Abstract
Background
Endocan, also known as endothelial cell specific molecule-1 (ESM1), is a 50 kDa soluble proteoglycan which is frequently overexpressed in many cancer types. Whether it is dysregulated in head and neck squamous cell carcinoma (HNSCC) has not been investigated.
Methods
We analyzed the expression of ESM1 using bioinformatics analysis based on data from The Cancer Genome Atlas (TCGA), and then validated that ESM1 was significantly overexpressed in human HNSCC at the protein level using immunohistochemistry. We also analyzed the genes co-expressed with ESM1 in HNSCC.
Results
The most correlated gene was angiopoietin-2 (ANGPT2), a molecule which regulates physiological and pathological angiogenesis. Several transcription factor binding motifs including SMAD3, SMAD4, SOX3, SOX4, HIF2A and AP-1 components were significantly enriched in the promoter regions of the genes co-expressed with ESM1. Further analysis based on ChIP-seq data from the ENCODE (Encyclopedia of DNA Elements) project revealed that AP-1 is an important regulator of ESM1 expression.
Conclusions
Our results revealed a dysregulation of ESM1 and a potential regulatory mechanism for the co-expression network in HNSCC.
Similar content being viewed by others
Background
Head and neck squamous cell carcinoma (HNSCC) includes many cancers in the head and neck originating from a variety of sub-sites including the lip, oral cavity, nasopharynx, oropharynx, and larynx. HNSCC is the sixth most common cancer worldwide. There are about 650,000 new cases and nearly 350,000 patient deaths from HNSCC annually [1]. The most common causes include tobacco and alcohol consumption, but human papilloma virus (HPV) has been shown to be a primary cause of oropharyngeal cancers [2]. Our understanding of the molecular and genetic abnormalities leading to oncogenesis of HNSCC has greatly increased over the past decade. Many studies based on genomic and expression profiles have provided a more thorough understanding of the molecular abnormalities in head and neck cancer to help guide the development of new therapeutic agents [3]. For example, mutational analysis has revealed that many genes such as TP53, CDKN2A, PTEN, PIK3CA, HRAS, NOTCH1, IRF6, and TP63 are frequently mutated in HNSCC [4]. As for gene expression, many genes, such as βIII-tubulin (TUBB3) [5], TMEM16A/ANO1 [6], homeobox gene family (HOX) members [7] and metalloproteinases (MMPs) [8], have been found to be dysregulated in HNSCC. It is crucial to investigate novel molecular mechanisms involved in proliferation, apoptosis, and invasion of HNSCC and provide effective biomarkers or drug targets for diagnosis and prevention of the disease.
Endocan, also called endothelial cell specific molecule-1 (ESM-1), is an endothelial cell-associated proteoglycan [9]. It is up-regulated by pro-inflammatory cytokines, such as tumor necrosis factor-α (TNF-α), interleukin (IL)-1 and microbial lipopolysaccharide, as well as by proangiogenic molecules such as vascular endothelial growth factor (VEGF) [10]. ESM1 is possibly involved in neoangiogenesis, and as a promising biomarker of endothelial dysfunction and inflammation, it has being increasingly studied in recent years in a wide spectrum of healthy and pathophysiological processes [11,12,13,14]. ESM1 is preferentially expressed in tumor endothelium [15], and is dramatically overexpressed in many cancers including non-small cell lung cancer [16], colorectal cancer [17], clear cell renal cell carcinoma [18], gastric cancer [19], hepatocellular carcinoma [20], pituitary adenoma [21], ovarian cancer [22], and brain cancers [23]. In addition, serum endocan was reported to be a potential marker for cancer diagnosis and prognosis [19, 24,25,26,27,28]. Therefore, ESM-1 may be useful as a therapeutic cancer target.
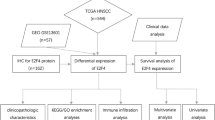
The differential expression of ESM1 has not been investigated in HNSCC. In this study, we analyzed the expression of ESM1 in cancerous and adjacent normal HNSCC tissue using RNA-seq data from The Cancer Genome Atlas (TCGA) [29], and we used immunohistochemistry to examine whether ESM1 was overexpressed at the protein level in HNSCC tissue. We also identified a set of genes co-expressed with ESM1, and found that transcription factor binding motifs including SMAD3, SMAD4, SOX3, SOX4, HIF2A and AP-1 components were significantly enriched in the promoter regions of these correlated genes. We further confirmed reliable motifs using ChIP-seq data from the ENCODE (Encyclopedia of DNA Elements) project via the University of California, Santa Cruz (UCSC) genome browser [30]. Our results show that AP-1 plays an important role in the regulation of ESM1 expression, and provide important functional clues about ESM1 dysregulation and its regulatory mechanism in HNSCC.
Materials and methods
Data set
The Cancer Genome Atlas (TCGA) data related to HNSCC were downloaded from Xena public data hubs (http://xena.ucsc.edu/). In the UCSC-hosted database, TCGA data sets are normalized and can be explored and downloaded.
TCGA copy number profile was measured experimentally using whole genome microarray. Gene-level copy number variation (CNV) was estimated using the GISTIC2 method [31]. GISTIC2 further thresholded the estimated values to − 2, − 1, 0, 1, 2, representing homozygous deletion, single copy deletion, diploid normal copy, low-level copy number amplification, or high-level copy number amplification.
The BioXpress database, which also uses TCGA data, was used to query differential expression [32].
Samples and immunohistochemical analysis
After informed consent had been obtained, all specimens were collected from patients. Twenty-one cases of laryngeal or hypopharyngeal squamous cell carcinoma were studied. Paraffin embedded cancer tissue and peri-cancerous tissue were selected for the immunohistochemical tests. After dehydration, transparent, paraffin embedded, frozen tissues were made into 2 μm serial sections. Slides of tissue were incubated for 40 min at 70 °C, rehydrated in alcohol solution, and then washed with water. Then the slides were treated with 3% H2O2 for 10 min, and then EDTA pH 9.0 for 1 min 50 s. For immunohistochemical analysis, the slides were incubated with anti-ESM1 (ab56914, Abcam, Cambridge, England) (1:300) for 1 h at 37 °C. After thorough washing with PBS, the slides were incubated with horseradish peroxidase (HRP) conjugated anti-rabbit IgG at 37 °C for 15 min, and then thoroughly washed again. After washing, bound antibody was detected using the 3,3′-diaminobenzidine (DAB) reaction. Nuclear counterstaining was performed with hematoxylin. Control sections were subjected to the same procedure except that the first antibody was eliminated from the incubation. Positive staining was seen as a brown color of varying intensity, and a positivity score was assigned for statistical analysis (Chi squared test).
Immunofluorescence assay
For immunofluorescence staining of ESM1 and ANGPT2, paraffin-embedded 3 μm serial sections of five cases of laryngeal or hypopharyngeal squamous cell carcinoma samples were deparaffinized and rehydrated. Preheat EDTA 8.0 was used for repairing in the high pressure cooker. Polyclonal rabbit anti-human primary antibodies anti-ESM1/FITC (ab103590, Abcam, Cambridge, England) and anti-ANGPT2/TRITC (Abcam, Cambridge, England) (1:100) were applied overnight at 4 °C. After washing, fluorescently conjugated secondary antibodies were used. Nuclear counterstain was achieved using DAPI staining. All fluorescently stained images were taken using an Olympus BX-51 upright light microscope (Olympus, Tokyo, Japan). Each site was imaged in all channels and overlaid in DPViewer version before examination in Photoshop.
Transcription factor binding motifs
The HOMER (Hypergeometric Optimization of Motif EnRichment) program package (v4.9, http://homer.ucsd.edu/) [33] was used for transcription factor binding motif analysis according to the procedure in the online guide. The region – 500 bp to + 100 bp from the transcription start site (TSS) in gene sets of interest was searched for enriched motifs against random background regions using the findMotifs.pl program. Enriched motifs were further validated by ChIP-seq data integrated in the transcription factor ChIP-seq (161 factors) track on the UCSC genome browser (http://genome.ucsc.edu).
Results
ESM1 is overexpressed in HNSCC
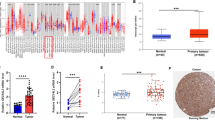
The Cancer Genome Atlas (TCGA) data have become an important and widely used resource in cancer research [29]. As for HNSCC, currently there are 522 cancerous and 44 normal samples that have been sequenced at the RNA level using high-throughput sequencing technology. As shown in Fig. 1a, the RNA-seq revealed that ESM1 was dramatically overexpressed in HNSCC. Because genetic instability such as gene copy number alteration is a general potential factor affecting gene expression in cancers, we therefore also examined the relationship between ESM1 copy number and gene expression in 514 common HNSCC samples. As shown in Fig. 1b, ESM1 has frequent heterozygous loss of copy number in HNSCC with a ratio of about 36.97% (193/522) compared to gain of copy number (about 9.39%, 49/522). However, there is no apparent correlation between copy number variation (CNV) and gene expression (Fig. 1b), suggesting that some other mechanisms may control the up-regulated expression of ESM1 in HNSCC. The overexpression of ESM1 in HNSCC and other cancers was also confirmed based on paired analysis of TCGA data (Fig. 1c).
ESM1 is overexpressed in HNSCC from TCGA data. a Comparison of expression levels between HNSCC and normal tissues. b Copy number does not affect gene expression of ESM1. Positive and negative values indicate gain and loss of copy number, respectively. c The BioXpress database reveals that ESM1 is widely overexpressed in human cancers. The frequencies of patients who have an over- (blue) or under- (orange) expression of ESM1 in each cancer type are shown. During paired analysis between cancerous and adjacent tissues, all log2 fold change (log2FC) values greater than zero for ESM1 are considered to be overexpression, less than zero to be under-expression. The abbreviations are as follows: BLCA: urinary bladder cancer; BRCA: breast cancer; CESC: cervical squamous cell carcinoma; COAD: colon adenocarcinoma; ESCA: esophageal cancer; HNSC: head and neck cancer; KICH: kidney chromophobe adenocarcinoma; KIRC: kidney renal clear cell carcinoma; KIRP: kidney papillary renal cell carcinoma; LIHC: liver cancer; LUAD: lung adenocarcinoma; LUSC: lung squamous cell carcinoma; PAAD: pancreas adenocarcinoma; PRAD: prostate cancer; READ: rectum adenocarcinoma; SARC: sarcoma; STAD: stomach cancer; THCA: thyroid cancer; UCEC: uterine cancer
Because these results from TCGA data were at the RNA level, we then detected ESM1 expression at the protein level in the 21 laryngeal or hypopharyngeal cancer samples. As shown in Table 1, Fig. 2 and Additional file 1, ESM1 was significantly overexpressed at the protein level in these cancers but there was no apparent correlation with clinical or pathologic stage.
Identification of ANGPT2 as the gene most correlated with ESM1 in HNSCC
Since up-regulation of ESM1 was not associated with copy number alteration in HNSCC, we next investigated the potential regulatory mechanisms, mainly focusing on transcription factors (TFs). Generally, transcription factor search tools identify potential TF binding sites (TFBSs) by sequence matching, which often results in dozens or even hundreds of candidate TFBSs and thus it is difficult to identify the true transcription factors that have important regulatory roles. Therefore, we first identified the co-expressed genes based on Pearson correlation. In total, there were 85 genes with r ≥ 0.45 and all of these genes were significantly overexpressed in HNSCC based on our analysis (Table 2). Some of them have been reported to be associated with HNSCC. The gene most correlated was ANGPT2 (angiopoietin 2, also known as Ang-2) with a correlation coefficient (r) of 0.7133 (p value = 3.95E−89) (Fig. 3a), suggesting that a tightly co-regulated mechanism exists between ESM1 and ANGPT2. ANGPT2 was also up-regulated in HNSCC (Fig. 3b, Table 2).
ANGPT2 is the gene most correlated with ESM1 and is also overexpressed in HNSCC. a The distribution of Pearson correlation coefficients between ESM1 and other genes. The dash lines in red indicate confidence intervals. The black dash line represents a cut-off r value with 0.45. b Kernel density distribution of all r values in a. c. The expressional correlation between ESM1 and ANGPT2, with a linear regression estimation shown. The shade band indicates a 95% confidence interval. d ANGPT2 is also overexpressed in HNSCC
We further confirmed the co-expression of ANGPT2 and ESM1 using immunofluorescence assay. The results showed that both of ESM1 and ANGPT2 could be expressed in the same tissues, either in the cancerous epithelial cells (Fig. 4a) or interstitial tissues (Fig. 4b).
Identification of AP-1 as an important regulator of ESM1
Next, we used the Homer program to identify possible enriched motifs in the promoter regions from – 500 to + 100 bp around the transcription start site (TSS) of the 85 correlated genes. As shown in Fig. 5a, seven motifs including Smad3, Smad4, c-Jun, AP-1, Sox3, Sox4 and HIF2α were significantly enriched, suggesting they play important roles in regulating the correlated network of ESM1.
AP-1 is an important regulator of ESM1 expression. a Homer known motif enrichment result. b Transcription factor ChIP-seq result on UCSC genome browser. In the ChIP-seq track, each block represents a peak bound by the corresponding transcription factor. c AP-1 binding sites in the promoter region of ESM1. Sequence 500 bp before the transcription start site (TSS) is shown. The shaded base A indicates TSS (+ 1 position). The underlined bold bases indicate potential AP-1 binding sites matching the second and fifth motifs in the enriched known Homer motifs
We then used ChIP-seq data from the ENCODE project to filter the results. We found that only AP-1, which is a heterodimer composed of proteins belonging to the c-Fos, c-Jun, ATF and JDP families, overlapped in the promoter region of ESM1 (Fig. 5b). We also observed that AP-1 or its subunit binding sites exist in the promoter region (Fig. 5c). These results further confirmed that AP-1 is an important regulator of ESM1.
Discussion
Endocan is a 50 kDa soluble proteoglycan secreted by vascular endothelial cells, especially from the inflamed endothelium, thereby it is also thought to play a role in the pathogenesis of vascular disorders, inflammation and endothelium dysfunction [9]. It can bind to the leukocyte integrin LFA-1 (CD11a/CD18), and prevents the specific binding of ICAM-1 to LFA-1, and may therefore influence both the recruitment of circulating lymphocytes to inflammatory sites and LFA-1-dependent leukocyte adhesion and activation [34]. Endocan is clearly overexpressed in many cancers and has also been shown to be directly involved in tumor progression as observed in mouse models of human tumor xenografts [9]. In the current study, we have confirmed that endocan is also dramatically overexpressed in HNSCC. A recent study revealed that ESM1 could mediate nerve growth factor receptor (NGFR)-induced invasion and metastasis in murine oral squamous cell carcinoma [35]. All these results indicate that ESM1 may be a potential therapeutic target in HNSCC.
An early study showed that Ets-binding motifs were mainly responsible for endothelial-cell-specific expression of ESM1 in vitro, though putative binding sites for GATA, AP1, AP4, NF1, and CREB/ATF transcription factors were also speculated [36]. We also investigated the regulatory mechanism using publically available data and found that AP-1 may be a key regulator of ESM1, particularly for the co-expressed network centered on ESM1. ESM1 can be activated by inflammation, cytokines and vascular growth factors, and in fact, AP-1 activity is also regulated by a broad range of physiological and pathological stimuli, including cytokines, growth factors, stress signals and infections, as well as oncogenic stimuli [37]. AP-1 mediates regulation involved in many biological processes such as proliferation, differentiation, apoptosis and transformation. A typical upstream signal pathway for activation of AP-1 that has been widely studied is the Ras-MAPK-ERK pathway, which is one of several important pathways for targeting therapy in HNSCC [38].
Besides AP-1, ChIP-seq from the ENCODE project also suggests that other transcription factors such as STAT3 (signal transducer and activator of transcription 3), TBP (TATA-box binding protein), GATA2 (GATA binding protein 2), RAD21 (RAD21 cohesin complex component) and MYC (MYC proto-oncogene, bHLH transcription factor) are also potential regulators of ESM1. Considering the genes co-expressed with ESM1, AP-1 probably plays a key role but other factors may synergize the regulation. Further details still need investigation.
We identified the genes co-expressed with ESM1 in HNSCC and the most correlated gene is ANGPT2. ANGPT2 can also be regulated by Ets-1 and AP-1 [39, 40], further confirming their correlation. As shown in Fig. 4, although the expressional patterns of ESM1 and ANGPT2 are not fully overlapped, co-expression in some of the same cells can be truly observed. However, relatively lower immunofluorescence positivity for ESM1 in Fig. 4 was observed as compared with the DAB positivity pattern in Fig. 2. This may be due to different specimen and antibodies used in two assays. On the other hand, correlation doesn’t mean co-expression in the same cells when bulk RNA-seq data were used, they can be expressed in different cell types but could also show positive correlation. A recent study shows ANGPT2 can be regulated by the synaptic protein neuroligin 2 (NLGN2) [41], whether ESM1 is also regulated by NLGN2 needs further investigation. Angiopoietins, including ANGPT1, ANGPT2, ANGPT3 and ANGPT4, are vascular growth factors that control microvascular permeability, vasodilation, and vasoconstriction by signaling smooth muscle cells. Antiangiogenic agents can normalize the tumor microenvironment, combining antiangiogenic therapies with immune-checkpoint inhibitors potentially improve patient outcomes for the treatment of a range of solid tumors [42].
ANGPT1 is critical for vessel maturation, adhesion, migration, and survival, but ANGPT2 is an antagonist of ANGPT1 promoting cell death and disrupting vascularization; [43] however, VEGF and ANGPT2 appear to play crucial roles in the balance between vascular regression and growth of this subset of tumors, and the combination can promote neo-vascularization. [42, 44] Mice deficient in ANGPT2 have abnormalities in the blood and lymphatic vasculatures, and also show deficits in rapid leukocyte recruitment to sites of inflammation [45]. This function is very similar to ESM1; however, whether ESM1 and ANGPT2 can be mutually regulated still awaits further investigation.
Conclusions
In conclusion, we have identified that ESM1 is overexpressed in HNSCC and investigated the regulatory mechanism of ESM1-centered co-expression. These results provide important functional clues for ESM1 dysregulation and regulation in cancers.
Abbreviations
- ESM1:
-
endothelial cell specific molecule-1
- HNSCC:
-
head and neck squamous cell carcinoma
- TCGA:
-
The Cancer Genome Atlas
- ANGPT2:
-
angiopoietin-2
- ENCODE:
-
Encyclopedia of DNA Elements
- HPV:
-
human papilloma virus
- TUBB3:
-
βIII-tubulin
- HOX:
-
homeobox gene family
- MMPs:
-
metalloproteinases
- TNF-α:
-
tumor necrosis factor-α
- IL:
-
interleukin
- VEGF:
-
vascular endothelial growth factor
- UCSC:
-
University of California, Santa Cruz
- HRP:
-
horseradish peroxidase
- DAB:
-
diaminobenzidine
- HOMER:
-
Hypergeometric Optimization of Motif EnRichment
- TSS:
-
transcription start site
- CNV:
-
copy number variation
- TFBSs:
-
TF binding sites
- NGFR:
-
nerve growth factor receptor
- STAT3:
-
signal transducer and activator of transcription 3
- TBP:
-
TATA-box binding protein
- GATA2:
-
GATA binding protein 2
References
Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127(12):2893–917.
Gillison ML, Koch WM, Capone RB, Spafford M, Westra WH, Wu L, Zahurak ML, Daniel RW, Viglione M, Symer DE, et al. Evidence for a causal association between human papillomavirus and a subset of head and neck cancers. J Natl Cancer Inst. 2000;92(9):709–20.
Riaz N, Morris LG, Lee W, Chan TA. Unraveling the molecular genetics of head and neck cancer through genome-wide approaches. Genes Dis. 2014;1(1):75–86.
Stransky N, Egloff AM, Tward AD, Kostic AD, Cibulskis K, Sivachenko A, Kryukov GV, Lawrence MS, Sougnez C, McKenna A, et al. The mutational landscape of head and neck squamous cell carcinoma. Science. 2011;333(6046):1157–60.
Nienstedt JC, Grobe A, Clauditz T, Simon R, Muenscher A, Knecht R, Sauter G, Moebius C, Blessmann M, Heiland M, et al. High level betaIII-tubulin overexpression occurs in most head and neck cancers but is unrelated to clinical outcome. J Oral Pathol Med. 2017;46(10):986–90.
Dixit R, Kemp C, Kulich S, Seethala R, Chiosea S, Ling S, Ha PK, Duvvuri U. TMEM16A/ANO1 is differentially expressed in HPV-negative versus HPV-positive head and neck squamous cell carcinoma through promoter methylation. Sci Rep. 2015;5:16657.
de Barros ELBR, Ramao A, Pinheiro DG, Alves CP, Kannen V, Jungbluth AA, de Araujo LF, Muys BR, Fonseca AS, Placa JR, et al. HOX genes: potential candidates for the progression of laryngeal squamous cell carcinoma. Tumour Biol. 2016;37(11):15087–96.
Scurry WC Jr, Stack BC Jr. Role of metalloproteins in the clinical management of head and neck squamous cell carcinoma. Head Neck. 2007;29(12):1144–55.
Sarrazin S, Adam E, Lyon M, Depontieu F, Motte V, Landolfi C, Lortat-Jacob H, Bechard D, Lassalle P, Delehedde M. Endocan or endothelial cell specific molecule-1 (ESM-1): a potential novel endothelial cell marker and a new target for cancer therapy. Biochem Biophys Acta. 2006;1765(1):25–37.
Rennel E, Mellberg S, Dimberg A, Petersson L, Botling J, Ameur A, Westholm JO, Komorowski J, Lassalle P, Cross MJ, et al. Endocan is a VEGF-A and PI3K regulated gene with increased expression in human renal cancer. Exp Cell Res. 2007;313(7):1285–94.
Kechagia M, Papassotiriou I, Gourgoulianis KI. Endocan and the respiratory system: a review. Int J Chron Obstruct Pulmon Dis. 2016;11:3179–87.
Balta S, Mikhailidis DP, Demirkol S, Ozturk C, Celik T, Iyisoy A. Endocan: a novel inflammatory indicator in cardiovascular disease? Atherosclerosis. 2015;243(1):339–43.
Afsar B, Takir M, Kostek O, Covic A, Kanbay M. Endocan: a new molecule playing a role in the development of hypertension and chronic kidney disease? J Clin Hypertens. 2014;16(12):914–6.
Yoon KH, Kim SY, Moon YS, Roh D, Lee SK, Kim DH. The relationship between serum endocan levels and depression in alzheimer’s disease. Dis Markers. 2016;2016:8254675.
Abid MR, Yi X, Yano K, Shih SC, Aird WC. Vascular endocan is preferentially expressed in tumor endothelium. Microvasc Res. 2006;72(3):136–45.
Grigoriu BD, Depontieu F, Scherpereel A, Gourcerol D, Devos P, Ouatas T, Lafitte JJ, Copin MC, Tonnel AB, Lassalle P. Endocan expression and relationship with survival in human non-small cell lung cancer. Clin Cancer Res. 2006;12(15):4575–82.
Zuo L, Zhang SM, Hu RL, Zhu HQ, Zhou Q, Gui SY, Wu Q, Wang Y. Correlation between expression and differentiation of endocan in colorectal cancer. World J Gastroenterol. 2008;14(28):4562–8.
Leroy X, Aubert S, Zini L, Franquet H, Kervoaze G, Villers A, Delehedde M, Copin MC, Lassalle P. Vascular endocan (ESM-1) is markedly overexpressed in clear cell renal cell carcinoma. Histopathology. 2010;56(2):180–7.
Liu N, Zhang LH, Du H, Hu Y, Zhang GG, Wang XH, Li JY, Ji JF. Overexpression of endothelial cell specific molecule-1 (ESM-1) in gastric cancer. Ann Surg Oncol. 2010;17(10):2628–39.
Chen LY, Liu X, Wang SL, Qin CY. Over-expression of the Endocan gene in endothelial cells from hepatocellular carcinoma is associated with angiogenesis and tumour invasion. J Int Med Res. 2010;38(2):498–510.
Cornelius A, Cortet-Rudelli C, Assaker R, Kerdraon O, Gevaert MH, Prevot V, Lassalle P, Trouillas J, Delehedde M, Maurage CA. Endothelial expression of endocan is strongly associated with tumor progression in pituitary adenoma. Brain Pathol. 2012;22(6):757–64.
El Behery MM, Seksaka MA, Ibrahiem MA, Saleh HS, El Alfy Y. Clinicopathological correlation of endocan expression and survival in epithelial ovarian cancer. Arch Gynecol Obstet. 2013;288(6):1371–6.
Atukeren P, Kunbaz A, Turk O, Kemerdere R, Ulu MO, Turkmen Inanir N, Tanriverdi T. Expressions of endocan in patients with meningiomas and gliomas. Dis Markers. 2016;2016:7157039.
Ji NY, Kim YH, Jang YJ, Kang YH, Lee CI, Kim JW, Yeom YI, Chun HK, Choi YH, Kim JH, et al. Identification of endothelial cell-specific molecule-1 as a potential serum marker for colorectal cancer. Cancer Sci. 2010;101(10):2248–53.
Kang YH, Ji NY, Lee CI, Lee HG, Kim JW, Yeom YI, Kim DG, Yoon SK, Kim JW, Park PJ, et al. ESM-1 silencing decreased cell survival, migration, and invasion and modulated cell cycle progression in hepatocellular carcinoma. Amino Acids. 2011;40(3):1003–13.
Lv Z, Fan Y, Chen H, Zhao D. Endothelial cell-specific molecule-1: a potential serum marker for gastric cancer. Tumour Biol. 2014;35(10):10497–502.
Jiang H, Fu XG, Chen YT. Serum level of endothelial cell-specific molecule-1 and prognosis of colorectal cancer. Genet Mol Res. 2015;14(2):5519–26.
Sagara A, Igarashi K, Otsuka M, Kodama A, Yamashita M, Sugiura R, Karasawa T, Arakawa K, Narita M, Kuzumaki N, et al. Endocan as a prognostic biomarker of triple-negative breast cancer. Breast Cancer Res Treat. 2017;161(2):269–78.
Cancer Genome Atlas Research N, Weinstein JN, Collisson EA, Mills GB, Shaw KR, Ozenberger BA, Ellrott K, Shmulevich I, Sander C, Stuart JM. The Cancer Genome Atlas Pan-Cancer analysis project. Nat Genet. 2013;45(10):1113–20.
Raney BJ, Cline MS, Rosenbloom KR, Dreszer TR, Learned K, Barber GP, Meyer LR, Sloan CA, Malladi VS, Roskin KM, et al. ENCODE whole-genome data in the UCSC genome browser (2011 update). Nucleic Acids Res. 2011;39(Database issue):D871–5.
Mermel CH, Schumacher SE, Hill B, Meyerson ML, Beroukhim R, Getz G. GISTIC2.0 facilitates sensitive and confident localization of the targets of focal somatic copy-number alteration in human cancers. Genome Biol. 2011;12(4):R41.
Wan Q, Dingerdissen H, Fan Y, Gulzar N, Pan Y, Wu TJ, Yan C, Zhang H, Mazumder R. BioXpress: an integrated RNA-seq-derived gene expression database for pan-cancer analysis. Database (Oxford). 2015;2015:bav019.
Heinz S, Benner C, Spann N, Bertolino E, Lin YC, Laslo P, Cheng JX, Murre C, Singh H, Glass CK. Simple combinations of lineage-determining transcription factors prime cis-regulatory elements required for macrophage and B cell identities. Mol Cell. 2010;38(4):576–89.
Bechard D, Scherpereel A, Hammad H, Gentina T, Tsicopoulos A, Aumercier M, Pestel J, Dessaint JP, Tonnel AB, Lassalle P. Human endothelial-cell specific molecule-1 binds directly to the integrin CD11a/CD18 (LFA-1) and blocks binding to intercellular adhesion molecule-1. J Immunol. 2001;167(6):3099–106.
Chen C, Shin JH, Eggold JT, Chung MK, Zhang LH, Lee J, Sunwoo JB. ESM1 mediates NGFR-induced invasion and metastasis in murine oral squamous cell carcinoma. Oncotarget. 2016;7(43):70738–49.
Tsai JC, Zhang J, Minami T, Voland C, Zhao S, Yi X, Lassalle P, Oettgen P, Aird WC. Cloning and characterization of the human lung endothelial-cell-specific molecule-1 promoter. J Vasc Res. 2002;39(2):148–59.
Hess J, Angel P, Schorpp-Kistner M. AP-1 subunits: quarrel and harmony among siblings. J Cell Sci. 2004;117(Pt 25):5965–73.
Du Y, Peyser ND, Grandis JR. Integration of molecular targeted therapy with radiation in head and neck cancer. Pharmacol Ther. 2014;142(1):88–98.
Hasegawa Y, Abe M, Yamazaki T, Niizeki O, Shiiba K, Sasaki I, Sato Y. Transcriptional regulation of human angiopoietin-2 by transcription factor Ets-1. Biochem Biophys Res Commun. 2004;316(1):52–8.
Ye FC, Blackbourn DJ, Mengel M, Xie JP, Qian LW, Greene W, Yeh IT, Graham D, Gao SJ. Kaposi’s sarcoma-associated herpesvirus promotes angiogenesis by inducing angiopoietin-2 expression via AP-1 and Ets1. J Virol. 2007;81(8):3980–91.
Pergolizzi M, Bizzozero L, Riccitelli E, Pascal D, Samarelli AV, Bussolino F, Arese M. Modulation of Angiopoietin 2 release from endothelial cells and angiogenesis by the synaptic protein Neuroligin 2. Biochem Biophys Res Commun. 2018;501(1):165–71.
Fukumura D, Kloepper J, Amoozgar Z, Duda DG, Jain RK. Enhancing cancer immunotherapy using antiangiogenics: opportunities and challenges. Nat Rev Clin Oncol. 2018;15(5):325–40.
Thurston G, Daly C. The complex role of angiopoietin-2 in the angiopoietin-tie signaling pathway. Cold Spring Harb Perspect Med. 2012;2(9):a006550.
Fagiani E, Christofori G. Angiopoietins in angiogenesis. Cancer Lett. 2013;328(1):18–26.
Fiedler U, Reiss Y, Scharpfenecker M, Grunow V, Koidl S, Thurston G, Gale NW, Witzenrath M, Rosseau S, Suttorp N, et al. Angiopoietin-2 sensitizes endothelial cells to TNF-alpha and has a crucial role in the induction of inflammation. Nat Med. 2006;12(2):235–9.
Authors’ contributions
ZH provided the histological specimen and interpreted the patient data regarding the head and neck cancer patients. XC performed the immunohistochemistry examination, and HX was an experimental designer and a major contributor in writing the manuscript. All authors read and approved the final manuscript.
Acknowledgements
We thank Xena public data hubs (http://xena.ucsc.edu/) for providing such a rich and useful resource of analyzed TCGA data. We also thank Pingzhang Wang at the Department of Immunology, Peking University for guidance about bioinformatics analysis in the study.
Competing interests
The authors declare that they have no competing interests.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Consent for publication
All authors agree to publish the paper in your journal.
Ethics approval and consent to participate
Not applicable.
Funding
This research was supported by National Natural Science Foundation of China (No.81670946) and Beijing Municipal Administration of Hospitals Clinical Medicine Development of Special Funding Support (code. XMLX201507) and Beijing Tongren Hospital, Capital Medical University scientific research backbone fund (2015-YJJ-GGL-002).
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Author information
Authors and Affiliations
Corresponding authors
Additional file
Additional file 1.
ESM1 expression of 21 paired samples of HNSCC with clinical and pathological features.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Xu, H., Chen, X. & Huang, Z. Identification of ESM1 overexpressed in head and neck squamous cell carcinoma. Cancer Cell Int 19, 118 (2019). https://doi.org/10.1186/s12935-019-0833-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12935-019-0833-y