Abstract
Advanced research in health science has broadened our view in approaching and understanding the pathophysiology of diseases and has also revolutionised diagnosis and treatment. Ever since the establishment of Braak’s hypothesis in the propagation of alpha-synuclein from the distant olfactory and enteric nervous system towards the brain in Parkinson’s Disease (PD), studies have explored and revealed the involvement of altered gut microbiota in PD. This review recapitulates the gut microbiome associated with PD severity, duration, motor and non-motor symptoms, and antiparkinsonian treatment from recent literature. Gut microbial signatures in PD are potential predictors of the disease and are speculated to be used in early diagnosis and treatment. In brief, the review also emphasises on implications of the prebiotic, probiotic, faecal microbiota transplantation, and dietary interventions as alternative treatments in modulating the disease symptoms in PD.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Parkinson’s disease (PD) is the second most common neurodegenerative disorder, Alzheimer’s disease being the most common. PD afflicts approximately ten million people globally (Statistics | Parkinson’s Foundation 2021; Kaur et al. 2019). PD’s prevalence and incidence increase with the advancing age, and the incident rate is higher in men (Statistics | Parkinson’s Foundation 2021). It has been two centuries in the wake of a prologue to PD with an observational essay on the ‘Shaking Palsy’ by James Parkinson (Parkinson 2002; Jost and Reichmann 2017), the investigation to identify the trigger that prompts the disease is at its prime. However, since Lewy discovered the eosinophilic inclusion body and established the involvement of alpha-synucleinopathy, researchers have uncovered most aspects of PD pathophysiology and have described it as a multifactorial disease. Environmental factors, exposure to chemicals (insecticides, pesticides), xenobiotic toxins, genetic predisposition, altered dopamine metabolism, mitochondrial dysfunction, oxidative stress, neuroinflammation, and aging play a crucial interactive role in the pathogenesis of PD (Riess and Krüger 1999; Kaur et al. 2019; Pang et al. 2019).
PD’s key cardinal motor features include resting tremors, rigidity, slowness of movement, and gait disturbance (Kasper et al. 2014). Most PD patients start experiencing non-motor symptoms (NMS) markedly decades before the motor symptom surfaces (Poewe 2008; Heintz-Buschart et al. 2018). Gastrointestinal (GI) symptoms such as dry mouth, constipation, and defecatory dysfunction are highly reported NMS prior to the onset of motor symptoms (Cersosimo et al. 2013). Most of these NMS are overlooked during this prodromal stage. Treatment for PD is initiated only when the motor symptoms appear; by then, more than fifty percent of the dopaminergic neuron might have degenerated in the substantia nigra (Cheng et al. 2010).
Recent advancements in research demonstrated gut dysbiosis could initiate the neurodegenerative process in PD and serve as potential biomarkers for diagnostics and treatment modulation (Keshavarzian et al. 2015; Scheperjans et al. 2015). This review is structured to address the involvement of gut microbiota and its metabolites associated with intestinal integrity, genetic predisposition, motor and non-motor aspects, antiparkinsonian treatment, and diet in PD by considering the available global data.
Alpha-synucleinopathy
Alpha-synuclein (α-syn) is a monomeric protein of 140 amino acids encoded by the SNCA (synuclein alpha) gene (Stefanis 2012). α-syn is found in small quantities in the heart, muscles, and other different tissues; however, it is abundantly found in the brain, especially in the presynaptic terminal tips of the neurons (Moons et al. 2020). In neurons, α-syn plays a role by inhibiting the release of neurotransmitters when over-expressed (Bendor et al. 2013). α-synucleinopathy is the confirmational change of the soluble protein α-syn into a pathological oligomeric beta-sheet structure (Meade et al. 2019) that loses its membrane binding capacity (Pang et al. 2019) and aggregates in the cytoplasm of neurons, glial cells, or nerve fibers, disrupting the cellular homeostasis inducing cytotoxicity (Luna and Luk 2015).
A few studies have demonstrated that α-syn can induce microglia or monocytes to produce proinflammatory cytokines (TNF-α and IL-1β) (Lee et al. 2010; Couch et al. 2011). Likewise, a study by Stolzenberg et al. reports a positive correlation between intestinal inflammation and expression of α-syn and observed α-syn to exhibit immune-modulatory or chemo-attractive properties that induce migration of neutrophils and monocytes and stimulate dendritic cells maturation (Stolzenberg et al. 2017). Hence these findings suggest α-syn is a component of the innate immune response of the GI enteric nervous system (ENS), and excessive exposure to α-syn can initiate PD (Stolzenberg et al. 2017). Immunohistochemical analysis of tissues of the spinal cord and peripheral nervous system by Beach et al. have divulged the high occurrence of phosphorylated α-syn in paraspinal sympathetic ganglia, the vagus nerve, and the GI tract of PD subjects (Beach et al. 2010). Similarly, α-syn has been demonstrated in the neurons of sigmoid mucosal samples collected two to five years before the onset of PD (Keshavarzian et al. 2015).
Holmqvist et al. verified Braak’s hypothesis by demonstrating the retrograde transfer of α-syn from the ENS to the brain in an animal experimental model. They injected different forms of α-syn obtained from human PD brain lysate and a recombinant α-syn into the intestinal walls of mice to demonstrate their transport via vagal nerve to the brain (Holmqvist et al. 2014). α-syn can directly activate microglia to upregulate Toll-like receptors (TLRs) and proinflammatory cytokines by serving as endogenous damage-associated molecular patterns (DAMPS). TLR signalling induces nuclear factor kappa B (NF-κB) activation, which is vital to dopaminergic neuron apoptosis. Microgliosis also increases nitric oxide production and further promotes α-syn pathology by inducing nitration of α-syn in neighbouring neurons and cell death (Béraud et al. 2011).
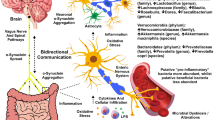
Drinking well water and exposure to herbicides and pesticides in a farm or cultivation setting have been associated with increased risk factors in PD (Fig. 1) (Gatto et al. 2009; Freire and Koifman 2012). Hill-Burns et al. reported an increased xenobiotics degradation pathway, specifically for herbicides and pesticides in the PD patient’s gut (Hill-Burns et al. 2017). An animal model also demonstrates that exposure to such compounds can cause cell death of dopaminergic neurons and lead to movement disorder in mice (Blesa et al. 2012). Forsyth et al. hypothesised that the dysbiosis and exposure to bacterial endotoxin possibly initiate α-syn misfolding by prompting GI inflammation and hyperpermeability in PD patients (Forsyth et al. 2011). Researchers have also observed that exposure to bacteria-producing amyloid protein curli can induce α-syn accumulation in the gut and brain of mice (Chen et al. 2016). In addition, exposing mice to environmental toxins such as pesticide rotenone triggered the release of α-syn of enteric neurons into the extracellular matrix. These α-syn are shown to be taken up by presynaptic neurons or other non-neuronal cells, proving the transneuronal retrograde movement of α-syn in tissue culture study (Pan-Montojo et al. 2012).
Microbiome, blood brain barrier and gut-brain communication
The microbiome comprises of bacteria, viruses, fungi, archaea, protozoa, and bacteriophages in their natural habitat. The human body is inhabited by 10–100 trillion bacterial cells, which outnumber the human cells by 1–10 folds (NIH Human Microbiome Project defines normal bacterial makeup of the body | National Institutes of Health (NIH) 2012); however, the number of bacterial and human cells are debatable. According to Sender et al., the human body contains roughly the same number of bacterial cells as human cells (i.e., the body contains 3.8 × 1013 bacteria and 3 × 1013 human cells) (Sender et al. 2016). Microbial community varies in diversity and composition at different body sites. The human gut harbours a vast majority of the microbial species encoding unique genes more than the human genome (Ley et al. 2006). Gut microbial diversity mostly remains stable in an individual through adulthood, maintaining a core microbiome unless there is a significant change in dietary habits. A dynamic shift in microbial diversity and composition is noted with debilitating immune system among the elderly as a health consequence (Dinan and Cryan 2017).
The Blood–Brain Barrier (BBB) develops during the foetal stage, and it is composed of multiple cell components: capillary endothelial cells, tightly sealed junctions, basement membrane (fibrous matrix), neuroglial membrane, glial podocytes (projection of astrocytes), and pericytes (Braniste et al. 2014), which work in concert to protect the central nervous system (CNS) by limiting the entry of various substances. Braniste et al. have defined the primary role of the gut microbiota in influencing the BBB during the early stage of development in their work on germ-free mice. This study has reported that BBB was highly permeable in germ-free mice, which continued to adulthood and had reduced tight junction protein expression compared to mice with normal intestinal microbiota (Braniste et al. 2014). Short-chain fatty acids (SCFA) neuroactive metabolites, like butyrate, a fermentation product of gut microbiota, can regulate tight junction protein expression and influence histone deacetylase activity of BBB epigenetically (Al-Asmakh and Hedin 2015; Hasan Mohajeri et al. 2018).
The bidirectional communication between the gut and the brain exists via the central vagal nerve and systemic metabolic routes (Hasan Mohajeri et al. 2018). Likewise, microbiota-gut-brain bidirectional interaction occurs through neural, endocrine, immune, and humoral networks (Carabotti et al. 2015). ENS innervates the GI system and is in close proximity to the intestinal lumen, which offers a vast area for significant interaction with microorganisms (Cosma-Grigorov et al. 2020). Faecal micro-RNA of the intestinal epithelial cells has been proposed to enter the gut microbial cells and control gene expression, eventually shaping the microbiome (Liu et al. 2016).
The influence of gut microbial colonisation on intestinal sensory-motor function has been demonstrated in experimental animal models exhibiting delayed gastric emptying and intestinal transit in germ-free mice (Carabotti et al. 2015). Furthermore, gut microbiota may impact the production of neurotransmitters like gamma-aminobutyric acid, and their metabolic by-products like butyrate promote the functioning of the nervous system (Bienenstock et al. 2015). In addition, bacterial fermentation product SCFA may control microglial by influencing its maturation and function in the CNS and maintaining homeostasis (Erny et al. 2015). Besides, bacterial SCFA-butyrate regulates the biosynthesis of serotonin (Yano et al. 2015), which is responsible for initiating peristalsis (Sikander et al. 2009), and thus gut microbiota influence GI motility (Yano et al. 2015).
Intestinal inflammation and barrier dysfunction
The intestinal mucosa is the inner lining of the GI tract. It is a physical and an immunological barrier between the environment and the internal host environs blood circulation, which is semipermeable and essential in the uptake of nutrients. It consists of an outer mucosal layer, a middle epithelial layer, and an inner lamina propria. The mucosal layer overlaying the epithelium is in close proximity to the external environment, communicates with gut microbiota, and keeps a check on pathogenic bacteria with immune sensing antimicrobial peptides and secretory IgA. A monolayer of epithelial cells with tight junction and adherens junction transport molecules and maintain the barrier’s integrity, respectively. Immune cells such as T-cells, B-cells, macrophages, and dendritic cells are in the lamina propria forming a defence barrier (Vancamelbeke and Vermeire 2017).
Intestinal bacteria and their metabolic by-products are one of the various factors contributing to the impairment of intestinal barrier function and hyperpermeability (Massier et al. 2021). Bacteria can damage the tight junction, alter the permeability and translocate through Peyer’s patches. Certain autoimmune disorders of the intestine can also trigger barrier dysfunction (Forsyth et al. 2011). Intestinal bacterial dysbiosis increases permeability and induces an intestinal inflammatory response, which activates neuroinflammation (Sun and Shen 2018). Researchers propose that TLRs are activated during dysbiosis and barrier dysfunction, which recognise bacterial lipopolysaccharides (LPS) presented by microglia and astrocytes. Thus, LPS hampers the intestinal barrier function and activates different immune cells, which in turn produce proinflammatory cytokines that cross the BBB and act on neurons and glial cells, leading to neuroinflammation and neuronal cell death (Sun and Shen 2018).
Markers of intestinal inflammation and barrier dysfunction in PD
Investigation on the intestinal biopsies of the ascending colon has revealed intestinal inflammation is associated with higher concentrations of proinflammatory markers among PD cases (Devos et al. 2013). A study by Devos et al. on the stool immunological profile provides evidence of intestinal inflammation in PD, and their work also supports the association of intestinal dysfunction and altered microbiota in PD (Devos et al. 2013). The immune system can activate intestinal barrier dysfunction on exposure to bacterial endotoxins or environmental triggers leading to α-syn deposition, a hallmark of intestinal hyper-permeability in PD (Forsyth et al. 2011). Some of the studies have reported that the detection of calprotectin, a faecal marker of inflammation by ELISA, may be helpful in detecting early signs of the activated colonic immune system in PD (Schwiertz et al. 2018; Mulak et al. 2019). Calprotectin is secreted into the lumen by neutrophils on their migration to the inflammation site. Thus, calprotectin can serve as a faecal marker of inflammation as it can be found in subclinical inflammation and can resist degradation.
A protease inhibitor, alpha-1-antitrypsin concentrations are elevated in faeces of PD, indicating the damage in the GI barrier and the loss of proteins to the lumen. Similarly, zonulin modulates the tight junction and maintains intestinal barrier integrity. Alpha-1-antitrypsin and zonulin are markers of intestinal hyperpermeability in PD (Schwiertz et al. 2018). Though these markers are not specific to PD, they can be used as a non-invasive means for spotting intestinal inflammation and hyperpermeability in the early stage of the disease.
Genetic predisposition and microbiome
Approximately 10 to 15% of PD are hereditary, and the rest is idiopathic. Multiple mutations and genes have been associated with PD. Most studies on gene mutation in PD are focused on LRRK2 (leucine-rich repeat kinase-2), PINK-1 (phosphatase and tensin [PTEN] homologue-induced kinase-1), and SNCA genes. Epigenetic alteration induced by exposure to the toxic chemical, environmental factors, pathogenic bacteria, or bacterial metabolites that interact with genes has been proposed to be involved in most sporadic PD cases (Kasper et al. 2014). LLRK2 gene has been linked to both PD and Crohn's disease (CD), an inflammatory bowel disease (IBD). Inflamed colonic tissue from PD and CD has higher levels of LRRK2, indicating the importance of the immune system in intestinal inflammation (Herrick and Tansey 2021). A systematic review and meta-analysis by Zhu et al. report a 28% and 30% increased risk of PD in patients with CD and ulcerative colitis, respectively (Zhu et al. 2019).
Genetic predisposition with microbiome interaction and association are sparsely studied in PD. SNCA is the α-syn encoding gene. Mutation in the SNCA gene affects its expression and is a risk factor for PD. SNCA has been associated with elevated opportunistic pathogens of the gut in PD. Certain bacterial species of the genus Corynebacterium, Porphyromonas, and Prevotella have been noted to be significantly abundant in PD and associated with SCNA genes (Wallen et al. 2021). These trios have been speculated as an opportunistic group with specific species of C. amycolatum, C. lactis of genus Corynebacterium, P. asaccharolytica, P. bennonis, P. somerae, P. unonis of genus Porphyromonas and P. birria, P. buccalis, P. disiens, P. timonensis of genus Prevotella, which are drastically altered in PD (Wallen et al. 2020, 2021). An overabundance of the unusually low Corynebacterium and endotoxins of the Porphyromonas and Prevotella can trigger the PD pathology in the gut (Wallen et al. 2021). Yet, there remains an enigma to solve and understand the underlying relationship between the gut microbiome and genetic predisposition in PD.
PINK1 and PRKN (parkin RBR E3 ubiquitin protein ligase) genes play a role in clearing damaged mitochondria. PINK1 and PRKN gene mutations are associated with mitochondrial dysfunction in the early-onset form of PD ((Kasper et al. 2014). Matheoud et al. have shown intestinal infection with Gram-negative bacteria in PINK1 − / − mice engage mitochondrial antigen presentation and autoimmune mechanisms to stimulate an inflammatory response in the periphery and the brain, triggering the PD-like motor symptoms in mice emphasising the gut-brain axis in the disease (Matheoud et al. 2019).
Peptidoglycan is a prominent cell wall component of the gram-negative bacterial cell. Microbes with peptidoglycan recognition protein encoding genes (PGLYRP) maintain a healthy gut by regulating immune responses to commensal and harmful bacteria. Variations in three PGLYRP genes of four have been linked with PD risk (Goldman et al. 2014).
Gut microbiota associated with PD
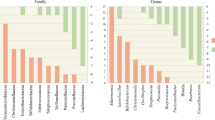
Studies on the gut microbiome of PD have been carried out on diverse populations worldwide by following different protocols, most of which are case–control studies, a few are follow-up studies, some target a specific group of organisms, some others deal with shotgun metagenomics, and most of the others have employed 16S rRNA sequencing (Fig. 2). Various studies have analysed the relative abundance of bacterial taxa in PD and compared it with healthy controls (HC) using diverse platforms and databases. This review focuses on the intraluminal microbiome encountered in faecal samples of PD compared to HC, as stated in Table 1. Putative cellulose-degrading bacterial taxa Prevotellaceae (Prevotella), Ruminococcaceae (Faecalibacterium), Lachnospiraceae (Blautia, Roseburia) that produce SCFA and help in the synthesis of mucin to maintain the intestinal integrity are considerably lower in abundance in PD (Hill-Burns et al. 2017; Li et al. 2017; Aho et al. 2019; Cosma-Grigorov et al. 2020; Lubomski et al. 2021). On the contrary, a few putative pathobionts of the family Enterobacteriaceae, Enterococcaceae, which are assumed to possibly reduce the production of SCFA, produce endotoxins and neurotoxins that promote intestinal inflammation, are enriched in PD (Li et al. 2017; Barichella et al. 2019). An altered microbial composition, a decrease in bacteria associated with SCFA synthesis that is bacteria related to anti-inflammation, and a higher abundance of proinflammatory pathobionts of phylum Proteobacteria in PD, are similar to that of the changes observed in IBD (Keshavarzian et al. 2015).
Mucin degrading genus Akkermansia of the phylum Verrucomicrobia has been widely reported to be significantly abundant in PD by most studies. Akkermansia and Christensenellaceae may symbiotically play a role in PD pathology and progression (Nishiwaki et al. 2020). Intestinal mucus layer is rich in protein mucin. Akkermansia utilises mucin as a nutritional source and degrades it into SCFA acetate, which acts as a substrate for other beneficial bacteria to produce butyrate, an energy source for the intestinal epithelial cells (Belzer et al. 2017). Akkermansia is a symbiont that degrades mucin and encourages cells to produce more mucin (Derrien et al. 2017) and, in turn, enhances the mucosal integrity and modulates the immune system. If the intestinal cells fail to produce mucin, it would eventually lead to detrimental effects, a leaky gut and inflammation. A few studies that reported a lower abundance of putative complex cellulose-degrading bacteria and have also found a higher abundance in Akkermanisa (Hill-Burns et al. 2017; Cirstea et al. 2020; Vidal-martinez et al. 2020). A compensatory effect of richness in Akkermansia is possibly due to depleting cellulose-degrading bacteria in the PD gut.
Various studies have also reported a correlation in the abundance of bacterial taxa with respect to disease duration, disease severity, motor symptom score, non-motor symptoms, particularly constipation, and antiparkinsonian treatment in PD (Table 2). Bradykinesia, rigidity, resting tremor, and gait impairment are cardinal motor symptoms of Parkinson’s that aid in the diagnosis and prognosis of the disease. Unified Parkinson’s disease rating scale (UPDRS) is broadly used to evaluate the disease state, and UPDRS part III is specific for motor symptoms (Goetz et al. 2007). An abundance of specific microbiota has been linked with motor impairment, as mentioned in Table 2. Aquabacterium, Peptococcus, and Sphingomonas are associated with motor complications in PD (Qian et al. 2018). Keshavarzian et al. have reported a higher abundance of phyla Proteobacteria and a lower abundance of Firmicutes with PD duration (Keshavarzian et al. 2015). Keshavarzian et al. and Hill-Burns et al. found Lachnospiraceae to be negatively correlated with duration (Keshavarzian et al. 2015; Hill-Burns et al. 2017), although Barichella et al. reported reduced levels of Lachnospiraceae in all duration of PD (Barichella et al. 2019). Barichella et al. have also observed an effect of disease duration on microbiota, indicating increasing levels of family Lactobacillaceae and a co-abundant genus Akkermansia (Barichella et al. 2019). Hasegawa et al. suggest an increase in Lactobacillus gasseri subgroup can predict disease duration in PD (Hasegawa et al. 2015). Lin et al. observed Pasteurellaceae, Alcaligenaceae, and Fusobacteria were more abundant in the early onset of PD, whereas Comamonas and Anaerotruncus were abundant in the late onset of PD (Lin et al. 2018).
Some studies have also noted variation in microbial diversity between tremor dominant and non-tremor PD subjects. Roseburia (Lin et al. 2018), Flavobacterium, Bacteroidia, Propionibacterium, and Alcaligenaceae (Lin et al. 2019) are abundant in non-tremor subjects. Leptotrichia (Lin et al. 2018), Clostridium, Verrucomicrobia, and Akkermansia (Lin et al. 2019) are abundant in tremor dominant PD subjects, while the family Ruminococcaceae (Weis et al. 2019) is low. Increased levels of Lactobacillaceae (Barichella et al. 2019) and Enterobacteriaceae (Scheperjans et al. 2015; Lin et al. 2018; Pietrucci et al. 2019) but a reduced level of Lachnospiraceae (Barichella et al. 2019) is associated with postural instability and gait disturbance in PD. Reduced abundance of genus Prevotella in patients with faster disease progression (Aho et al. 2019) has been observed. Symbiotically Akkermansia and Christensenellaceae are predicted to play a role in the advancement of PD (Nishiwaki et al. 2020). Hill-Burns et al. describe family Ruminococcaceae increases in abundance as a consequence of disease duration as they observed Ruminococcaceae not to be high in patients within the first ten years of the disease but to be highly elevated in patients with the disease for more than ten years (Hill-Burns et al. 2017).
Anosmia, sensory disturbance, mood disorders, sleep disturbance, and autonomic disturbance are additional pre-motor and non-motor features in PD. Barichella et al. observed increased levels of Lactobacillaceae and reduced Lachnospiraceae and related genera with intellectual impairment (Barichella et al. 2019). A study by Rem et al. have analysed the gut microbiota in PD with cognitive impairment by employing the mini-mental state examination (MMSE) and Montreal cognitive assessment (MoCA) questionnaires. Genus Alistipes and Odoribatcer are negatively correlated with MoCA scores. Barnesiella is negatively associated with MMSE scores. Butyricimonas have been negatively correlated with MMSE and MoCA scores. Genus Blautia of the family Lachnospiraceae was observed to be depleted in patients with mild cognitive impairment, whereas family Rikenellaceae and Ruminococcaceae were rich (Ren et al. 2020). Reduced abundance of Bifidobacterium is associated with depression in PD (Qian et al. 2018).
GI dysfunction is one of the autonomic disturbances in PD. A range of PD-associated GI dysfunctions has been clinically identified, involving weight loss, gastroparesis, constipation, and defecation dysfunction (Kim and Sung 2015). James Parkinson has mentioned constipation as one of the symptoms experienced by his first shaking palsy subject, which is now attributed as one of the prodromal non-motor symptoms in PD (Parkinson 2002). Constipation has been reported in approximately 60 percent of patients with PD (Kaye et al. 2006). Recent research has indicated that gut microbiota may contribute to constipation and related symptoms (Zhao and Yu 2016). A richness of Lactobacillaceae (Nishiwaki et al. 2020), Verrucomicrobiaceae, Bradyrhizobiaceae (Scheperjans et al. 2015), Bifidobacterium (Baldini et al. 2020), and Akkermansia (Heintz-Buschart et al. 2018; Baldini et al. 2020; Cirstea et al. 2020; Lubomski et al. 2021), and lower abundance of Lachinospiraceae (Nishiwaki et al. 2020), Roseburia (Cirstea et al. 2020), and Faecalibacterium (Cirstea et al. 2020; Nishiwaki et al. 2020) have been correlated with constipation or bristol score in PD. Akkermansia has been associated with slow transit time (Heintz-Buschart et al. 2018; Baldini et al. 2020), firmness of stool (Cirstea et al. 2020), and constipation severity (Lubomski et al. 2021).
Antiparkinsonian treatment
Therapeutic drugs targeting humans affect the gut microbiome, which is also relevant the other way round, referred to as bacteria-drug interactions. Gut bacteria render the drug less available for the target, either by bioaccumulation and biotransformation or biodegradation. Intestinal bacteria store drugs intracellularly during bioaccumulation without modifying the chemical, where bacterial growth remains unaffected. In biotransformation, bacteria chemically metabolise the drug by their enzymatic secretions, making the drug ineffective and altering the microbial community composition by metabolic cross-feeding (Klünemann et al. 2021).
Various studies describe the different impacts of antiparkinsonian drugs on the gut microbiome. Different classes of drugs are employed in PD treatment. Some studies examined the impact of dopamine therapy on microbial composition and reported a lower abundance of Faecalibacterium, an anti-inflammatory SCFA producing bacteria (Weis et al. 2019; Melis et al. 2021). Several studies have reported a strong impact of the catechol-O-methyltransferase inhibitors (iCOMT) on microbial diversity and compositions and are considered as confounding factors while analysing the PD gut microbiome and confirmed by metanalysis (Nishiwaki et al. 2020). The richness of Enterobacteriaceae has been significantly associated with iCOMT (Scheperjans et al. 2015; Lin et al. 2018; Ren et al. 2020; Melis et al. 2021), and Bifidobacterium is more common in iCOMT users (Lin et al. 2018; Aho et al. 2019; Weis et al. 2019). However, Lachnospiraceae has been noted to be lower in abundance (Hill-Burns et al. 2017; Barichella et al. 2019).
Two studies have analysed the influence of device-assisted therapy in PD on microbial composition, and both studies describe the over-representation of Enterobacteriaceae in PD subjects on the levodopa-carbidopa intestinal gel (LCIG) (Lubomski et al. 2021; Melis et al. 2021). The abundance of Enterobacteriaceae has been assumed as a result of inflammation by LCIG usage (Melis et al. 2021). An abundance of Clostridium cluster XlVa, Bilophila, Parabacteroides, and Pseudoflavonifractor and a lower abundance of Dorea have been reported in patients on deep brain stimulation (DBS) therapy (Lubomski et al. 2021).
Microbes associated with PD drug metabolism
Helicobacter pylori, the causative organism of peptic ulcers, has been associated with different GI infections. H pylori infection is correlated with motor fluctuations, impaired levodopa absorption with worsening symptoms, and aggravating neurodegenerative process in PD (Pierantozzi et al. 2006; Tan et al. 2014). Randomised control trials on eliminating H. pylori infection have generated conflicting results. Lolekha et al. report a decrease in wearing off symptoms post medication and significant clinical improvement in the symptoms (Lolekha et al. 2021), contradicting the study by Tan et al. that did not notice any substantial changes in the symptoms (Tan et al. 2020). Recently Clostridium sporogenes have been associated with deamination of unabsorbed residual levodopa in the intestine to 3-(3,4-dihydroxyphenyl) propionic acid, which has been shown to exert an inhibitory effect on ileal motility in an ex-vivo model and speculated to increase the transit time (Van Kessel et al. 2020).
Levodopa, a primary drug for the treatment of PD, is absorbed in the jejunum (Gundert-Remy et al. 1983). Levodopa must enter the brain and be converted to the neurotransmitter dopamine by the human enzyme aromatic amino acid decarboxylase (AADC) in order to be effective. Intestinal bacteria, Enterococcus faecalis, can convert levodopa to dopamine in the gut before crossing the BBB rendering the drug ineffective as dopamine is incapable of crossing the BBB. Rekdal et al. demonstrated that Enterococcus faecalis and Eggerthella lenta metabolise levodopa sequentially to m-tyramine employing the enzymes tyrosine decarboxylase and dopamine decarboxylase, respectively, affecting the pharmacokinetics of the drug. They have also discovered that intestinal bacterial levodopa decarboxylation can be inactivated by (S)-α-fluoromethyltyrosine (AFMT) (Rekdal et al. 2019).
A rat in situ study model discovered the presence of bacterial tyrosine decarboxylase in the small bowel contributes to interindividual variation in drug efficacy and a higher abundance of the microbial tyrosine decarboxylase gene in the small bowel of rats diminishes the levels of levodopa in small intestinal and plasma. The same study has also found a positive correlation between higher bacterial tyrosine decarboxylase gene in PD faecal samples with disease duration and levodopa daily dose (Van Kessel et al. 2019). This may possibly explain how some PD patients require a higher dosage regimen of levodopa treatment.
Intestinal bacterial metabolites
Bacteria inhabiting the gut metabolise carbohydrates and proteins to SCFA and amino acids to acquire energy and proliferate. SCFA, such as butyrate, propionate, and acetate, in addition to amino acids such as p-cresol and phenylacetylglutamine, are generated on carbohydrate and protein metabolism by intestinal bacteria (Oliphant and Allen-Vercoe 2019). Recent research has established that some GI abnormalities are contributed by or related to changes in the composition of the gut microbiota and their metabolites (Zhao and Yu 2016). The gut microbiome analysis revealed that the PD microbiota composition, function, and metabolic pathways are skewed toward proteolytic metabolism, which is linked with GI dysfunction in PD (Cirstea et al. 2020).
Microbiota responsible for nucleic acid and amino acid metabolism pathways is enhanced in PD subjects, whereas microbiota carrying out the carbohydrate degradation pathway are abundant in healthy subjects (Cirstea et al. 2020). Unger et al. confirmed a significant reduction in the SCFA in the faeces of PD subjects, consistent with a lower abundance of SCFA producing bacteria and suggesting it can contribute to constipation in PD. SCFA are produced as a metabolic product of complex carbohydrate metabolism by the beneficial gut microbiota (Unger et al. 2016). SCFAs are signalling molecules with anti-inflammatory and antioxidant properties (Huuskonen et al. 2004). Depleting SCFA in the intestine results in devastating effects on the GI barrier function with enhanced inflammation and intensifies the risk of α-syn deposition and brain microglial activation (Mulak 2018).
Melis et al. showed altered microbial composition matched with metabolic changes affecting the metabolism of lipids and proteins. They report an upsurge in metabolites cadaverine and putrescine with proinflammatory activity in PD subjects. They have found an abundance of 4-hydroxyphenylpropionic acid in patients on levodopa therapy and speculate the existence of numerous metabolic pathways to degrade the drug (Melis et al. 2021). Cristea et al. have noted enriched fucose degradation, which possibly indicates host mucin breakdown. They observed elevated levels of para-cresol and phenylacetylglutamine and their association with stool consistency (Cirstea et al. 2020).
Diet and microbiome in PD
Diet is the most important factor determining the composition and function of the microbiome and its metabolites in the gut (Heiman and Greenway 2016; Jackson et al. 2019). Change in diet can alter the gut microbial profile of an individual, which can be transient if the modifications are for a short duration or if the amendment is continued for an extended period, the established bacteria will be replaced according to the diet. However, it would revert to the original composition once the dietary modifications are terminated (Wu et al. 2011; David et al. 2014). Consumption of an animal-based diet increases the abundance of bile-tolerant microorganisms and lowers the mass of Firmicutes that utilize dietary plant polysaccharides (David et al. 2014). Bacteroides enterotypes are associated with protein and animal fat consumption (Wu et al. 2011). A plant-based diet that is a higher intake of fibres is associated with increased production of SCFA and positively correlated with levels of Prevotella in the gut (Wu et al. 2011; David et al. 2014; De Filippis et al. 2016).
Derkinderen et al. postulate that reduced risk of PD with cigarette smoking and coffee consumption may be associated with shaping the gut microbial composition that exhibits anti-inflammatory properties by their metabolites that reduces intestinal inflammation (Derkinderen et al. 2014). In a dietary intervention by adding whole-grain barley and brown rice to the diet, Martinez et al. report increased microbial diversity and immunological and metabolic improvement (Martínez et al. 2013). Two studies on the American and Greece populations have revealed that individuals on a strict Mediterranean diet have a reduced risk of prodromal PD symptoms (Maraki et al. 2019; Molsberry et al. 2020). A survey by Metcalfe-Roach et al. suggests the Mediterranean diet can be a nutritional strategy to delay the onset of PD (Metcalfe-Roach et al. 2021).
Prebiotic, probiotic, and faecal microbiome transplantation approach in PD therapeutics
As reported in the review, studies have demonstrated altered gut microbiome is potentially associated with PD pathology and outcome. Faecal microbiome transplantation (FMT), prebiotics, and probiotics are potential options for restoring the altered microbiome in PD. An interventional study on a small group of PD subjects by Hegelmaier et al. suggests the inclusion of dietary SCFA has a positive influence on the gut microbiome and has a positive outcome on the course of the PD (Hegelmaier et al. 2020). Similarly, prebiotic intervention with the inclusion of resistant starch in the diet resulted in a decline in non-motors symptoms, stabilised faecal microbial diversity, improved butyrate levels, and reduced calprotectin concentration in PD (Becker et al. 2021). In clinical trials, increase in spontaneous bowel movements in PD patients with constipation (Ibrahim et al. 2020; Tan et al. 2021), an improvement in abdominal pain and bloating (Georgescu et al. 2016), and a decrease in UPDRS score (Tamtaji et al. 2019) has been observed on using different strains of multi-strain probiotics. A randomized controlled trial showed the intake of fermented milk with prebiotic fibers and different probiotic strains (Lactobacillus spp, Bifidobacterium spp, Streptococcus salivarius, and Enterococcus faecium) significantly increased the frequency of complete bowel movements in PD patients improving constipation (Barichella et al. 2016). Combination therapy is the only treatment recommended as “efficacious” and “clinically useful” by the latest MDS evidence-based guidelines (Seppi et al. 2019).
Following the significant success in treating recurrent Clostridium difficile infections with FMT (Silverman et al. 2010), scientists have focused on treating non-curable diseases by modifying the gut microbiome (Merenstein et al. 2014). FMT involves the transfer of screened stool that contains microbes and their metabolites from a healthy donor to a patient (Merenstein et al. 2014). Some of the studies have thrived on revealing the practical implication of FMT in PD. Both Xue et al. and Kuai et al. report FMT improves motor and nonmotor symptoms in PD (Xue et al. 2020; Kuai et al. 2021). Xue et al. compared FMT via the colonoscopy and nasointestinal tube and recommend colonoscopy over the latter since it had a relatively more satisfactory impact on PD symptoms, improving anxiety, depression, and sleep quality (Xue et al. 2020). Kuai et al. have analysed the gut microbiome of PD subjects before and after FMT and report an increased abundance of Blautia, Lachnospiraceae, and Prevotella in PD, whereas a decrease in abundance of Bacteroidetes post-FMT (Kuai et al. 2021). Recovery in GI symptoms and remission in constipation was documented after FMT therapy in PD subjects with constipation (Huang et al. 2019; Kuai et al. 2021). Further confirmatory studies are essential on the application of prebiotics, probiotics, and FMT as therapeutics in PD, which may also be personalised as per the requirements of the patients.
A recent intriguing study has brought together a new hypothesis of brain-first PD centered on prodromal rapid eye movement sleep behaviour disorder conflicting with the existing body-first PD. This Danish research reports two PD subtypes based on the initial starting point and spread of α-syn pathology: brain-first PD, neuropathology begins in the brain and then spreads downward, while other being the body-first PD subtype, as suggested by Braak that PD pathology originates in the body from GI and olfactory peripheral nervous systems and ascends towards the brainstem (Horsager et al. 2020). Their investigation has opened a new horizon for microbiome scientists to explore and understand the microbial diversity in the two subtypes. Microbes and their metabolites may vary in PD subtypes and even have a differential impact on the disease progression. Since none of the studies have considered the possibilities of the different microbial compositions within PD or have grouped them based on the origin of the synucleinopathy, any interventions aimed to modify the gut microbiota would be ineffective if the disease started in the brain. It would be relevant for the researchers to look into the microbiome involvement in the different subtypes and stages of PD for effective personalised therapy.
Conclusion
In recent years, there has been an increased interest in discovering the genesis of PD and identifying markers that can assist in detecting the condition early to tailor treatment strategies accordingly. Despite this, none of the research covered in this review has consistently predicted the common microbial signature associated with PD, and it could be the result of the brain-first and body-first PD subtypes. In general, the negative impact of the imbalance in the bacterial community that promotes inflammatory response and protective bacterial communities producing SCFA has been documented in PD. Gut microbiota and their metabolic products are associated with motor and non-motor symptoms of PD, with genetic predisposition, and with antiparkinsonian drug metabolism. However, the findings are quite heterogeneous across the studies due to differences in the study population and methodology. Therefore, we should move beyond microbiome composition and potential metabolic entities to direct the evaluation of active metabolic pathways. A multi-omics approach is likely required to understand the function of the gut microbiota in PD progression for better diagnosis and treatment. Dietary and microbial interventions are promising stratagems for disease modulation and therapy. Additional clinical trials on personalised FMT and nutritional modifications in PD would probably enhance our knowledge of the gut microbiota and brain interactions for better treatment approaches.
References
Aho VTE, Pereira PAB, Voutilainen S et al (2019) Gut microbiota in Parkinson’s disease: Temporal stability and relations to disease progression. EBioMedicine 44:691–707. https://doi.org/10.1016/j.ebiom.2019.05.064
Al-Asmakh M, Hedin L (2015) Microbiota and the control of blood-tissue barriers. Tissue Barriers 3:e1039691. https://doi.org/10.1080/21688370.2015.1039691
Baldini F, Hertel J, Sandt E et al (2020) Parkinson’s disease-associated alterations of the gut microbiome predict disease-relevant changes in metabolic functions. BMC Biol 18:62. https://doi.org/10.1186/s12915-020-00775-7
Barichella M, Pacchetti C, Bolliri C et al (2016) Probiotics and prebiotic fiber for constipation associated with Parkinson disease. Neurology 87:1274–1280. https://doi.org/10.1212/WNL.0000000000003127
Barichella M, Severgnini M, Cilia R et al (2019) Unraveling gut microbiota in Parkinson’s disease and atypical parkinsonism. Mov Disord 34:396–405. https://doi.org/10.1002/mds.27581
Beach TG, Adler CH, Sue LI et al (2010) Multi-organ distribution of phosphorylated α-synuclein histopathology in subjects with Lewy body disorders. Acta Neuropathol 119:689–702. https://doi.org/10.1007/s00401-010-0664-3
Becker A, Pierre Schmartz G, Gröger L et al (2021) Effects of Resistant Starch on Symptoms, Fecal Markers and Gut Microbiota in Parkinson’s Disease – the RESISTA-PD Trial. Genomics Proteomics Bioinformatics S1672–0229(21)00245-X. https://doi.org/10.1016/j.gpb.2021.08.009
Belzer C, Chia LW, Aalvink S et al (2017) Microbial Metabolic Networks at the Mucus Layer Lead to Diet-Independent Butyrate and Vitamin B 12 Production by Intestinal Symbionts. mBio 8:e00770–17. https://doi.org/10.1128/mBio.00770-17
Bendor J, Logan T, Edwards RH (2013) The Function of α-Synuclein. Neuron 79:1044–1066. https://doi.org/10.1016/J.NEURON.2013.09.004
Béraud D, Twomey M, Bloom B et al (2011) α-Synuclein Alters Toll-Like Receptor Expression. Front Neurosci 5:80. https://doi.org/10.3389/fnins.2011.00080
Bienenstock J, Kunze W, Forsythe P (2015) Microbiota and the gut-brain axis. Nutr Rev 73:28–31. https://doi.org/10.1093/nutrit/nuv019
Blesa J, Phani S, Jackson-Lewis V, Przedborski S (2012) Classic and new animal models of Parkinson’s disease. J Biomed Biotechnol 2012:845618. https://doi.org/10.1155/2012/845618
Braniste V, Al-Asmakh M, Kowal C et al (2014) The gut microbiota influences blood-brain barrier permeability in mice. Sci Transl Med 6:263ra158. https://doi.org/10.1126/scitranslmed.3009759
Carabotti M, Scirocco A, Maselli MA, Severi C (2015) The gut-brain axis: Interactions between enteric microbiota, central and enteric nervous systems. Ann Gastroenterol 28:203–209
Cersosimo MG, Raina GB, Pecci C et al (2013) Gastrointestinal manifestations in Parkinson’s disease: prevalence and occurrence before motor symptoms. J Neurol 260:1332–1338. https://doi.org/10.1007/s00415-012-6801-2
Chen SG, Stribinskis V, Rane MJ et al (2016) Exposure to the Functional Bacterial Amyloid Protein Curli Enhances Alpha-Synuclein Aggregation in Aged Fischer 344 Rats and Caenorhabditis elegans. Sci Rep 6:34477. https://doi.org/10.1038/srep34477
Cheng HC, Ulane CM, Burke RE (2010) Clinical progression in Parkinson disease and the neurobiology of axons. Ann Neurol 67:715–725. https://doi.org/10.1002/ANA.21995
Cirstea MS, Yu AC, Golz E et al (2020) Microbiota Composition and Metabolism Are Associated With Gut Function in Parkinson’s Disease. Mov Disord 35:1208–1217. https://doi.org/10.1002/mds.28052
Cosma-Grigorov A, Meixner H, Mrochen A et al (2020) Changes in Gastrointestinal Microbiome Composition in PD: A Pivotal Role of Covariates. Front Neurol 11:1041. https://doi.org/10.3389/fneur.2020.01041
Couch Y, Alvarez-Erviti L, Sibson NR et al (2011) The acute inflammatory response to intranigral α-synuclein differs significantly from intranigral lipopolysaccharide and is exacerbated by peripheral inflammation. J Neuroinflammation 8:166. https://doi.org/10.1186/1742-2094-8-166
David LA, Maurice CF, Carmody RN et al (2014) Diet rapidly and reproducibly alters the human gut microbiome. Nature 505:559–563. https://doi.org/10.1038/nature12820
De Filippis F, Pellegrini N, Vannini L et al (2016) High-level adherence to a Mediterranean diet beneficially impacts the gut microbiota and associated metabolome. Gut 65:1812–1821. https://doi.org/10.1136/gutjnl-2015-309957
Derkinderen P, Shannon KM, Brundin P (2014) Gut feelings about smoking and coffee in Parkinson’s disease. Mov Disord 29:976–979. https://doi.org/10.1002/mds.25882
Derrien M, Belzer C, de Vos WM (2017) Akkermansia muciniphila and its role in regulating host functions. Microb Pathog 106:171–181. https://doi.org/10.1016/j.micpath.2016.02.005
Devos D, Lebouvier T, Lardeux B et al (2013) Colonic inflammation in Parkinson’s disease. Neurobiol Dis 50:42–48. https://doi.org/10.1016/j.nbd.2012.09.007
Dinan TG, Cryan JF (2017) Gut instincts: microbiota as a key regulator of brain development, ageing and neurodegeneration. J Physiol 595:489–503. https://doi.org/10.1113/JP273106
Erny D, De Angelis ALH, Jaitin D et al (2015) Host microbiota constantly control maturation and function of microglia in the CNS. Nat Neurosci 18:965–977. https://doi.org/10.1038/nn.4030
Forsyth CB, Shannon KM, Kordower JH et al (2011) Increased intestinal permeability correlates with sigmoid mucosa alpha-synuclein staining and endotoxin exposure markers in early Parkinson’s disease. PLoS ONE 6:e28032. https://doi.org/10.1371/journal.pone.0028032
Freire C, Koifman S (2012) Pesticide exposure and Parkinson’s disease: Epidemiological evidence of association. Neurotoxicology 33:947–971. https://doi.org/10.1016/j.neuro.2012.05.011
Gatto NM, Cockburn M, Bronstein J et al (2009) Well-Water Consumption and Parkinson’s Disease in Rural California. Environ Health Perspect 117:1912. https://doi.org/10.1289/ehp.0900852
Georgescu D, Ancusa OE, Georgescu LA et al (2016) Nonmotor gastrointestinal disorders in older patients with Parkinson’s disease: is there hope? Clin Interv Aging 11:1601–1608. https://doi.org/10.2147/CIA.S106284
Goetz CG, Fahn S, Martinez-Martin P et al (2007) Movement Disorder Society-sponsored revision of the Unified Parkinson’s Disease Rating Scale (MDS-UPDRS): Process, format, and clinimetric testing plan. Mov Disord 22:41–47. https://doi.org/10.1002/mds.21198
Goldman SM, Kamel F, Ross GW et al (2014) Peptidoglycan recognition protein genes and risk of Parkinson’s disease. Mov Disord 29:1171–1180. https://doi.org/10.1002/mds.25895
Gundert-Remy U, Hildebrandt R, Stiehl A et al (1983) Intestinal absorption of levodopa in man. Eur J Clin Pharmacol 25:69–72. https://doi.org/10.1007/BF00544017
Mohajeri MH, La Fata G, Steinert RE et al (2018) Relationship between the gut microbiome and brain function. Nutr Rev 76:481–496. https://doi.org/10.1093/nutrit/nuy009
Hasegawa S, Goto S, Tsuji H et al (2015) Intestinal dysbiosis and lowered serum lipopolysaccharide-binding protein in Parkinson’s disease. PLoS ONE 10:e0142164. https://doi.org/10.1371/journal.pone.0142164
Hegelmaier T, Lebbing M, Duscha A et al (2020) Interventional Influence of the Intestinal Microbiome Through Dietary Intervention and Bowel Cleansing Might Improve Motor Symptoms in Parkinson’s Disease. Cells 9:376. https://doi.org/10.3390/cells9020376
Heiman ML, Greenway FL (2016) A healthy gastrointestinal microbiome is dependent on dietary diversity. Mol Metab 5:317–320. https://doi.org/10.1016/j.molmet.2016.02.005
Heintz-Buschart A, Pandey U, Wicke T et al (2018) The nasal and gut microbiome in Parkinson’s disease and idiopathic rapid eye movement sleep behavior disorder. Mov Disord 33:88–98. https://doi.org/10.1002/mds.27105
Herrick MK, Tansey MG (2021) Is LRRK2 the missing link between inflammatory bowel disease and Parkinson’s disease? NPJ Parkinsons Dis 7:26. https://doi.org/10.1038/s41531-021-00170-1
Hill-Burns EM, Debelius JW, Morton JT et al (2017) Parkinson’s Disease and Parkinson’s Disease Medications Have Distinct Signatures of the Gut Microbiome. Mov Disord 32:739–749. https://doi.org/10.1002/mds.26942
Holmqvist S, Chutna O, Bousset L et al (2014) Direct evidence of Parkinson pathology spread from the gastrointestinal tract to the brain in rats. Acta Neuropathol 128:805–820. https://doi.org/10.1007/s00401-014-1343-6
Horsager J, Andersen KB, Knudsen K et al (2020) Brain-first versus body-first Parkinson’s disease: a multimodal imaging case-control study. Brain 143:3077–3088. https://doi.org/10.1093//brain/awaa238
Huang H, Xu H, Luo Q et al (2019) Fecal microbiota transplantation to treat Parkinson’s disease with constipation: A case report. Medicine (baltimore) 98:e16163. https://doi.org/10.1097/MD.0000000000016163
Huuskonen J, Suuronen T, Nuutinen T et al (2004) Regulation of microglial inflammatory response by sodium butyrate and short-chain fatty acids. Br J Pharmacol 141:874–880. https://doi.org/10.1038/sj.bjp.0705682
Ibrahim A, Raja Ali RA, Abdul Manaf MR et al (2020) Multi-strain probiotics (Hexbio) containing MCP BCMC strains improved constipation and gut motility in Parkinson’s disease: A randomised controlled trial. PLoS ONE 15:e0244680. https://doi.org/10.1371/journal.pone.0244680
Jackson A, Forsyth CB, Shaikh M et al (2019) Diet in Parkinson’s Disease: Critical Role for the Microbiome. Front Neurol 10:1245. https://doi.org/10.3389/fneur.2019.01245
Jin M, Li J, Liu F et al (2019) Analysis of the Gut Microflora in Patients With Parkinson’s Disease. Front Neurosci 13:1184. https://doi.org/10.3389/fnins.2019.01184
Jost WH, Reichmann H (2017) “An essay on the shaking palsy” 200 years old. J Neural Transm (vienna) 124:899–900. https://doi.org/10.1007/s00702-017-1684-0
Kasper D, Fauci A, Hauser S et al (2014) Harrison’s Principles of Internal Medicine 19th edn (Vols 1+2). McGraw Hill
Kaur R, Mehan S, Singh S (2019) Understanding multifactorial architecture of Parkinson’s disease: pathophysiology to management. Neurol Sci 40:13–23. https://doi.org/10.1007/s10072-018-3585-x
Kaye J, Gage H, Kimber A et al (2006) Excess burden of constipation in Parkinson’s disease: a pilot study. Mov Disord 21:1270–1273. https://doi.org/10.1002/mds.20942
Keshavarzian A, Green SJ, Engen PA et al (2015) Colonic bacterial composition in Parkinson’s disease. Mov Disord 30:1351–1360. https://doi.org/10.1002/mds.26307
Kim JS, Sung HY (2015) Gastrointestinal Autonomic Dysfunction in Patients with Parkinson’s Disease. J Mov Disord 8:76–82. https://doi.org/10.14802/jmd.15008
Klünemann M, Andrejev S, Blasche S et al (2021) Bioaccumulation of therapeutic drugs by human gut bacteria. Nature 597:533–538. https://doi.org/10.1038/s41586-021-03891-8
Kuai XY, Yao XH, Xu LJ et al (2021) Evaluation of fecal microbiota transplantation in Parkinson’s disease patients with constipation. Microb Cell Fact 20:98. https://doi.org/10.1186/s12934-021-01589-0
Lee E-J, Woo M-S, Moon P-G et al (2010) Alpha-synuclein activates microglia by inducing the expressions of matrix metalloproteinases and the subsequent activation of protease-activated receptor-1. J Immunol 185:615–623. https://doi.org/10.4049/jimmunol.0903480
Ley RE, Peterson DA, Gordon JI (2006) Ecological and evolutionary forces shaping microbial diversity in the human intestine. Cell 124:837–848. https://doi.org/10.1016/j.cell.2006.02.017
Li W, Wu X, Hu X et al (2017) Structural changes of gut microbiota in Parkinson’s disease and its correlation with clinical features. Sci China Life Sci 60:1223–1233. https://doi.org/10.1007/s11427-016-9001-4
Lin A, Zheng W, He Y et al (2018) Gut microbiota in patients with Parkinson’s disease in southern China. Parkinsonism Relat Disord 53:82–88. https://doi.org/10.1016/j.parkreldis.2018.05.007
Lin CH, Chen CC, Chiang HL et al (2019) Altered gut microbiota and inflammatory cytokine responses in patients with Parkinson’s disease. J Neuroinflammation 16:129. https://doi.org/10.1186/s12974-019-1528-y
Liu S, Da Cunha AP, Rezende RM et al (2016) The Host Shapes the Gut Microbiota via Fecal MicroRNA. Cell Host Microbe 19:32–43. https://doi.org/10.1016/j.chom.2015.12.005
Lolekha P, Sriphanom T, Vilaichone R-K (2021) Helicobacter pylori eradication improves motor fluctuations in advanced Parkinson’s disease patients: A prospective cohort study (HP-PD trial). PLoS One 16:e0251042. https://doi.org/10.1371/journal.pone.0251042
Lubomski M, Xu X, Holmes AJ et al (2021) The impact of device-assisted therapies on the gut microbiome in Parkinson’s disease. J Neurol 269:780–795. https://doi.org/10.1007/s00415-021-10657-9
Luna E, Luk KC (2015) Bent out of shape: α-Synuclein misfolding and the convergence of pathogenic pathways in Parkinson’s disease. FEBS Lett 589:3749–3759. https://doi.org/10.1016/J.FEBSLET.2015.10.023
Maraki MI, Yannakoulia M, Stamelou M et al (2019) Mediterranean diet adherence is related to reduced probability of prodromal Parkinson’s disease. Mov Disord 34:48–57. https://doi.org/10.1002/mds.27489
Martínez I, Lattimer JM, Hubach KL et al (2013) Gut microbiome composition is linked to whole grain-induced immunological improvements Subject Category: microbe-microbe and microbe-host interactions. ISME J 7:269–280. https://doi.org/10.1038/ismej.2012.104
Massier L, Blüher M, Kovacs P, Chakaroun RM (2021) Impaired Intestinal Barrier and Tissue Bacteria: Pathomechanisms for Metabolic Diseases. Front Endocrinol (lausanne) 12:616506. https://doi.org/10.3389/fendo.2021.616506
Matheoud D, Cannon T, Voisin A et al (2019) Intestinal infection triggers Parkinson’s disease-like symptoms in Pink1 −/− mice. Nature 571:565–569. https://doi.org/10.1038/s41586-019-1405-y
Meade RM, Fairlie DP, Mason JM (2019) Alpha-synuclein structure and Parkinson’s disease – lessons and emerging principles. Mol Neurodegener 14:1–14. https://doi.org/10.1186/S13024-019-0329-1
Melis M, Vascellari S, Santoru ML et al (2021) Gut microbiota and metabolome distinctive features in Parkinson disease: Focus on levodopa and levodopa-carbidopa intrajejunal gel. Eur J Neurol 28:1198–1209. https://doi.org/10.1111/ene.14644
Merenstein D, El-Nachef N, Lynch SV (2014) Fecal Microbial Therapy – Promises and Pitfalls. J Pediatr Gastroenterol Nutr 59:157–161. https://doi.org/10.1097/MPG.0000000000000415
Metcalfe-Roach A, Yu AC, Golz E et al (2021) MIND and Mediterranean Diets Associated with Later Onset of Parkinson’s Disease. Mov Disord 36:977–984. https://doi.org/10.1002/mds.28464
Molsberry S, Bjornevik K, Hughes KC et al (2020) Diet pattern and prodromal features of Parkinson disease. Neurology 95:e2095–e2108. https://doi.org/10.1212/WNL.0000000000010523
Moons R, Konijnenberg A, Mensch C et al (2020) Metal Ions Shape α-Synuclein Sci Rep 10:1–13. https://doi.org/10.1038/s41598-020-73207-9
Mulak A (2018) A controversy on the role of short-chain fatty acids in the pathogenesis of Parkinson’s disease. Mov Disord 33:398–401. https://doi.org/10.1002/MDS.27304
Mulak A, Koszewicz M, Panek-Jeziorna M et al (2019) Fecal calprotectin as a marker of the gut immune system activation is elevated in parkinson’s disease. Front Neurosci 13:992. https://doi.org/10.3389/fnins.2019.00992
NIH Human Microbiome Project defines normal bacterial makeup of the body | National Institutes of Health (NIH) (2012) News Release-June13, 2012. https://www.nih.gov/news-events/news-releases/nih-human-microbiome-project-defines-normal-bacterial-makeup-body. Accessed 5 Apr 2022
Nishiwaki H, Ito M, Ishida T et al (2020) Meta-Analysis of Gut Dysbiosis in Parkinson’s Disease. Mov Disord 35:1626–1635. https://doi.org/10.1002/mds.28119
Oliphant K, Allen-Vercoe E (2019) Macronutrient metabolism by the human gut microbiome: Major fermentation by-products and their impact on host health. Microbiome 7:91. https://doi.org/10.1186/s40168-019-0704-8
Pan-Montojo F, Schwarz M, Winkler C et al (2012) Environmental toxins trigger PD-like progression via increased alpha-synuclein release from enteric neurons in mice. Sci Rep 2:898. https://doi.org/10.1038/srep00898
Pang SY, Ho PW, Liu HF et al (2019) The interplay of aging, genetics and environmental factors in the pathogenesis of Parkinson’s disease. Transl Neurodegener 8:23. https://doi.org/10.1186/S40035-019-0165-9
Parkinson J. An essay on the shaking palsy. 1817 (2002) . J Neuropsychiatry Clin Neurosci 14:223–222. https://doi.org/10.1176/jnp.14.2.223
Pierantozzi M, Pietroiusti A, Brusa L et al (2006) Helicobacter pylori eradication and l-dopa absorption in patients with PD and motor fluctuations. Neurology 66:1824–1829. https://doi.org/10.1212/01.wnl.0000221672.01272.ba
Pietrucci D, Cerroni R, Unida V et al (2019) Dysbiosis of gut microbiota in a selected population of Parkinson’s patients. Parkinsonism Relat Disord 65:124–130. https://doi.org/10.1016/j.parkreldis.2019.06.003
Poewe W (2008) Non-motor symptoms in Parkinson’s disease. Eur J Neurol 15(Suppl 1):14–20. https://doi.org/10.1111/j.1468-1331.2008.02056.x
Qian Y, Yang X, Xu S et al (2018) Alteration of the fecal microbiota in Chinese patients with Parkinson’s disease. Brain Behav Immun 70:194–202. https://doi.org/10.1016/j.bbi.2018.02.016
Rekdal VM, Bess EN, Bisanz JE et al (2019) Discovery and inhibition of an interspecies gut bacterial pathway for Levodopa metabolism. Science 364:eaau6323. https://doi.org/10.1126/science.aau6323
Ren T, Gao Y, Qiu Y et al (2020) Gut Microbiota Altered in Mild Cognitive Impairment Compared With Normal Cognition in Sporadic Parkinson’s Disease. Front Neurol 11:137. https://doi.org/10.3389/fneur.2020.00137
Riess O, Krüger R (1999) J Neural Transm Suppl 56:113–125. https://doi.org/10.1007/978-3-7091-6360-3_6
Scheperjans F, Aho V, Pereira PA et al (2015) Gut microbiota are related to Parkinson’s disease and clinical phenotype. Mov Disord 30:350–358. https://doi.org/10.1002/mds.26069
Schwiertz A, Spiegel J, Dillmann U et al (2018) Fecal markers of intestinal inflammation and intestinal permeability are elevated in Parkinson’s disease. Parkinsonism Relat Disord 50:104–107. https://doi.org/10.1016/j.parkreldis.2018.02.022
Sender R, Fuchs S, Milo R (2016) Revised Estimates for the Number of Human and Bacteria Cells in the Body. PLoS Biol 14:e1002533. https://doi.org/10.1371/journal.pbio.1002533
Seppi K, Ray Chaudhuri K, Coelho M et al (2019) Update on treatments for nonmotor symptoms of Parkinson’s disease—an evidence-based medicine review. Mov Disord 34:180–198. https://doi.org/10.1002/mds.27602
Sikander A, Rana SV, Prasad KK (2009) Role of serotonin in gastrointestinal motility and irritable bowel syndrome. Clin Chim Acta 403:47–55. https://doi.org/10.1016/j.cca.2009.01.028
Silverman MS, Davis I, Pillai DR (2010) Success of Self-Administered Home Fecal Transplantation for Chronic Clostridium difficile Infection. Clin Gastroenterol Hepatol 8:471–473. https://doi.org/10.1016/j.cgh.2010.01.007
Statistics | Parkinson’s Foundation (2021) https://www.parkinson.org/Understanding-Parkinsons/Statistics. Accessed 26 Oct 2021
Stefanis L (2012) α-Synuclein in Parkinson’s disease. Cold Spring Harb Perspect Med 2:1–23. https://doi.org/10.1101/cshperspect.a009399
Stolzenberg E, Berry D, Yang D et al (2017) A Role for Neuronal Alpha-Synuclein in Gastrointestinal Immunity. J Innate Immun 9:456–463. https://doi.org/10.1159/000477990
Sun MF, Shen YQ (2018) Dysbiosis of gut microbiota and microbial metabolites in Parkinson’s Disease. Ageing Res Rev 45:53–61. https://doi.org/10.1016/j.arr.2018.04.004
Tamtaji OR, Taghizadeh M, Daneshvar Kakhaki R et al (2019) Clinical and metabolic response to probiotic administration in people with Parkinson’s disease: A randomized, double-blind, placebo-controlled trial. Clin Nutr 38:1031–1035. https://doi.org/10.1016/j.clnu.2018.05.018
Tan AH, Lim S-Y, Mahadeva S et al (2020) Helicobacter pylori Eradication in Parkinson’s Disease: A Randomized Placebo-Controlled Trial. Mov Disord 35:2250–2260. https://doi.org/10.1002/MDS.28248
Tan AH, Lim SY, Chong KK et al (2021) Probiotics for Constipation in Parkinson Disease: A Randomized Placebo-Controlled Study. Neurology 96:e772–e782. https://doi.org/10.1212/WNL.0000000000010998
Tan AH, Mahadeva S, Marras C et al (2014) Helicobacter pylori infection is associated with worse severity of Parkinson’s disease. Parkinsonism Relat Disord 21:221–225. https://doi.org/10.1016/j.parkreldis.2014.12.009
Unger MM, Spiegel J, Dillmann KU et al (2016) Short chain fatty acids and gut microbiota differ between patients with Parkinson’s disease and age-matched controls. Parkinsonism Relat Disord 32:66–72. https://doi.org/10.1016/j.parkreldis.2016.08.019
Van Kessel SP, de Jong HR, Winkel SL et al (2020) Gut bacterial deamination of residual levodopa medication for Parkinson’s disease. BMC Biol 18:137. https://doi.org/10.1186/s12915-020-00876-3
Van Kessel SP, Frye AK, El-Gendy AO et al (2019) Gut bacterial tyrosine decarboxylases restrict levels of levodopa in the treatment of Parkinson’s disease. Nat Commun 10:310. https://doi.org/10.1038/S41467-019-08294-Y
Vancamelbeke M, Vermeire S (2017) The intestinal barrier: a fundamental role in health and disease. Expert Rev Gastroenterol Hepatol 11:821–824. https://doi.org/10.1080/17474124.2017.1343143
Vidal-martinez G, Chin B, Camarillo C et al (2020) A Pilot Microbiota Study in Parkinson’s Disease Patients versus Control Subjects, and Effects of FTY720 and FTY720-Mitoxy Therapies in Parkinsonian and Multiple System Atrophy Mouse Models. J Parkinsons Dis 10:185–192. https://doi.org/10.3233/JPD-191693
Wallen ZD, Appah M, Dean MN et al (2020) Characterizing dysbiosis of gut microbiome in PD: evidence for overabundance of opportunistic pathogens. NPJ Parkinsons Dis 6:11. https://doi.org/10.1038/s41531-020-0112-6
Wallen ZD, Stone WJ, Factor SA et al (2021) Exploring human-genome gut-microbiome interaction in Parkinson’s disease. NPJ Parkinsons Dis 7:74. https://doi.org/10.1038/s41531-021-00218-2
Weis S, Schwiertz A, Unger MM et al (2019) Effect of Parkinson’s disease and related medications on the composition of the fecal bacterial microbiota. NPJ Parkinsons Dis 5:28. https://doi.org/10.1038/s41531-019-0100-x
Wu GD, Chen J, Hoffmann C et al (2011) Linking long-term dietary patterns with gut microbial enterotypes. Science 334:105–108. https://doi.org/10.1126/science.1208344
Xue LJ, Yang XZ, Tong Q et al (2020) Fecal microbiota transplantation therapy for Parkinson’s disease: A preliminary study. Medicine (baltimore) 99:e22035. https://doi.org/10.1097/MD.0000000000022035
Yano JM, Yu K, Donaldson GP et al (2015) Indigenous bacteria from the gut microbiota regulate host serotonin biosynthesis. Cell 161:264–276. https://doi.org/10.1016/j.cell.2015.02.047
Zhao Y, Yu YB (2016) Intestinal Microbiota and Chronic Constipation Springerplus 5:1130. https://doi.org/10.1186/S40064-016-2821-1
Zhu F, Li C, Gong J et al (2019) The risk of Parkinson’s disease in inflammatory bowel disease: A systematic review and meta-analysis. Dig Liver Dis 51:38–42. https://doi.org/10.1016/J.DLD.2018.09.017
Acknowledgements
Thankfully acknowledge the Manipal Academy of Higher Education.
Funding
Open access funding provided by Manipal Academy of Higher Education, Manipal. No funding was received to assist with the preparation of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors have no conflicts of interest associated with this publication.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Pavan, S., Prabhu, A.N., Prasad Gorthi, S. et al. Exploring the multifactorial aspects of Gut Microbiome in Parkinson’s Disease. Folia Microbiol 67, 693–706 (2022). https://doi.org/10.1007/s12223-022-00977-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12223-022-00977-2