Abstract
Parkinson’s disease (PD) is the second most prevalent neurodegenerative disease, and in an effort to identify novel therapeutic target for this disease in recent years, human microbiota has attracted much interest. This paper briefly summarizes the main findings concerning the differences of human microbiome across several important mucosal interfaces, including nose, mouth, and gut between PD patients and controls as obtained from a total of 13 studies published since 2015, which covered a total of 943 PD patients and 831 matched controls from 6 countries. Overall, these studies supported the differences of gut microbiota between PD patients and matched controls, while significantly altered bacterial taxa among studies were not identical. Due to relatively limited number of available studies and covered patients, the associations between oral and nasal microbiota and PD remain inconclusive. The therapeutic and diagnostic potentials of gut microbiota for PD are discussed. More well-designed clinical studies recruiting large-scale PD patients are encouraged in future.
Similar content being viewed by others
Introduction
Parkinson’s disease (PD) is the second most prevalent neurodegenerative disease in the elderly after Alzheimer’s disease. PD is estimated to affect about 1% of populations over the age of 60 [1]. Clinically, PD is characterized primarily by severe and progressing tremors, rigidity, posture instability, and cognitive impairment. Neuropathologically, the hallmarks of PD mainly include the progressive degeneration of dopaminergic nigrostriatal neurons and the formation of aggregated α-synuclein, called Lewy bodies, in the brain [2, 3]. Despite much progress has been made to understand the genetic and environmental factors contributing to PD development in the past decades, the pathogenesis of PD remains far to be fully elucidated [4, 5]. Currently, available drugs for PD are symptomatic, and disease progression is inexorable, and patients will ultimately suffer from disability. As a result, identifying novel targets to develop new agents to combat PD has gained much interest.
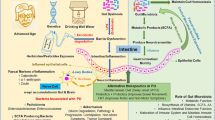
In recent years, the association between human gut microbiota, which comprises approximately 1014 microbes, and PD development has sparked increasing attentions [5,6,7,8,9]. Gut microbiota has been proposed to be a potential therapeutic target and also has diagnostic biomarker potential. In addition, as nasal and oral cavities constitute two important ports of entry for a possible pathogenic agent spreading to the central nervous system, which may be involved in the pathogenesis of PD, the oral and nasal microbiota of PD patients have been investigated [10,11,12]. This paper summarizes the recent literature on the differences of human microbiome across several important mucosal interfaces, including nose, mouth, and gut, between PD patients and controls, and their therapeutic and diagnostic potentials.
Summary of included studies
Through retrieving the PubMed database, a total of 13 eligible studies, which were published between 2015 and 2018, are included in the present review (Table 1). The 13 studies were conducted in 6 countries (4 studies from Germany, 3 from China, 3 from USA, 2 from Finland, 1 from Russia and 1 from Japan, respectively). These studies cover 943 PD patients and 831 controls. The samples range from 38 to 327 cases and controls combined. The 13 studies report 16 sets of microbiota data, 13 are about gut microbiota, 2 are about nasal microbiota, and 1 is about oral microbiota (Fig. 1).
Gut microbiota
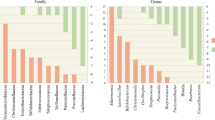
There are a total of 13 studies focusing on the gut microbiota of 726 PD patients. The first study was conducted by Scheperjans et al. which compared the gut microbiota of 72 Finnish PD patients and 72 controls by means of 16S rRNA gene amplicon sequencing [8]. The altered gut microbiota of PD patients was demonstrated. A significant reduction by 77.6% of the relative abundance of Prevotellaceae in the feces of PD patients compared with controls, and the relative abundance of Enterobacteriaceae was identified to be positively associated with the severity of postural instability and gait difficulty [8]. Keshavarzian et al. investigated the colonic bacterial composition of 38 American PD patients and 34 controls by means of 16S rRNA gene amplicon sequencing [13]. Significant difference was observed between the fecal microbiota of PD patients and controls. Further analysis indicated significantly higher relative abundances of some butyrate-producing bacteria, which included genera Blautia, Coprococcus and Roseburia, in the feces of controls in comparison with PD patients [13]. Hasegawa et al. analyzed the gut microbiota of 52 Japanese PD patients and compared with those of 36 controls by employing rRNA-targeted reverse transcription-quantitative PCR [14]. It was found that the abundances of Clostridium coccoides and Bacteroides fragilis decreased, while that of Lactobacillus increased in the gut microbiota of PD patients in comparison with controls. In 2016, Unger et al. reported a comparative analysis of the gut microbiota of 34 German PD patients and 34 age-matched controls by means of quantitative PCR [9]. It was indicated that the abundances of Bacteroidetes and Prevotellaceae decreased, while that of Enterobacteriaceae was enriched in the gut microbiota of PD patients in comparison with controls. In 2017, Li et al. conducted a comparative analysis of the gut microbiota of 24 Chinese patients and 14 healthy controls by 16S rRNA gene amplicon sequencing [15]. It was revealed that the relative abundances of cellulose degraders like Blautia, Faecalibacterium and Ruminococcus significantly decreased, and those of pathobionts, including Escherichia-Shigella, Streptococcus, Proteus, and Enterococcus, significantly increased in PD subjects compared with healthy controls. Hopfner et al. analyzed the gut microbiota of 29 German PD patients and 29 age-matched controls by 16S rRNA gene amplicon sequencing [16]. It was reported that there was significant difference in beta diversity indices between PD patients and controls, while no significant difference was observed for alpha diversity indices. Lactobacillaceae, Barnesiellaceae and Enterococcacea were found to be more abundant in patients than in controls. The gut microbiota analysis by Bedarf et al. covered 31 early stage, l-DOPA-naïve PD patients and 28 age-matched controls from Germany. Significant difference was observed for the gut microbiota composition between PD patients and controls at all taxonomic levels [17]. It was indicated that PD patients possessed increased abundances of Errucomicrobiaceae (Akkermansia muciniphila) and unclassified Firmicutes, while reduced abundances of Prevotellaceae (Prevotella copri) and Erysipelotrichaceae (Eubacterium biforme). In the study of Hill-Burns et al. relatively larger samples (197 American PD patients and 130 controls) were covered [18]. By 16S rRNA gene amplicon sequencing, it was found that the abundances of Bifidobacteriaceae, Christensenellaceae, [Tissierellaceae], Lachnospiraceae, Lactobacillaceae, Pasteurellaceae and Verrucomicrobiaceae differed significantly between PD patients and controls. Petrov et al. compared the gut microbiota of 89 Russian PD patients and 66 controls by means of 16S rRNA gene amplicon sequencing [19]. It was indicated that the gut microbiota diversity reduced in PD patients in comparison with controls. The abundances of 14 bacterial taxa Dorea, Bacteroides, Prevotella, Faecalibacterium, Bacteroides massiliensis, Stoquefichus massiliensis, Bacteroides coprocola, Blautia glucerasea, Dorea longicatena, Bacteroides dorei, Bacteroides plebeus, Prevotella copri, Coprococcus eutactus, and Ruminococcus callidus decreased, while those of Christensenella, Catabacter, Lactobacillus, Oscillospira, Bifidobacterium, Christensenella minuta, Catabacter hongkongensis, Lactobacillus mucosae, Ruminococcus bromii, and Papillibacter cinnamivorans increased in PD patients in comparison with controls. In 2018, Qian et al. investigated the differences in gut microbiota between 45 Chinese PD patients and their healthy spouses by means of 16S rRNA gene amplicon sequencing [20]. It was indicated that the richness and diversity of the gut microbiota in PD patients were significantly higher compared with those of control group. Several enriched genera were identified in the feces of PD patients, which include Clostridium IV, Aquabacterium, Holdemania, Sphingomonas, Clostridium XVIII, Butyricicoccus and Anaerotruncus. The genera Escherichia/Shigella were found to be negatively associated with disease duration. Lin et al. investigated the gut microbiota of 75 Chinese PD patients and 45 age-matched controls by means of 16S rRNA gene amplicon sequencing [21]. It was found the alpha and beta diversity between PD patients and controls did not differ significantly. The abundances of four bacterial families significantly increased and those of seventeen ones decreased in PD patients in comparison with controls. Heintz-Buschart et al. compared the gut microbiota of 76 PD patients and 78 matched healthy individuals by means of 16S and 18S rRNA gene amplicon sequencing [22]. They demonstrated that PD patients possessed significantly increased abundance of Akkermansia sp. and Prevotella sp. in gut microbiota compared with healthy controls. In addition, Tetz et al. analyzed the gut microbiota of 31 American PD patients and 28 controls by means of shotgun metagenomics sequencing [23]. A depletion of Prevotellaceae and Lachnospiraceae and decreased abundances of Lactobacillaceae and Streptococcaceae in the feces of PD patients in comparison with the controls were found.
Oral and nasal microbiota
The oral and nasal microbiota of PD patients has gained growing attentions and been investigated by three studies. Pereira et al. investigated the oral microbiota of 72 Finnish PD patients and 76 controls employing 16S rRNA gene amplicon sequencing [12]. Through comparative analysis, significant difference in beta diversity of oral microbiota was found between PD patients and control groups. Further analysis identified the increased abundances of Prevotella, Prevotellaceae, Veillonella, Solobacterium, Veillonellaceae, Lactobacillaceae, and Coriobacteriaceae, and decreased abundances of Capnocytophaga, Rothia, Kingella, Leptotrichia, Actinomyces, and Leptotrichiaceae, in oral microbiota of PD patients compared with controls.
As to nasal microbiota, the study of Pereira et al. also compared the nasal microbiota of 69 PD patients and 67 controls using 16S rRNA gene amplicon sequencing [12]. It was indicated that no alpha or beta differences existed between the nasal microbiota of PD patients and control groups. In addition, through 16S and 18S rRNA gene amplicon sequencing, Heintz-Buschart et al. analyzed the microbiota of nasal wash samples from 76 Russian PD patients and 78 matched healthy controls, and they found no strong differences in nasal microbiota between PD patients and controls [22].
Conclusions and perspectives
The associations between PD and human microbiome across several important mucosal interfaces, including nose, mouth, and gut, have sparked much interest in recent years [23,24,25,26]. According to the above discussion, the currently available studies support the alterations in gut microbiota in PD patients compared with controls. Nevertheless, despite a few significantly differed bacterial taxa are common in selected studies, the altered bacterial taxa reported in each study was not completely consistent overall. This may derive from the facts that these studies differed in PD patient inclusion criteria, severity of disease, sequencing methodologies, and the treatment of confounders. As to oral microbiota, there is only one study on this issue and found differed beta diversity and some bacterial taxa between patients and controls. Two studies have explored the nasal microbiota, and both indicated no obvious differences in nasal microbiota between PD patients and controls. However, currently, we cannot give an affirmative and negative conclusion concerning the association between oral/nasal microbiota and PD due to the rather limited number of studies and patients.
There should be multiple molecular mechanisms underlying the association between gut microbiota and PD. As one main metabolic product of gut bacteria, the concentrations of short chain fatty acids (SCFAs) were observed to be altered accompanied by altered gut microbiota composition in several studies. Several studies have found less SCFA butyrate-producing bacteria in the feces of PD patients [9, 13], while it has been suggested that decreased levels of SCFAs might decrease colonic motility, and also elevate the gut barrier leakiness [27, 28]. In addition, several studies have identified the decreased abundance of Prevotellaceae [8, 9, 17, 19, 23]. It was inferred that decreased Prevotellaceae levels could decrease mucin synthesis, and resulted in increased gut permeability. Exposure to bacterial endotoxin (e.g., lipopolysaccharide) caused by increased gut permeability could induce excessive expression and aggregation of α-synuclein, which is crucial in PD development [29,30,31].
Several animal studies have provided further insights into the association between gut microbiota dysbiosis and pathogenesis of PD. Employing the α-synuclein overexpressing mice model of PD, Sampson et al. found the important role of gut microbiota for motor deficits, microglia activation, and α-synuclein pathology [24]. This was supported by the interesting findings that oral gavaging with specific microbial metabolites to germ-free mice promoted neuroinflammation and motor symptoms, and colonization with microbiota from PD patients could enhance physical impairments in α-synuclein-overexpressing mice [24]. Yang et al. reported that oral administration of rotenone led to gastrointestinal dysfunction and microbiome dysbiosis prior to motor dysfunction of mice model of PD induced by rotenone, and gut microbiota dysbiosis might contribute to rotenone toxicity in PD initiation [26]. Similarly, Perez-Pardo et al. also revealed that the gut microbiota of mice model of PD induced by rotenone was characterized by a significant decrease in the relative abundance of the genus Bifidobacterium, and gut microbiota dysbiosis might play an important role in the disruption of intestinal epithelial integrity as well as intestinal inflammation, which are potentially associated with PD pathology [25].
The following aspects may deserve attentions in future studies. First, most studies discussed above employed 16S rRNA gene amplicon sequencing, and only one used the shotgun metagenomics sequencing during gut microbiota analysis. As we know, 16S rRNA gene amplicon sequencing may be biased owing to unequal amplification of species’ 16S rRNA genes, and is not deep enough to detect all species. Second, the number of samples in some included studies are relatively small, and the inclusion criteria of PD patients varies among the included studies. Thus, more studies recruiting large-scale patients with new generation sequencing methodology, are encouraged to investigate the association between human microbiota and PD. Third, several clinical studies have indicated the benefits of supplying probiotics or in combination with prebiotics for PD [32, 33]. For instance, Barichella et al. conducted a randomized, double-blind, placebo-controlled trial and it was found that consumption of fermented milk containing probiotics and prebiotics could increase the frequency of complete bowel movements in PD patients with constipation [33]. Future studies are warranted to verify and optimize the efficacy of gut microbiota-modulation based strategy against PD. Forth, a certain degree of consistency of microbiota along the gastrointestinal tract has been observed and an individual’s salivary microbiota was found to share some similarity with gut microbiota of the same individual [34]. In addition, the oral bacterial strain is proven to colonize in the gut, which is involved in the disease pathogenesis [35]. This suggests that the oral microbiota should also be considered to understand the “gut-brain axis” [36]. Fifth, although the alterations of gut microbiota in PD patients in comparison with controls have been demonstrated by a series of studies, and several pathways have been proposed to be involved, including the initiation of α-synuclein pathology in the gut, microbial products initiating inflammation and oxidative stress in the brain [30, 37,38,39], the relationship between gut microbiota and PD still remains to be fully elucidated. This will benefit from better understanding of the molecular basis underlying the “gut-brain axis”. In addition, as the number of studies concerning the relationships between gut microbiota and PD is relatively limited, we can also obtain larger sets of microbiota data to gain implications from the studies on gut microbiota and other neurodegenerative diseases with similar pathogenesis to PD, such as Alzheimer’s disease and amyotrophic lateral sclerosis in future.
In summary, in view of the great potential of gut, oral and nasal microbiota as diagnostically biomarker, and the therapeutic potential of gut microbiota, more well-designed clinical studies recruiting large-scale patients are encouraged on these issues under the condition of steadily increasing prevalence and lack of effective treatment options of PD.
Availability of data and materials
Not applicable.
Abbreviations
- PD:
-
Parkinson’s disease
- SCFAs:
-
Short chain fatty acids
References
Bekris LM, Mata IF, Zabetian CP. The genetics of Parkinson disease. J Geriatr Psychiatry Neurol. 2010;23:228–42.
Obeso JA, Stamelou M, Goetz CG, Poewe W, Lang AE, Weintraub D, Burn D, Halliday GM, Bezard E, Przedborski S, Lehericy S, Brooks DJ, Rothwell JC, Hallett M, DeLong MR, Marras C, Tanner CM, Ross GW, Langston JW, Klein C, Bonifati V, Jankovic J, Lozano AM, Deuschl G, Bergman H, Tolosa E, Rodriguez-Violante M, Fahn S, Postuma RB, Berg D, Marek K, Standaert DG, Surmeier DJ, Olanow CW, Kordower JH, Calabresi P, Schapira AHV, Stoessl AJ. Past, present, and future of parkinson’s disease: a special essay on the 200th anniversary of the shaking palsy. Mov Disord. 2017;32:1264–310.
Aarsland D, Creese B, Politis M, Chaudhuri KR, Ffytche DH, Weintraub D, Ballard C. Cognitive decline in Parkinson disease. Nat Rev Neurol. 2017;13:217–31.
Olanow CW, Tatton WG. Etiology and pathogenesis of Parkinson’s disease. Annu Rev Neurosci. 1999;22:123–44.
Edwards LL, Quigley EM, Pfeiffer RF. Gastrointestinal dysfunction in Parkinson’s disease: frequency and pathophysiology. Neurology. 1992;42:726–32.
Wang Y, Kasper LH. The role of microbiome in central nervous system disorders. Brain Behav Immun. 2014;38:1–12.
Scheperjans F, Derkinderen P, Borghammer P. The gut and Parkinson’s disease: hype or hope? J Parkinsons Dis. 2018;8:S31–9.
Scheperjans F, Aho V, Pereira PA, Koskinen K, Paulin L, Pekkonen E, Haapaniemi E, Kaakkola S, Eerola-Rautio J, Pohja M, Kinnunen E, Murros K, Auvinen P. Gut microbiota are related to Parkinson’s disease and clinical phenotype. Mov Disord. 2015;30:350–8.
Unger MM, Spiegel J, Dillmann KU, Grundmann D, Philippeit H, Bürmann J, Faßbender K, Schwiertz A, Schäfer KH. Short chain fatty acids and gut microbiota differ between patients with Parkinson’s disease and age-matched controls. Parkinsonism Relat Disord. 2016;32:66–72.
Müller T, Palluch R, Jackowski J. Caries and periodontal disease in patients with Parkinson’s disease. Spec Care Dentist. 2011;31:178–81.
Rey NL, Petit GH, Bousset L, Melki R, Brundin P. Transfer of human α-synuclein from the olfactory bulb to interconnected brain regions in mice. Acta Neuropathol. 2013;126:555–73.
Pereira PAB, Aho VTE, Paulin L, Pekkonen E, Auvinen P, Scheperjans F. Oral and nasal microbiota in Parkinson’s disease. Parkinsonism Relat Disord. 2017;38:61–7.
Keshavarzian A, Green SJ, Engen PA, Voigt RM, Naqib A, Forsyth CB, Mutlu E, Shannon KM. Colonic bacterial composition in Parkinson’s disease. Mov Disord. 2015;30:1351–60.
Hasegawa S, Goto S, Tsuji H, Okuno T, Asahara T, Nomoto K, Shibata A, Fujisawa Y, Minato T, Okamoto A, Ohno K, Hirayama M. Intestinal dysbiosis and lowered serum lipopolysaccharide-binding protein in Parkinson’s disease. PLoS ONE. 2015;10:e0142164.
Li W, Wu X, Hu X, Wang T, Liang S, Duan Y, Jin F, Qin B. Structural changes of gut microbiota in Parkinson’s disease and its correlation with clinical features. Sci China Life Sci. 2017;60:1223–33.
Hopfner F, Künstner A, Müller SH, Künzel S, Zeuner KE, Margraf NG, Deuschl G, Baines JF, Kuhlenbäumer G. Gut microbiota in Parkinson disease in a northern German cohort. Brain Res. 2017;1667:41–5.
Bedarf JR, Hildebrand F, Coelho LP, Sunagawa S, Bahram M, Goeser F, Bork P, Wüllner U. Functional implications of microbial and viral gut metagenome changes in early stage L-DOPA-naïve Parkinson’s disease patients. Genome Med. 2017;9:39.
Hill-Burns EM, Debelius JW, Morton JT, Wissemann WT, Lewis MR, Wallen ZD, Peddada SD, Factor SA, Molho E, Zabetian CP, Knight R, Payami H. Parkinson’s disease and PD medications have distinct signatures of the gut microbiome. Mov Disord. 2017;32:739–49.
Petrov VA, Saltykova IV, Zhukova IA, Alifirova VM, Zhukova NG, Dorofeeva YB, Tyakht AV, Kovarsky BA, Alekseev DG, Kostryukova ES, Mironova YS, Izhboldina OP, Nikitina MA, Perevozchikova TV, Fait EA, Babenko VV, Vakhitova MT, Govorun VM, Sazonov AE. Analysis of gut microbiota in patients with Parkinson’s disease. Bull Exp Biol Med. 2017;162:734–7.
Qian Y, Yang X, Xu S, Wu C, Song Y, Qin N, Chen SD, Xiao Q. Alteration of the fecal microbiota in Chinese patients with Parkinson’s disease. Brain Behav Immun. 2018;70:194–202.
Lin A, Zheng W, He Y, Tang W, Wei X, He R, Huang W, Su Y, Huang Y, Zhou H, Xie H. Gut microbiota in patients with Parkinson’s disease in southern China. Parkinsonism Relat Disord. 2018;53:82–8.
Heintz-Buschart A, Pandey U, Wicke T, Sixel-Döring F, Janzen A, Sittig-Wiegand E, Trenkwalder C, Oertel WH, Mollenhauer B, Wilmes P. The nasal and gut microbiome in Parkinson’s disease and idiopathic rapid eye movement sleep behavior disorder. Mov Disord. 2018;33:88–98.
Tetz G, Brown SM, Hao Y, Tetz V. Parkinson’s disease and bacteriophages as its overlooked contributors. Sci Rep. 2018;8:10812.
Sampson TR, Debelius JW, Thron T, Janssen S, Shastri GG, Ilhan ZE, Challis C, Schretter CE, Rocha S, Gradinaru V, Chesselet MF, Keshavarzian A, Shannon KM, Krajmalnik-Brown R, Wittung-Stafshede P, Knight R, Mazmanian SK. Gut microbiota regulate motor deficits and neuroinflammation in a model of Parkinson’s disease. Cell. 2016;167:1469–80.
Perez-Pardo P, Dodiya HB, Engen PA, Naqib A, Forsyth CB, Green SJ, Garssen J, Keshavarzian A, Kraneveld AD. Gut bacterial composition in a mouse model of Parkinson’s disease. Benef Microbes. 2018;9:799–814.
Yang X, Qian Y, Xu S, Song Y, Xiao Q. Longitudinal analysis of fecal microbiome and pathologic processes in a rotenone induced mice model of Parkinson’s disease. Front Aging Neurosci. 2018;9:441.
Tazoe H, Otomo Y, Kaji I, Tanaka R, Karaki SI, Kuwahara A. Roles of short-chain fatty acids receptors, GPR41 and GPR43 on colonic functions. J Physiol Pharmacol. 2008;59(Suppl 2):251–62.
Soret R, Chevalier J, De Coppet P, Poupeau G, Derkinderen P, Segain JP, Neunlist M. Short-chain fatty acids regulate the enteric neurons and control gastrointestinal motility in rats. Gastroenterology. 2010;138:1772–82.
Koopman H, Jackson S, Anichtchik O, Carroll C. Lps induces aggregation of α-synuclein in monocytes. J Neurol Neurosur PS. 2015;86:e4.188–e4.
Forsyth CB, Shannon KM, Kordower JH, Voigt RM, Shaikh M, Jaglin JA, Estes JD, Dodiya HB, Keshavarzian A. Increased intestinal permeability correlates with sigmoid mucosa alpha-synuclein staining and endotoxin exposure markers in early Parkinson’s disease. PLoS ONE. 2011;6:e28032.
Sergeyeva TN, Sergeyev VG. Administration of LPS-stimulated autologous macrophages induces α-synuclein aggregation in dopaminergic neurons of rat brain. Bull Exp Biol Med. 2011;150:406–8.
Cassani E, Privitera G, Pezzoli G, Pusani C, Madio C, Iorio L, Barichella M. Use of probiotics for the treatment of constipation in Parkinson’s disease patients. Minerva Gastroenterol Dietol. 2011;57:117–21.
Barichella M, Pacchetti C, Bolliri C, Cassani E, Iorio L, Pusani C, Pinelli G, Privitera G, Cesari I, Faierman SA, Caccialanza R, Pezzoli G, Cereda E. Probiotics and prebiotic fiber for constipation associated with Parkinson disease: an RCT. Neurology. 2016;87:1274–80.
Ding T, Schloss PD. Dynamics and associations of microbial community types across the human body. Nature. 2014;509:357–60.
Atarashi K, Suda W, Luo C, Kawaguchi T, Motoo I, Narushima S, Kiguchi Y, Yasuma K, Watanabe E, Tanoue T, Thaiss CA, Sato M, Toyooka K, Said HS, Yamagami H, Rice SA, Gevers D, Johnson RC, Segre JA, Chen K, Kolls JK, Elinav E, Morita H, Xavier RJ, Hattori M, Honda K. Ectopic colonization of oral bacteria in the intestine drives TH1 cell induction and inflammation. Science. 2017;358:359–65.
Mulak A, Bonaz B. Brain-gut-microbiota axis in Parkinson’s disease. World J Gastroenterol. 2015;21:10609–20.
Shannon KM, Keshavarzian A, Dodiya HB, Jakate S, Kordower JH. Is alpha-synuclein in the colon a biomarker for premotor Parkinson’s disease? Evidence from 3 cases. Mov Disord. 2012;27:716–9.
Holmqvist S, Chutna O, Bousset L, Aldrin-Kirk P, Li W, Björklund T, Wang ZY, Roybon L, Melki R, Li JY. Direct evidence of Parkinson pathology spread from the gastrointestinal tract to the brain in rats. Acta Neuropathol. 2014;128:805–20.
Houser MC, Tansey MG. The gut-brain axis: is intestinal inflammation a silent driver of Parkinson’s disease pathogenesis? NPJ Parkinsons Dis. 2017;3:3.
Acknowledgements
This work was supported by the Shandong Provincial Natural Science Foundation (Grant No. ZR2018MH010), University Youth Innovation Team of Shandong Province (Grant No. 2019KJK017), and Shandong Provincial Key Research and Development Program (Grant No. 2018GSF121001).
Funding
This work was supported by the Shandong Provincial Natural Science Foundation (Grant No. ZR2018MH010), University Youth Innovation Team of Shandong Province (Grant No. 2019KJK017), and Shandong Provincial Key Research and Development Program (Grant No. 2018GSF121001).
Author information
Authors and Affiliations
Contributions
LS designed the study, collected the data and wrote the manuscript. The author read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Yes.
Competing interests
The author declares no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Shen, L. Gut, oral and nasal microbiota and Parkinson’s disease. Microb Cell Fact 19, 50 (2020). https://doi.org/10.1186/s12934-020-01313-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12934-020-01313-4