Abstract
Background
The recent emergence in Southeast Asia of artemisinin resistance poses major threats to malaria control and elimination globally. Green nanotechnologies can constitute interesting tools for discovering anti-malarial medicines. This systematic review focused on the green synthesis of metal nanoparticles as potential source of new antiplasmodial drugs.
Methods
Seven electronic database were used following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.
Results
A total of 17 papers were included in the systematic review. 82.4% of the studies used plant leaves to produce nanoparticles (NPs) while three studies used microorganisms, including bacteria and fungi. Silver was the main metal precursor for the synthesis of NPs. The majority of studies obtained nanoparticles spherical in shape, with sizes ranging between 4 and 65 nm, and reported no or little cytotoxic effect of the NPs. Results based on 50% inhibitory concentration (IC50) varied between studies but, in general, could be divided into three NP categories; (i) those more effective than positive controls, (ii) those more effective than corresponding plant extracts and, (iii) those less effective than the positive controls or plant extracts.
Conclusions
This study highlights the high antiplasmodial potential of green-synthesized metal nanoparticles thereby underscoring the possibility to find and develop new anti-malarial drugs based on green synthesis approaches. However, the review also highlights the need for extensive in vitro and in vivo studies to confirm their safety in humans and the elucidation of the mechanism of action.
Graphical abstract

Similar content being viewed by others
Background
In 2015, nearly 9.2 million deaths occurred in Africa with 56.4% being due to communicable, maternal, perinatal or nutritional conditions and, malaria ranked as one of the most devastating infectious diseases characterized by intermittent high fevers, severe anaemia, convulsions, neurological complications such as brain injury and coma [1]. Malaria is caused by protozoan parasites of the genus Plasmodium that are transmitted to humans through the bite of infected female Anopheles mosquitoes [2]. Plasmodium falciparum causes most of the deaths, whereas Plasmodium vivax is the most widespread. Plasmodium malariae, Plasmodium ovale, Plasmodium knowlesi, and Plasmodium cynomolgi are other species that infect or cause disease in humans [2,3,4]. Malaria remains a very important public health problem, especially in sub-Saharan Africa where the disease has significantly delayed economic development. In 2017, approximately 219 million malaria cases and 435,000 related deaths were recorded worldwide; the majority (92%) of which occurred in sub-Saharan Africa [3].
Despite significant global efforts in the fight against malaria through increased funding for malaria research and development, delivery and scaling up of control interventions (diagnosis, prevention and treatment), the Global Technical Strategy (GTS) goals for malaria morbidity and mortality for 2020 are far from being achieved [3]. The World Malaria Report 2018 reported that only 70% of cases were avoided from 2000 to 2015, and also showed an increase in malaria cases in some countries from 2016 to 2017.
Unfortunately, one of the major barriers to successful global malaria control (GMC) is the emergence and the propagation of parasites resistant to currently used anti-malarial drugs. Artemisinin-based combination therapy (ACT), which is the most effective treatment available today, has been an integral part of the recent successes in GMC [2,3,4]. However, the future of these artemisinin-based combinations is endangered by the emergence of artemisinin resistant P. falciparum strains primarily reported in western Cambodia and subsequently in the Greater Mekong Subregion (GMS) and Southern China [5,6,7]. The circulation of artemisinin (ART) resistant parasites and/or resistant to partner drugs in ACT has greatly hindered the management of malarious patients and control strategies in these areas. Many studies reported increased failure rates following ACT due to the presence of ART-resistant parasites [8,9,10].
The resistance phenotype against artemisinin loci seems to be under positive selection within the propeller domain of the P. falciparum kelch (k13) gene, but other studies have indicated that additional single nucleotide substitutions on chromosomes 10, 13, and 14 may also be responsible for this resistance phenotype [11,12,13]. This suggest the exact genes which confer this delayed clearance or involved in artemisinin resistance are yet unknown, although 13 nonsynonymous mutations have been validated as associated markers [5, 14,15,16,17,18,19]. Moreover, mortality rates as well as recurrent malaria cases increased following the spread of artemisinin resistant parasites in these areas [1].
The emergence or spread of artemisinin resistance from Asia to Africa, as observed previously for older anti-malarial drugs including chloroquine and sulfadoxine–pyrimethamine [20,21,22], would be devastating to global malaria elimination efforts. Despite numerous fears on the potential emergence or spread of artemisinin resistance-associated k13 mutations in Africa, the so far identified mutations are rare and unrelated to k13 polymorphisms found to be associated with reduced susceptibility in Asia [5, 23,24,25,26,27,28,29,30,31]. Thus, in this situation and in the context where many anti-malarial treatments are paid for by non-profit organizations and governments, the future of malaria control and global elimination would depend on the ability of research and development to deliver the next generation of anti-malarial drugs [32]. If unsuccessful, this could greatly jeopardize the hope to efficiently control and eliminate malaria, particularly in the African continent as outlined in the Global Technical Strategy 2016–2030 [1].
Diverse strategies exist for the development of novel anti-malarial drugs, and some have come from living organisms. Basically the synthesis of metal NPs requests the combination of three elements namely: the metal source (generally noble metals such as silver, gold, palladium and titanium salt), the reducing agent and the capping agent. Metal nanoparticles are traditionally produced using chemical and physical methods. However, these methods are challenging as they are costly, time-consuming and request for utilization of reagents harmful to environment [33, 34]. In this regard, new NPs synthesis methods referred to as green synthesis have been developed to overcome these issues. Green synthesis consists in the production of metal NPs by exploiting the reducing and capping natural potential of biomolecules from living organisms such as plants and microorganisms. The method is simple, cost-effective and eco-friendly [33, 34].
Nanoproducts and metal nanoparticles are highly useful, safe in nature with numerous applications in renewable energies, catalysis, cosmetics, food, electronics, environmental remediation, biomedical devices and health [35, 36]. Metal NPs were mainly tested for their biocidal activity against bacteria [37,38,39], fungi [40, 41], and viruses [42, 43]. Little is reported on antiplasmodial potential of metal NPs [44]. In this systematic review, the living organisms mediated synthesis of nanoparticles (NPs) is presented as a source for new medicines to overcome the possible loss of ACT in the future. A recent systematic review by Barabadi and co-workers [45] addressed the utilization of biosynthesized NPs as control tool of malaria vectors and parasites. The authors did not address some of the gaps and challenges existing in this emerging line of research as well as the toxicity of green nanoparticles against non-target organisms (humans, for example). However, the authors reported interesting biocidal antiplasmodial activity of NPs but unfortunately, information concerning the efficacy of NPs compared to the positive control (anti-malarial drug or plant extract) is lacking.
Thus, data from 17 studies on the antiplasmodial activity of green-synthesized metal nanoparticles were comprehensively analysed with aims (1) to present commonly used biological material and main methodological aspects for green synthesis of metal nanoparticles; (2) to summarize the main findings of the selected studies; (3) to outline difficulties encountered in the synthesis of green-synthesized metal nanoparticles and, evaluation of their antiplasmodial and cytotoxic potential and, (4) to highlight future challenges and gaps in green technology driven anti-malarial drug discovery.
Methods
Data source and eligibility criteria
Two authors of the research team developed a strategy to search for articles to be included in the systematic review. Between 25th September and 25th November 2018 seven electronic databases including Medline, Scopus, Excerpta Medica Database (EMBASE), African Index Medicus, Popline, Africa wide information and the Cochrane library were used to search for potentially eligible publications. Supplemental sources included Boolean operators that helped to conduct more efficient searches from these databases. In addition, search engines such as Google and Google Scholar were also used to identify all potentially eligible publications. Another search was performed between 7th February and 30th March 2019.
The review included (i) studies focused on the effectiveness of green-synthesized metal-based nanoparticles against malaria parasites, (ii) published peer reviewed and research articles between January 1999 and March 2019 and, written in English or French. Articles were thereafter independently reviewed, rated and data were abstracted by two persons. Studies focused mainly on malaria vectors, letters to the editor, editorials, conference papers, preprints, reports or comment publication type sources were excluded from the review.
Screening strategy
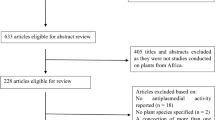
Titles and abstracts of retrieved studies from electronic database of interest were reviewed by two persons and the terms used during the search process were distributed in four groups (Table 1) and combined with Boolean operators “AND”/“OR” during the searching using the above mentioned electronic databases. The full texts of each study were retrieved, analysed and the screened studies were included in the review. Corresponding authors of relevant documents were asked to provide full texts when not free or inaccessible. When it was not possible, i.e. non-reply or negative reply from corresponding authors these full texts were purchased. In addition, the references list of relevant documents was also examined to increase the chances of finding eligible papers. The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flowchart was used to depict the entire stepwise process of screening strategy (Additional files 1 and 2) [46].
Data of interest
All information collected in the different articles collected from the seven different databases used are classified into six major groups (Table 2). Two investigators independently extracted data and any discrepancies were resolved through discussion and consensus.
Data verification for consistency
Data of interest were independently keyed in an Excel spreadsheet (Microsoft Office 2016, USA) by two persons to ensure internal quality control of database. These data were also checked for consistency by two additional persons for external quality control of database. When discrepancy between the two Excel sheets occurred, two more people checked the data again.
Results
Characteristics of the studies included in the review
In total, 17 studies were eligible based on the selection process summarized in Fig. 1 [47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63]. All these studies clearly synthesized, characterized and assessed the antiplasmodial potential of biologically produced metal-based nanoparticles (NPs). Fourteen of them used plants as biological material for producing NPs, while the three remaining used microorganism especially bacteria (Table 3). Most of included studies (16/17; 94.11%) were conducted by Indian research teams.
Information on living organism used for synthesis of metal nanoparticles
Globally, 17 plant species distributed into 16 families were investigated for their ability to elicit nano-sized materials with antiplasmodial properties (Table 3). These plants included Andrographis paniculata, Azadirachta indica and Pteridium aquilinum. These are popularly known as “King of Bitters”, “Neem” and “Bracken fern” respectively by populations who use them. The morphological type of plants included tree, herb, herbaceous herb and algae (Table 3). Of the three studies that used microorganisms, two focused on bacteria and the remaining one on worms. Bacteria consisted of Streptomyces sp LK-3 (JF710608) and Magnetospirillum gryphiswaldense which were isolated from marine sediments and laboratory-maintained respectively (Table 3).
Methods used for green synthesis of metal nanoparticles within included studies
Leaves were used as biological material for synthesis of metal NPs in 11 out of 14 studies having used plants. The other plant parts included flowers, seeds and barks [50, 53, 57]. Decantation was predominantly used by authors to produce aqueous extracts which had to be mixed with metal precursor for NPs synthesis (Table 4). Indeed, biological material (4–10 g) was boiled following careful washing (tap water and double-distilled water) and cutting. Water was mainly used as extraction solvent and the mixture was then decanted and filtered using Whatman N°1 filter paper (Table 4). The mode of preparation of NPs among studies having used microorganisms was available in two studies. For instance, Kharthik and colleagues inoculated their bacterium of interest, incubated with metal precursor in aqueous medium consisting of 50% sea water and the mixture was then centrifuged [61].
Methods used for studying nanoparticles
The physical characterization of metal nanoparticles was studied on four aspects namely shape, size and distribution size, chemical composition, and structure and stability (Table 4). Ultraviolet spectroscopy (UV–Vis) proved the formation of the nanoparticles by showing the characteristic plasmon vibration. Scanning electron microscopy (SEM) and transmission electron microscopy (TEM) were used to determine the shape and size of the nanoparticles. SEM is coupled with energy dispersive X-ray spectroscopy (EDX, EDS) which provides elemental mapping in terms of atomic composition while TEM is coupled with selected area electron diffraction (SAED) which shows crystallographic planes. Fourier-transform infrared spectroscopy (FTIR) was used to study the nanoparticles/secondary metabolites interface by providing molecular vibrations. X-ray diffraction (XRD) was used for nature, crystallinity as well as shape and size determination. Dynamic light scattering (DLS) provides size distributions in term of hydrodynamic radius. Few studies studied the nanoparticles stability by determining their Zeta potential and the silver content by Atomic absorption spectroscopy (AAS) [53, 57, 59, 61].
Characteristics of plant-synthesized nanoparticles
As depicted in Table 4, silver nitrate (AgNO3) was mainly used as metal precursor for synthesis of plant-related nanoparticles. Other noble metals including gold, titanium and palladium were also used for NPs synthesis [51, 53, 57, 59, 61]. The colour change outlining the obtainment of NPs was achieved between 10 and 150 min after mixture between precursor metal and plant extract.
UV–Vis spectroscopy appears as one of the key method to investigate nanoparticles behaviour such as formation, development or aggregation. Characteristic plasmon vibration occurs because of the free oscillation of electrons at the metallic surface. They are situated at 400–450 nm for silver [47,48,49,50, 52, 54,55,56,57,58], 540 and 560 nm for gold [51, 61], 360 for TiO2 [59].
A majority of studies obtained spherical-shaped nanoparticles (Table 4). Globally, the size of NPs ranged between 4 and 65 nm and a few studies reported an aggregation phenomenon during synthesis [49, 56]. Some studies reported the appearance of additional Braggs peaks [48,49,50, 56]. The presence of energy dispersive X ray-related signals associated with oxygen or carbon atoms [52, 56], while additional chlorine signal may appear too [54]. Selected area electron diffraction (SAED) showed diffraction dots and crystallographic planes of the obtained nanoparticles (Table 5) [57].
Powder X-ray diffraction is one of the most important characterization tool used in solid state chemistry [64]. It is used to determine the nature of the crystalline phases and then of the synthesized nanoparticles starting from the biological extracts (plant, earthworm). More antiplasmodial properties have been evaluated with nanosilver (57%) and nanogold (29%) (Table 4). This determination is possible by comparison of the obtained pattern to the International Centre for Diffraction Data (ICDD) patterns, formerly the Joint Committee on Powder Diffraction Standards (JCPDS) patterns. If the biosynthesis leads to pure palladium, gold or titanium dioxide nanoparticles, it is not the case for silver where nanosilver, silver chloride nanocrystallites or their mixture are obtained [35, 36, 43].
FTIR was carried out to investigate biomolecules extracts at the metallic interface of silver, gold, palladium and the metal oxide interface for titanium. The method shows molecular vibrations at the surface or the synthesized nanoparticles. FTIR spectroscopy revealed absorption frequencies that can be well correlated with characteristic tables. For example, O–H (stretch, H-bonded) at 3200–3600 and C–O (stretch) at 1050–1150 for alcohols (Table 5). C–H (stretch) at 2860–3000 and –C–H (bending) at 1350–1480 for alkanes. C=C (stretch) ae 1620–1680 for alkenes. N–H (stretch) at 3300–3500 and N–H (bending) at 1600 but also C–N (stretch) at 1080–1360 for amines. C–H (stretch) at 3000–3100 and C=C (stretch) at 1400–1600 for aromatics or C=O (stretch) at 1670–1820 for carbonyls [65].
Evaluation methods and findings on antiplasmodial activity of nanoparticles
The studies were mainly designed as in vitro even though a few studies were in vivo [51, 59], or a combination of both [56, 63]. Most studies evaluated the susceptibility of laboratory strains of P. falciparum, such as INDO (CQ-resistance), 3D7 (CQ-sensitive), FcB1/Colombia (CQ-sensitive) and Dd2 (CQ-sensitive) using chloroquine as positive control (Table 3). Negative controls were included in the study design and consisted of distilled water, uninfected and infected red blood cells or medium culture. A few studies collected P. falciparum field isolates from patients attending health facilities [54, 55, 57]. Plasmodium berghei was used in studies based on animal model for appraising the malarial susceptibility and nanoparticles were administered either orally [56], or intraperitoneally [61].
The percentage of parasite growth suppression and 50% inhibitory concentration (IC50) were used as endpoints for evaluation of antiplasmodial activity of nanoparticles (Table 6). Panneerselvam and co-workers reported a reduction in parasite growth rate by 26% to 83% at doses 25 µg/mL and 100 µg/mL respectively [50]. A lower antiplasmodial activity comprises between 6.4 and 42.8% was reported by Murugan and colleagues [56]. Results based on IC50 were very contrasted between studies but generally, these met into three categories namely (i) nanoparticles were more efficient than positive control [52,53,54,55,56], (ii) nanoparticles were more efficient than plant extract [49, 51, 56, 57], and (iii) nanoparticles were less efficient than positive control (chloroquine) or plant extract [53,54,55, 57]. Nine of eleven studies having used chloroquine as control found that metal NPs were more efficient [49, 51,52,53,54,55,56, 62, 63]. For instance, Jaganathan et al. found their nanoparticles had IC50 of 49.3 µg/mL and 55.5 µg/mL against P. falciparum 3D7 (chloroquine-sensitive) and INDO (chloroquine-resistant) strains respectively compared to chloroquine (81.5 µg/mL and 86.5 µg/mL respectively) [62]. Murugan et al. reported an IC50 of 63.18 µg/mL and 69.24 µg/mL for nanoparticles compared to 82.41 µg/mL and 86.12 µg/mL for extracts against 3D7 and INDO strains respectively, thus outlining a higher antiplasmodial activity of nanoparticles compared to plant extract (Table 6) [56].
Cytotoxicity of nanoparticles
As presented in Table 7, seven out seventeen studies included cytotoxicity analysis of synthesized nanoparticles [49, 51, 61]. Of the seven studies, four reported no or little deleterious effect of nanoparticles on used cell lines [49, 50, 57, 59]. Conversely, the remaining studies three reported important adverse effects including tissue damages, behavioural changes, changes in physical appearance, deaths of laboratory animals [61], necrosis and cytopathic effects [51], and apoptosis [62] (Table 7).
Discussion
This systematic review focused on studies having evaluated the antiplasmodial activity of biologically synthesized metal nanoparticles. The production of nanoparticles (NPs) using living beings also known as green synthesis is much more interesting as it deals with environmental and economic issues. Indeed, this approach is environment friendly, rapid, nontoxic in most cases, cost-effective and easily scaled up for large scale production of NPs compared to their chemical and physical counterparts [35, 66]. In addition, compounds used for NPs chemical synthesis such as sodium borohydride (NaBH4) or Tollen’s reagent are non-biodegradable and very harmful to humans and expose them to cancer for instance [67].
Green synthesis of metal nanoparticles was mainly done using plant extracts. The utilization of plants is more advantageous as it limits the risk of biohazard and reduces costs imposed by isolation, purification of microorganisms as well as maintaining cell cultures [35, 68]. Furthermore, the critical need for creation of highly aseptic conditions and their maintenance impedes the possibility of using microbe-synthesized nanoparticles in a large-scale production perspective [35, 68]. Benelli [69] concluded in a precedent review that carbonyl groups had the stronger ability to bind metals, indicating that the proteins could form a capping layer on AgNPs, preventing agglomeration and thereby stabilizing the medium. Other molecules like (poly)phenols of enzymes and polysaccharides or flavonoids of proteins could perform that same role and build the metal interfaces.
Silver was mainly used as metal precursor for the synthesis of nanoparticles. This can be due to interesting properties of this atom such as its wide antimicrobial activity and chemical stability [70]. The synthesis leads generally to pure silver, silver chloride nanocrystallites or a mixture of both. The biosegregation of those entities by plant extract is not described in literature [71]. This atom has been known for having biocidal action against a broad range of microorganisms in ancient times. Nowadays, silver ions are used in a large number of medical situations including catheter disinfection, water purification, food hygiene and dental work for control of bacterial growth [72, 73].
The colour change outlining the obtainment of NPs was achieved between 10 and 150 min after mixture between precursor metal and plant extract. This colour change is attributed to the surface plasmon resonance phenomenon which occurs when free electrons present on the surface of nanoparticles enter in resonance with the wavelength of the incident light [57].
Nanoparticles were mainly spherical in shape even though other shapes were also reported. Furthermore, their size distribution was large enough. Both physical parameters are responsible for distinctive physico-chemical properties of NPs which underlie their biological activities against microorganisms [74,75,76]. The formation of NPs involves two stages namely (i) nucleation where nuclei form by self-assemblage of atoms and (ii) subsequent growth of this nuclei into a nanosized particle. Tran et al. [74] demonstrated that the size and shape of Ag-NPs were strongly dependent on these stages. Indeed, it is more likely to have monodispersed nanoparticles with uniform size distribution if all nuclei form at the same time. As a result, these nuclei will need to have the sale subsequent growth [74]. Additionally, factors such as reaction parameters (pH, ionic force, osmotic pressure and temperature), the nature of stabilizing agent and surface plasmon resonance can influence the shape and size of nanoparticles [77,78,79,80].
Importantly, a few studies reported an aggregation phenomenon during synthesis of metal nanoparticles outlining that nanoparticles were not stable during and/or after synthesis. These studies did not include methods such as energy dispersive X-ray (EDX) in their design in order to predict any possibility of aggregation. This phenomenon modulates the particular physico-chemical properties of nanosized particles and accordingly their biological actions [81]. However, a few authors outlined the importance of this phenomenon in toxicity against pathogenic microorganisms such as Escherichia coli induced by gold-based nanoparticles upon their intracellular penetration [82].
The studies were designed as in vitro, in vivo or a combination of both. In vitro studies have advantages to appraise the intrinsic susceptibility of malaria parasite to drugs compared to in vivo studies which results are strongly dependent on level of anti-malarial immunity of host. If not taken into account, one can believe illusively the effectiveness of tested molecules especially nanoparticles. Besides, most in vitro designed studies appraised the susceptibility of laboratory strains of P. falciparum such as INDO, 3D7 and Dd2. In a context of multidrug resistance in malaria parasites, it would be more interesting to test metal NPs against of P. falciparum field isolates in order to objectively appreciate their antiplasmodial action [47, 48, 50]. In the context of emergence and spread of resistance of malaria parasites to artemisinin and its derivatives, the possibility to develop new medicines through methods such as green nanotechnology is of utmost importance and interest. Furthermore, P. falciparum laboratory strains resistant to ART and its derivatives could be used as control instead of the above mentioned laboratory strains.
Most studies included in the review found that synthesized nanoparticles had antiplasmodial potential higher than used controls (chloroquine, extract). This finding indicates that these nanomaterials can be valuable tools for discovering and designing new medicines. The antiplasmodial activity of nanoparticles can be attributed to the presence of biological compounds such as flavonoids, alkaloids, terpenes, lignans, terpenoids, steroids, coumarins, phenolic acids, xanthones, proteins and anthraquinones [48, 62]. The Fourier-transform infrared spectroscopy-based results provided by the studies indicated the presence of functional groups hallmarking these compounds. These included N–H, C=O, C=C, COO−, N–O and C–N stretching. These compounds referred to as secondary metabolites had been shown previously to have biocidal activity against malaria parasites [83,84,85,86,87,88,89,90,91]. The mechanisms of action through which nanoparticles induce reduction in parasite growth rate and death are still clearly elusive. However, these could elicit their lethal action by operating on the genomic material of parasite, its surface membrane or even intracytoplasmic elements such as enzymes [35, 92]. The elucidation of mechanisms of antiplasmodial action is under intensive investigation. Karthik and colleagues reported the administration of gold NPs was associated with both a high TGF-β and low TNF production in mice infected with P. berghei; drawing the immunomodulatory role of metal NPs [61].
Finally, a few studies reported a toxic action of nanoparticles tested for their antiplasmodial potential. Indeed, a few of them reported nanoparticles had elicited toxic action against human cancer cell lines outlining thereby their possible but interesting anti-cancer potential. This is consistent with previous studies [93]. On the other hand, one study included in this review reported severe adverse effects and death cases caused by metal nanoparticles [61]. This brings back on the table the issue on the harmfulness of nanoparticles to humans. The question has been well documented [94,95,96,97], and implies that the evaluation of toxic potential of any new products is a crucial and composite step in the drug design. Thus, it would be important to include the evaluation of cytopathic effects of nanoparticles when their antimicrobial effect is evaluated.
Future considerations
The some following researches worth addressing in future:
-
1.
According to the 17 publications considered in the current review, most were conducted in India; and only one was conducted in Africa (South Africa). It is somewhat paradoxical as the African continent constitute the bulk of the total malaria burden and has an incredible diverse flora [3, 98]. Thus, there is need for more studies in this field in malaria endemic countries in this continent;
-
2.
The mode of action through which metal nanoparticles elicit their biological effects is still elusive; thereby calling out to address this issue in future;
-
3.
It is likely that many factors such as size and shape of NPs greatly influence their biological activities. Bioinformatics and modelling studies would be helpful to understand the real influence of these some abovementioned factors;
-
4.
Seven out of seventeen papers included in the review addressed the toxic potency of metal NPs; of which three reported significant toxicity against non-target organisms [51, 61, 62]. This finding put in light conflictual results on this issue and point out a need for more extensive studies on NPs toxicity prior to any development of anti-malarial drug.
-
5.
Finally, great discrepancies in methodological approaches were recorded in the 17 reviewed publications; from the process NPs synthesis to methods of evaluating their antiplasmodial activity. Thus, in order to compare the results of different studies, it would be interesting to standardize the methodology for evaluating the antiplasmodial activity of green nanoparticles.
Limitations of the study
Articles written in English and French were included in the present review and as a result may result in selection bias.
Conclusion
This review points out certain advantages in terms of rapidity and eco-friendliness of using living organisms such as plants for synthesis of metal nanoparticles. It provides a global overview on the antiplasmodial potential of these nanomaterials highlighting their usefulness as promising sources for new anti-malarial drugs. The review also highlights unanswered questions regarding the exact mechanism through which these NPs elicit their cytotoxic actions against the parasite, and the need for further studies addressing the issue. Lastly, the review underscores the need to conduct detail studies on the safety profiles of available nanoparticles prior to use in humans.
Availability of data and materials
All data generated or analysed during this study are included in this published article and its additional files.
Abbreviations
- AAS:
-
atomic absorption spectroscopy
- ACT:
-
artemisinin-based combination therapy
- AE:
-
aqueous extract
- AgNPs:
-
silver nanoparticles
- ART:
-
artemisinin
- AuNPs:
-
gold nanoparticles
- bw:
-
body weight
- CQ:
-
chloroquine
- DLS:
-
dynamic light scattering
- DRIFT:
-
diffuse reflectance infrared Fourier transform
- EDAX:
-
energy dispersive X-ray
- EDS:
-
Electron Diffraction Spectrophotometer
- EMBASE:
-
Excerpta Medica Database
- FESEM:
-
field emission scanning electron microscopy
- FTIR:
-
Fourier-transform infrared
- GMC:
-
global malaria control
- GMS:
-
Greater Mekong subregion
- GTS:
-
Global Technical Strategy
- HF:
-
health facility
- HIV/AIDS:
-
human immunodeficiency virus/acquired immunodeficiency syndrome
- HR-TEM:
-
high resolution transmission electron microscopy
- IC50 :
-
50% inhibitory concentration
- ICDD:
-
International Centre for Diffraction Data
- IPTp:
-
intermittent preventive therapy in pregnancy
- IRS:
-
indoor residual spraying
- JCPS:
-
Joint Committee on Powder Diffraction Standards
- LLINs:
-
long-lasting insecticides-treated nets
- MeSH:
-
Medical Subject Headings
- MHC10 :
-
minimum haemolytic concentration resulting in 10% haemolysis
- MTT:
-
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
- NC:
-
negative control
- PBMCs:
-
peripheral mononuclear cells
- PC:
-
positive control
- PRISMA:
-
Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- RBC:
-
red blood cells
- SAED:
-
size and elected area diffraction
- SEM:
-
scanning electron microscopy
- TEM:
-
transmission electron microscopy
- TFG:
-
tumour growth factor
- TNF:
-
tumour necrosis factor
- NPs:
-
nanoparticles
- WHO:
-
World Health Organization
References
WHO. Factsheet on leading causes of deaths in Africa. Geneva: World Health Organization; 2017.
White NJ, Pukrittayakamee S, Tinh Hien T, Abul Faiz M, Mokuolu OA, Dondorp AM. Malaria. Lancet. 2014;391:1608–21.
WHO. World malaria report 2018. Geneva: World Health Organization; 2018. http://www.who.int.
Ta TH, Hisam S, Lanza M, Jiram AI, Ismail N, Rubio JM. First case of a naturally acquired human infection with Plasmodium cynomolgi. Malar J. 2014;13:68.
WHO. Status report on artemisinin and ACT resistance (April 2017). WHO/Htm/Gmp/20179. 2017:11. http://www.who.int/malaria/publications/atoz/artemisinin-resistance-april2017/en/.
Souleymane D, Abdoulaye AD, Ogobara KD. Methods for monitoring artemisinin-based combination therapies efficacy. Clin Rev Opin. 2017;8:1–13.
Noedl H, Se Y, Schaecher K, Smith BL, Socheat D, Fukuda MM. Evidence of artemisinin-resistant malaria in Western Cambodia. N Engl J Med. 2008;359:2619–20.
Fairhurst RM, Dondorp AM. Artemisinin-resistant Plasmodium falciparum malaria. Microbiol Spectr. 2016;4:3.
Tilley L, Straimer J, Gnadig NF, Ralph SA, Fidock DA. Artemisinin action and resistance in Plasmodium falciparum. Trends Parasitol. 2017;32:682–96.
Woodrow CJ, White NJ. The clinical impact of artemisinin resistance in Southeast Asia and the potential for future spread. FEMS Microbiol Rev. 2017;41:34–48.
Cheeseman IH, Miller BA, Nair S, Nkhoma S, Tan A, Tan C, et al. A major genome region underlying artemisinin resistance in malaria. Science. 2012;336:79–82.
Miotto O, Almagro-garcia J, Manske M, Macinnis B, Campino S, Rockett KA, et al. Multiple populations of artemisinin-resistant Plasmodium falciparum in Cambodia. Nat Genet. 2013;45:648–55.
Takala-Harrison S, Clark TG, Jacob CG, Cummings MP, Miotto O, Dondorpe AM, et al. Genetic loci associated with delayed clearance of Plasmodium falciparum following artemisinin. Proc Natl Acad Sci USA. 2013;110:240–5.
Dondorp AM, Nosten F, Yi P, Das D, Phyo AP, Tarning J, et al. Artemisinin resistance in Plasmodium falciparum malaria. Drug Ther. 2009;361:455–67.
Witkowski B, Amaratunga C, Khim N, Sreng S, Chim P, Kim S, et al. Novel phenotypic assays for the detection of artemisinin-resistant Plasmodium falciparum malaria in Cambodia: in vitro and ex vivo drug-response studies. Lancet Infect Dis. 2013;13:1043–9.
Ashley E, Dhorda M, Fairhurst RM, Amaratunga C, Lim P, Suon S, et al. Spread of artemisinin resistance in Plasmodium falciparum malaria. N Engl J Med. 2015;371:411–23.
Takala-Harrison S, Jacob CG, Arze C, Cummings MP, Silva JC, Dondorp AM, et al. Independent emergence of artemisinin resistance mutations among Plasmodium falciparum in Southeast Asia. J Infect Dis. 2015;211:670–9.
Ariey F, Witkowski B, Amaratunga C, Beghain J, Langlois A-C, Khim N, et al. A molecular marker of artemisinin-resistant Plasmodium falciparum malaria. Nature. 2014;505:50–5.
Straimer J, Gnadig NF, Witkowski B, Amaratunga C, Duru V, Ramadani AP, et al. Plasmodium falciparum clinical isolates. Science. 2015;347:428–31.
Trape J-F. The public health impact of chloroquine resistance in Africa. Am J Trop Med Hyg. 2001;64:12–7.
Trape J-F, Pison G, Spiegel A, Enel C, Rogier C. Combating malaria in Africa. Trends Parasitol. 2002;18:224–30.
Wongsrichanalai C, Sibley CH. Fighting drug-resistant Plasmodium falciparum: the challenge of artemisinin resistance. Clin Microbiol Infect. 2013;19:908–16.
Djaman JA, Olefongo D, Ako AB, Roman J, Ngane VF, Basco LK, et al. Molecular epidemiology of malaria in Cameroon and Côte d’Ivoire. XXXI. Kelch 13 propeller sequences in Plasmodium falciparum isolates before and after implementation of artemisinin-based combination therapy. Am J Trop Med Hyg. 2017;97:222–4.
Kamau E, Campino S, Amenga-Etego L, Drury E, Ishengoma D, Johnson K, et al. K13-propeller polymorphisms in Plasmodium falciparum parasites from sub-saharan Africa. J Infect Dis. 2015;211:1352–5.
Murugan K, Anitha J, Suresh U, Rajaganesh R, Panneerselvam C, Aziz AT, et al. Chitosan-fabricated Ag nanoparticles and larvivorous fishes: a novel route to control the coastal malaria vector Anopheles sundaicus? Hydrobiologia. 2017;797:335–50.
Borrmann S, Straimer J, Mwai L, Abdi A, Rippert A, Okombo J, et al. Genome-wide screen identifies new candidate genes associated with artemisinin susceptibility in Plasmodium falciparum in Kenya. Sci Rep. 2013;3:3318.
Torrentino-Madamet M, Fall B, Benoit N, Camara C, Amalvict R, Fall M, et al. Limited polymorphisms in k13 gene in Plasmodium falciparum isolates from Dakar, Senegal in 2012–2013. Malar J. 2014;13:472.
Cooper RA, Conrad MD, Watson QD, Huezo SJ, Ninsiima H, Tumwebaze P, et al. Lack of artemisinin resistance in Plasmodium falciparum in Uganda based on parasitological and molecular assays. Antimicr Agents Chemother. 2015;59:5061–4.
Ouattara A, Kone A, Adams M, Fofana B, Maiga AW, Hampton S, et al. Polymorphisms in the K13-propeller gene in artemisinin-susceptible Plasmodium falciparum parasites from Bougoula-Hameau and Bandiagara, Mali. Am J Trop Med Hyg. 2015;92:1202–6.
Menard S, Tchoufack JN, Maffo CN, Nsango SE, Iriart X, Abate L, et al. Insight into k13-propeller gene polymorphism and ex vivo DHA-response profiles from Cameroonian isolates. Malar J. 2016;15:572.
Taylor SM, Parobek CM, De Conti DK, Kayentao K, Coulibaly SO, Greenwood BM, et al. Absence of putative artemisinin resistance mutations among Plasmodium falciparum in sub-Saharan Africa: a molecular epidemiologic study. J Infect Dis. 2015;211:680–8.
Tse EG, Korsik M, Todd MH. The past, present and future of anti-malarial medicines. Malar J. 2019;18:93.
Ealias AM, Saravanakumar M. A review on the classification, characterisation, synthesis of nanoparticles and their application. Mater Sci Eng. 2017;263:032019.
Gahlawat G, Choudhury AR. A review on the biosynthesis of metal and metal salt nanoparticles by microbes. RSC Adv. 2019;9:12944.
Shakeel A, Mudasir A, Babu Lal S, Saiqa I. A review on plants extract mediated synthesis of silver nanoparticles for antimicrobial applications: a green expertise. J Adv Res. 2016;7:17–28.
Khandel P, Shahi SK. Microbes mediated synthesis of metal nanoparticles: current status and future prospects. Int J Nanomater Biostruct. 2016;6:1–24.
Maiti S, Krishnan D, Barman G, Ghosh SK, Laha JK. Antimicrobial activities of silver nanoparticles synthesized from Lycopersicon esculentum extract. J Anal Sci Technol. 2014;5:1–7.
Patra JK, Baek KH. Biosynthesis of silver nanoparticles using aqueous extract of silky hairs of corn and investigation of its antibacterial and anticandidal synergistic activity and antioxidant potential. IET Nanobiotechnol. 2016;10:326–33.
Patra JK, Baek KH. Antibacterial activity and synergistic antibacterial potential of biosynthesized silver nanoparticles against foodborne pathogenic bacteria along with its anticandidal and antioxidant effects. Front Microbiol. 2017;8:167.
Mallmann EJJ, Cunha FA, Castro BNMF, Maciel AM, Menezes EA, Fechine PBA. Antifungal activity of silver nanoparticles obtained by green synthesis. Rev Inst Med Trop São Paulo. 2015;57:165–7.
Arciniegas-Grijalba PA, Patiño-Portela MC, Mosquera-Sánchez LP, Guerrero-Vargas JA, Rodríguez-Páez JE. ZnO nanoparticles (ZnO-NPs) and their antifungal activity against coffee fungus Erythricium salmonicolor. Appl Nanosci. 2017;7:225–41.
Narasimha G. Virucidal properties of silver nanoparticles synthesized from white button mushrooms (Agaricus bisporus). Int J Nano Dimens. 2016;3:181–4.
Broglie JJ, Alston B, Yang C, Ma L, Adcock AF, Chen W, et al. Antiviral activity of gold/copper sulfide core/shell nanoparticles against human norovirus virus-like particles. PLoS ONE. 2015;10:e0141050.
Dauda K, Busari Z, Morenikeji O, Afolayan F. Poly (d-l-lactic-co-glycolic acid) -based artesunate nanoparticles: formulation, antimalarial and toxicity assessments. J Zhejiang Univ Sci B. 2017;18:977–85.
Barabadi H, Alizadeh Z, Rahimi MT, Barac A, Maraolo AE, Robertson LJ, et al. Nanobiotechnology as an emerging approach to combat malaria: a systematic review. Nanomedicine. 2019;18:221–33.
Moher D, Liberati A, Tetzlaff J, Altman DG, Altman D, Antes G, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097.
Panneerselvam C, Ponarulselvam S, Murugan K. Potential anti-plasmodial activity of synthesized silver nanoparticle using Andrographis paniculata Nees (Acanthaceae). Arch Appl Sci Res. 2011;3:208–17.
Ponarulselvam S, Panneerselvam C, Murugan K, Aarthi N, Kalimuthu K, Thangamani S. Synthesis of silver nanoparticles using leaves of Catharanthus roseus Linn. G. Don and their antiplasmodial activities. Asian Pac J Trop Biomed. 2012;2:574–80.
Mishra A, Kaushik NK, Sardar M, Sahal D. Evaluation of antiplasmodial activity of green synthesized silver nanoparticles. Colloid Surf B. 2013;111:713–8
Panneerselvam C, Murugan K, Amerasan D. Biosynthesis of silver nanoparticles using plant extract and its anti-plasmodial property. Adv Mater Res. 2015;1086:11–30.
Rajakumar G, Rahuman AA, Chung IM, Kirthi AV, Marimuthu S, Anbarasan K. Antiplasmodial activity of eco-friendly synthesized palladium nanoparticles using Eclipta prostrata extract against Plasmodium berghei in Swiss albino mice. Parasitol Res. 2015;114:1397–406.
Murugan K, Samidoss CM, Panneerselvam C, Higuchi A, Roni M, Suresh U, et al. Seaweed-synthesized silver nanoparticles: an eco-friendly tool in the fight against Plasmodium falciparum and its vector Anopheles stephensi? Parasitol Res. 2015;114:4087–97.
Subramaniam J, Murugan K, Panneerselvam C, Kovendan K, Madhiyazhagan P, Dinesh D, et al. Multipurpose effectiveness of Couroupita guianensis-synthesized gold nanoparticles: high antiplasmodial potential, field efficacy against malaria vectors and synergy with Aplocheilus lineatus predators. Environ Sci Pollut Res. 2016;23:7543–58.
Panneerselvam C, Murugan K, Roni M, Aziz AT, Suresh U, Rajaganesh R, et al. Fern-synthesized nanoparticles in the fight against malaria: LC/ MS analysis of Pteridium aquilinum leaf extract and biosynthesis of silver nanoparticles with high mosquitocidal and antiplasmodial activity. Parasitol Res. 2016;115:997–1013.
Murugan K, Panneerselvam C, Subramaniam J, Madhiyazhagan P, Hwang JS, Wang L, et al. Eco-friendly drugs from the marine environment: spongeweed-synthesized silver nanoparticles are highly effective on Plasmodium falciparum and its vector Anopheles stephensi, with little non-target effects on predatory copepods. Environ Sci Pollut Res. 2016;23:16671–85.
Murugan K, Panneerselvam C, Samidoss CM, Madhiyazhagan P, Suresh U, Roni M, et al. In vivo and in vitro effectiveness of Azadirachta indica-synthesized silver nanocrystals against Plasmodium berghei and Plasmodium falciparum, and their potential against malaria mosquitoes. Res Vet Sci. 2016;106:14–22.
Dutta PP, Bordoloi M, Gogoi K, Roy S, Narzary B, Bhattacharyya DR, et al. Antimalarial silver and gold nanoparticles: green synthesis, characterization and in vitro study. Biomed Pharmacother. 2017;91:567–80.
Sardana M, Agarwal V, Pant A, Kapoor V, Pandey KC, Kumar S. Antiplasmodial activity of silver nanoparticles: a novel green synthesis approach. Asian Pac J Trop Biomed. 2018;8:268–72.
Gandhi PR, Jayaseelan C, Kamaraj C, Rajasree SRR, Regina Mary R. In vitro antimalarial activity of synthesized TiO2 nanoparticles using Momordica charantia leaf extract against Plasmodium falciparum. J Appl Biomed. 2018;16:378–86.
Rotimi L, Ojemaye MO, Okoh OO, Sadimenko A, Okoh AI. Synthesis, characterization, antimalarial, antitrypanocidal and antimicrobial properties of gold nanoparticle. Green Chem Lett Rev. 2019;12:61–8.
Karthik L, Kumar G, Keswani T, Bhattacharyya A, Reddy BP, Rao KVB. Marine actinobacterial mediated gold nanoparticles synthesis and their antimalarial activity. Nanomedicine. 2013;9:951–60.
Jaganathan A, Murugan K, Panneerselvam C, Madhiyazhagan P, Dinesh D, Vadivalagan C, et al. Earthworm-mediated synthesis of silver nanoparticles: a potent tool against hepatocellular carcinoma, Plasmodium falciparum parasites and malaria mosquitoes. Parasitol Int. 2016;65:276–84.
Murugan K, Wei J, Alsalhi MS, Nicoletti M, Paulpandi M, Samidoss CM, et al. Magnetic nanoparticles are highly toxic to chloroquine-resistant Plasmodium falciparum, dengue virus (DEN-2), and their mosquito vectors. Parasitol Res. 2017;116:495–502.
Das R, Ali ME, Hamid SBA. Current applications of x-ray powder diffraction—a review. Rev Adv Mater Sci. 2014;38:95–109.
Faculty of Hanson. Introduction to structure determination infrared: characteristic frequencies. 2013. p. 1–4.
Prabhu S, Poulose EK. Silver nanoparticles: mechanism of antimicrobial action, synthesis, medical applications, and toxicity effects. Int Nano Lett. 2012;2:32.
Peng L, Calton GJ, Burnett JW. Effect of borohydride reduction on antibodies. Appl Biochem Biotechnol. 1987;14:91–9.
Kalishwaralal K, Deepak V, Ram Kumar Pandian SB, Kottaisamy M, BarathManiKanth S, Kartikeyan B, et al. Biosynthesis of silver and gold nanoparticles using Brevibacterium casei. Colloid Surf B. 2010;77:257–62.
Benelli G. Plant-mediated biosynthesis of nanoparticles as an emerging tool against mosquitoes of medical and veterinary importance: a review. Parasitol Res. 2016;115:23–34.
Kaler A, Patel N, Banerjee UC. Green synthesis of silver nanoparticles. Curr Res Inform Pharm Sci. 2010;11:68–71.
Eya’ane Meva F, Okalla Ebongue C, Fannang SV, Segnou ML, Ntoumba AA, Belle Ebanda Kedi P, et al. Natural substances for the synthesis of silver nanoparticles against Escherichia coli: the case of Megaphrynium macrostachyum (Marantaceae), Corchorus olitorus (Tiliaceae), Ricinodendron heudelotii (Euphorbiaceae), Gnetum bucholzianum (Gnetaceae), and Ipomoea batatas (Convulvulaceae). J Nanomater. 2017;2017:1–6.
Jung WK, Hye CK, Ki WK, Shin S, So HK, Yong HP. Antibacterial activity and mechanism of action of the silver ion in Staphylococcus aureus and Escherichia coli. Appl Environ Microbiol. 2008;74:2171–8.
Swathy JR, Udhaya Sankar M, Chaudhary A, Aigal S, Anshup, Pradeep T. Antimicrobial silver: an unprecedented anion effect. Sci Rep. 2014;4:7161.
Tran QH, Nguyen VQ, Le A. Silver nanoparticles: synthesis, properties, toxicology. Adv Nat Sci Nanosci Nanotechnol. 2013;4:033001.
Pal S, Tak YK, Song JM. Does the antibacterial activity of silver nanoparticles depend on the shape of the nanoparticle? A study of the gram-negative bacterium Escherichia coli. Appl Environ Microbiol. 2007;73:1712–20.
Adams CP, Walker KA, Obare SO, Docherty KM. Size-dependent antimicrobial effects of novel palladium nanoparticles. PLoS ONE. 2014;9:e85981.
Dang TMD, Le TTT, Fribourg-Blanc E, Dang MC. Influence of surfactant on the preparation of silver nanoparticles by polyol method. Adv Nat Sci Nanosci Nanotechnol. 2012;3:035004.
Chen SF, Zhang H. Aggregation kinetics of nanosilver in different water conditions. Adv Nat Sci Nanosci Nanotechnol. 2012;3:035006.
Christy AJ, Umadevi M. Synthesis and characterization of monodispersed silver nanoparticles. Adv Nat Sci Nanosci Nanotechnol. 2012;3:035013.
Wang L, Hu C, Shao L. The antimicrobial activity of nanoparticles: present situation and prospects for the future. Int J Nanomed. 2017;12:1227–49.
Gatoo MA, Naseem S, Arfat MY, Mahmood Dar A, Qasim K, Zubair S. Physicochemical properties of nanomaterials: implication in associated toxic manifestations. Biomed Res Int. 2014;2014:498420.
Cui W, Li J, Zhang Y, Rong H, Lu W, Jiang L. Effects of aggregation and the surface properties of gold nanoparticles on cytotoxicity and cell growth. Nanomedicine. 2012;8:46–53.
Chokchaisiri R, Chaichompoo W, Chalermglin R, Suksamrarn A. Potent antiplasmodial alkaloids and flavonoids from Dasymaschalon acuminatum. Rec Nat Prod. 2015;9:243–6.
Ovenden SPB, Cobbe M, Kissell R, Birrell GW, Chavchich M, Edstein MD. Phenolic glycosides with antimalarial activity from Grewillea “Poorinda Queen”. J Nat Prod. 2011;74:74–8.
Messi AN, Ngo Mbing J, Ndongo JT, Nyegue MA, Tchinda AT, Yemeda FL, et al. Phenolic compounds from the roots of Ochna schweinfurthiana and their antioxidant and antiplasmodial activities. Phytochem Lett. 2016;17:119–25.
Azebaze AGB, Meyer M, Valentin A, Nguemfo EL, Fomum ZT, Nkengfack AE. Prenylated xanthone derivatives with antiplasmodial activity from Allanblackia monticola Staner L.C. Chem Pharm Bull. 2006;54:111–3.
Azebaze AGB, Mbosso Teinkela JE, Nguemfo EL, Valentin A, Dongmo AB, et al. Antiplasmodial activity of some phenolic compounds from Cameroonians Allanblackia parasite is transmitted by the female mosquito species. Afr Health Sci. 2015;15:835–40.
Kayembe J, Taba K, Ntumba K, Kazadi T. In vitro Antimalarial activity of 11 terpenes isolated from Ocimum gratissimum and Cassia alata leaves. Screening of their binding affinity with haemin. J Plant Stud. 2012;1:168–72.
Saito AY, Marin Rodriguez AA, Menchaca Vega DS, Sussmann RAC, Kimura EA, Katzin AM. Antimalarial activity of the terpene nerolidol. Int J Antimicrob Agents. 2016;48:641–6.
Yenjai C, Sripontan S, Sriprajun P, Kittakoop P, Jintasirikul A, Tanticharoen M, et al. Coumarins and carbazoles with antiplasmodial activity from Clausena harmandiana. Planta Med. 2000;66:277–9.
Abrantes M, Mil-Homens T, Duarte N, Lopes D, Cravo P, Do Céu Madureira M, et al. Antiplasmodial activity of lignans and extracts from Pycnanthus angolensis. Planta Med. 2008;74:1408–12.
Kamaraj C, Balasubramani G, Siva C, Raja M, Balasubramanian V, Raja RK, et al. Ag nanoparticles synthesized using β-caryophyllene isolated from Murraya koenigii: antimalarial (Plasmodium falciparum 3D7) and anticancer Activity (A549 and HeLa cell lines). J Clust Sci. 2017;28:1667–84.
Çiftci H, Turk M, Tamer U, Karahan S, Menemen Y. Silver nanoparticles: cytotoxic, apoptotic, and necrotic effects on MCF-7 cells. Turkish J Biol. 2013;37:573–81.
Panyala NR, Peña-Méndez EM, Havel J. Silver or silver nanoparticles: a hazardous threat to the environment and human health? J Appl Biomed. 2012;10:117–29.
Zeyons O. Etudes des interactions physicochimiques et biologiques entre des nanoparticules manufacturées et des bactéries de l’environnement. Thèse de doctorat de l’université Paris VI - Pierre et Marie Curie, Paris, France. 2008.
Satyavani K, Gurudeeban S, Ramanathan T, Balasubramanian T. Toxicity study of silver nanoparticles synthesized from Suaeda monoica on Hep-2 cell line. Avicenna J Med Biotechnol. 2012;4:35–9.
Zhang T, Wang L, Chen Q, Chen C. Cytotoxic potential of silver nanoparticles. Yonsei Med J. 2014;55:283–91.
Linder HP, Verboom GA. The evolution of regional species richness: the history of the Southern African flora. Annu Rev Ecol Evol Syst. 2015;46:393–412.
Acknowledgements
The authors are grateful to Mr. Innocent Damudu Peter (Faculty of Veterinary Medicine, Universiti Putra Malaysia, Malaysia) and Mr. Wepnje Godlove Bunda (Department of Zoology and Animal physiology, University of Buea, Cameroon) for proofreading the manuscript.
Funding
None.
Author information
Authors and Affiliations
Contributions
LPKF, FEM, CEEM and LGL conceived of and designed the study. LPK, AAN, MINN and KPBE carried out the screen of the literature and data extraction. LPKF analysed and interpreted the results with help of FEM, CEEM and LA. LPKF drafted the manuscript, and FEM, CEEM, LA and LGL revised the manuscript. FEM, CEEM and LGL supervised the work at all stages. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
This research was based on information/data extracted from published studies and no ethical approval was acquired.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Additional file 1.
PRISMA 2009 flow diagram.
Additional file 2.
PRISMA 2009 checklist.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Kojom Foko, L.P., Eya’ane Meva, F., Eboumbou Moukoko, C.E. et al. A systematic review on anti-malarial drug discovery and antiplasmodial potential of green synthesis mediated metal nanoparticles: overview, challenges and future perspectives. Malar J 18, 337 (2019). https://doi.org/10.1186/s12936-019-2974-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12936-019-2974-9