In conclusion, we provided evidence that there was a concerning high level of anemia and stunting clustering among infants and young children in Ethiopia. CAS was associated with various dietary and non-dietary factors, originating from community, maternal and child levels. Strengthening the existing comprehensive public health/nutrition interventions, with due emphasis on the multifactorial nature of CAS, might stand an important consideration to reduce the burden of CAS in Ethiopia and beyond.
Abbreviations
- AOB:
-
adjusted odds ratio
- CAS:
-
concurrent anemia and stunting
- CI:
-
confidence interval
- EDHS:
-
Ethiopian demographic health survey
- MDD:
-
minimum dietary diversity
- MMF:
-
minimum meal frequency
- SD:
-
standard deviation
- UNICEF:
-
United Nations International Children’s Emergency Fund
- VARFV:
-
vitamin A rich fruit and vegetables
- WHO:
-
World Health Organization
References
Central Statistical Agency [Ethiopia] and ICF International. Ethiopia Demographic and Health Survey 2016. https://dhsprogram.com/pubs/pdf/FR328/FR328.pdf. Accessed 20 June 2018.
Amare B, Moges B, Fantahun B, Tafess K, Woldeyohannes D, Yismaw G, et al. Micronutrient levels and nutritional status of school children living in Northwest Ethiopia. Nutr J. 2012;11:108.
World Health Organization. Iron deficiency anaemia: assessment, prevention and control: a guide for programme managers. World Health Organization, 2001. https://www.who.int/nutrition/publications/en/ida_assessment_prevention_control.pdf. Accessed 20 June 2018.
Smith LC, Haddad L. Reducing child undernutrition: past drivers and priorities for the post-MDG era. World Dev. 2015;68:180–204.
Bhutta ZA, Das JK, Rizvi A, Gaffey MF, Walker N, Horton S, et al. Evidence-based interventions for improvement of maternal and child nutrition: what can be done and at what cost? Lancet. 2013;382:452–77.
Olusanya BO, Wirz SL, Renner JK. Prevalence, pattern and risk factors for undernutrition in early infancy using the WHO multicentre growth reference: a community-based study. Paediatr Perinat Epidemiol. 2010;24:572–83.
Allali S, Brousse V, Sacri AS, Chalumeau M, de Montalembert M. Anemia in children: prevalence, causes, diagnostic work-up, and long-term consequences. Expert Rev Hematol. 2017;10:1023–8.
UNICEF. Strategy for improved nutrition of children and women in developing countries. United Nations Children's fund. Indian J Pediatr. 1991;58:13–24.
Lopez A, Cacoub P, Macdougall IC, Peyrin-Biroulet L. Iron deficiency anaemia. Lancet. 2016;387:907–16.
Akombi BJ, Agho KE, Hall JJ, Wali N, Renzaho AMN, Merom D. Stunting, wasting and underweight in sub-Saharan Africa: a systematic review. Int J Environ Res Public Health. 2017;14.
Victora CG, de Onis M, Hallal PC, Blossner M, Shrimpton R. Worldwide timing of growth faltering: revisiting implications for interventions. Pediatrics. 2010;125:e473–80.
Reinhardt K, Fanzo J. Addressing chronic malnutrition through multi-sectoral. Sustainable Approaches: A Review of the Causes and Consequences Front Nutr. 2014;1:13.
Gashu D, Stoecker BJ, Bougma K, Adish A, Haki GD, Marquis GS. Stunting, selenium deficiency and anemia are associated with poor cognitive performance in preschool children from rural Ethiopia. Nutr J. 2016;15:38.
Saaka M, Galaa SZ. Relationships between wasting and stunting and their concurrent occurrence in Ghanaian preschool children. J Nutr Metab. 2016;2016:4654920.
Castejon HV, Ortega P, Amaya D, Gomez G, Leal J, Castejon OJ. Co-existence of Anemia, vitamin a deficiency and growth retardation among children 24-84 months old in Maracaibo, Venezuela. Nutr Neurosci. 2013;7:113–9.
Gosdin L, Martorell R, Bartolini RM, Mehta R, Srikantiah S, Young MF. The co-occurrence of anaemia and stunting in young children. Matern Child Nutr. 2018;14:e12597.
Albalak R, Ramakrishnan U, Stein AD, Van der Haar F, Haber MJ, Schroeder D, et al. Co-occurrence of nutrition problems in Honduran children. J Nutr. 2000;130:2271–3.
Group WHOMGRS. WHO child growth standards based on length/height, weight and age. Acta Paediatr Suppl. 2006;450:76–85.
The Demographic and Health Surveys Program. Guide to DHS Statistics. 2018. https://dhsprogram.com/pubs/pdf/DHSG1/Guide_to_DHS_Statistics_DHS-7.pdf. Accessed 20 June 2018.
Ranganathan P, Pramesh CS, Aggarwal R. Common pitfalls in statistical analysis: logistic regression. Perspectives in clinical research. 2017;8:148–51.
Bursac Z, Gauss CH, Williams DK, Hosmer DW. Purposeful selection of variables in logistic regression. Source code for biology and medicine. 2008;3:17.
Victora CG, Huttly SR, Fuchs SC, Olinto MT. The role of conceptual frameworks in epidemiological analysis: a hierarchical approach. Int J Epidemiol. 1997;26:224–7.
Melaku YA, Gill TK, Taylor AW, Adams R, Shi Z, Worku A. Associations of childhood, maternal and household dietary patterns with childhood stunting in Ethiopia: proposing an alternative and plausible dietary analysis method to dietary diversity scores. Nutr J. 2018;17:14.
World Health Organization. Essential nutrition actions: improving maternal, newborn, infant and young child health and nutrition. 2013. http://www.who.int/nutrition/publications/infantfeeding/essential_nutrition_actions/en/. Accessed 20 June 2018.
Danaei G, Andrews KG, Sudfeld CR, Fink G, McCoy DC, Peet E, et al. Risk factors for childhood stunting in 137 developing countries: a comparative risk assessment analysis at global, regional, and country levels. PLoS Med. 2016;13:e1002164.
Mohammed SH, Esmaillzadeh A. The relationships among iron supplement use, Hb concentration and linear growth in young children: Ethiopian demographic and health survey. Br J Nutr. 2017;118:730–6.
Mwanri L, Worsley A, Ryan P, Masika J. Supplemental vitamin a improves anemia and growth in anemic school children in Tanzania. J Nutr. 2000;130:2691–6.
Semba RD, de Pee S, Sun K, Campbell AA, Bloem MW, Raju VK. Low intake of vitamin A-rich foods among children, aged 12-35 months, in India: association with malnutrition, anemia, and missed child survival interventions. Nutrition. 2010;26:958–62.
Semba RD, Bloem MW. The anemia of vitamin a deficiency: epidemiology and pathogenesis. Eur J Clin Nutr. 2002;56:271–81.
Sedgh G, Herrera MG, Nestel P, el Amin A, Fawzi WW. Dietary vitamin a intake and nondietary factors are associated with reversal of stunting in children. J Nutr. 2000;130:2520–6.
Esfarjani F, Roustaee R, Mohammadi-Nasrabadi F, Esmaillzadeh A. Major dietary patterns in relation to stunting among children in Tehran, Iran. J Health Popul Nutr. 2013;31:202–10.
Camaschella C. Iron-deficiency anemia. N Engl J Med. 2015;372:1832–43.
Ziegler EE. Consumption of cow's milk as a cause of iron deficiency in infants and toddlers. Nutr Rev. 2011;69(Suppl 1):S37–42.
Okada T. Effect of cow milk consumption on longitudinal height gain in children. Am J Clin Nutr. 2004;80:1088–9.
Agostoni C, Turck D. Is cow's milk harmful to a child's health? J Pediatr Gastroenterol Nutr. 2011;53:594–600.
Griebler U, Bruckmüller MU, Kien C, Dieminger B, Meidlinger B, Seper K, et al. Health effects of cow’s milk consumption in infants up to 3 years of age: a systematic review and meta-analysis. Public Health Nutr. 2016;19:293–307.
Sachdev H, Gera T, Nestel P. Effect of iron supplementation on physical growth in children: systematic review of randomised controlled trials. Public Health Nutr. 2006;9:904–20.
Allen LH, Rosado JL, Casterline JE, Lopez P, Munoz E, Garcia OP, et al. Lack of hemoglobin response to iron supplementation in anemic mexican preschoolers with multiple micronutrient deficiencies. Am J Clin Nutr. 2000;71:1485–94.
World Health Organization. Indicators for assessing infant and young child feeding practices part 3: country profiles. 2010. http://www.who.int/nutrition/publications/infantfeeding/9789241599757/en/. Accessed 20 June 2018.
Acknowledgments
SHM is a recipient of postgraduate scholarship, Tehran University of Medical Sciences-International Campus. We are also grateful for the USAID/DHS program for offering us free access to the survey dataset.
Funding
This research received no specific grant from any funding agency in public, commercial or not-for-profit sectors.
Availability of data and materials
The dataset supporting the conclusions of this article is available on the DHS International website: http://dhsprogram.com/data/dataset/Ethiopia_Standard-DHS_2016.cfm.
Author information
Authors and Affiliations
Contributions
SHM conceived and led the study. SHM extracted the dataset, prepared the analysis plan, performed the data analysis, and wrote the manuscript. AE and BL supervised the work, guided the analysis, and reviewed the manuscript critically. All authors reviewed and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
EDHS 2016 was approved by the Institutional Review Boards (IRB) of Ethiopian Public Health Institute and ICF International. Caregivers of the children provided consent before data collection [1]. For this particular work, we obtained additional ethical approval from the IRB of Tehran University of Medical Sciences, ethical code IR.TUMS.VCR.REC.1397.142, and approval to use the dataset from the DHS program through a project titled “trends and determinants of malnutrition in Ethiopia.”
Consent for publication
Not applicable.
Competing interests
The authors declared no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Mohammed, S., Larijani, B. & Esmaillzadeh, A. Concurrent anemia and stunting in young children: prevalence, dietary and non-dietary associated factors. Nutr J 18, 10 (2019). https://doi.org/10.1186/s12937-019-0436-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12937-019-0436-4