Abstract
Background
Erythritol is a four-carbon sugar alcohol with sweetening properties that is used by the agro-food industry as a food additive. In the yeast Yarrowia lipolytica, the last step of erythritol synthesis involves the reduction of erythrose by specific erythrose reductase(s). In the earlier report, an erythrose reductase gene (YALI0F18590g) from erythritol-producing yeast Y. lipolytica MK1 was identified (Janek et al. in Microb Cell Fact 16:118, 2017). However, deletion of the gene in Y. lipolytica MK1 only resulted in some lower erythritol production but the erythritol synthesis process was still maintained, indicating that other erythrose reductase gene(s) might exist in the genome of Y. lipolytica.
Results
In this study, we have isolated genes g141.t1 (YALI0D07634g) and g3023.t1 (YALI0C13508g) encoding two novel erythrose reductases (ER). The biochemical characterization of the purified enzymes showed that they have a strong affinity for erythrose. Deletion of the two ER genes plus g801.t1 (YALI0F18590g) did not prevent erythritol synthesis, suggesting that other ER or ER-like enzymes remain to be discovered in this yeast. Overexpression of the newly isolated two genes (ER10 or ER25) led to an average 14.7% higher erythritol yield and 31.2% higher productivity compared to the wild-type strain. Finally, engineering NADPH cofactor metabolism by overexpression of genes ZWF1 and GND1 encoding glucose-6-phosphate dehydrogenase and 6-phosphogluconate dehydrogenase, respectively, allowed a 23.5% higher erythritol yield and 50% higher productivity compared to the wild-type strain. The best of our constructed strains produced an erythritol titer of 190 g/L in baffled flasks using glucose as main carbon source.
Conclusions
Our results highlight that in the Y. lipolytica genome several genes encode enzymes able to reduce erythrose into erythritol. The catalytic properties of these enzymes and their cofactor dependency are different from that of already known erythrose reductase of Y. lipolytica. Constitutive expression of the newly isolated genes and engineering of NADPH cofactor metabolism led to an increase in erythritol titer. Development of fermentation strategies will allow further improvement of this productivity in the future.
Similar content being viewed by others
Background
Erythritol (1,2,3,4-butanetetrol) is a four-carbon sugar alcohol with sweetening properties and applications in the agro-food and pharmaceutical industries [1,2,3]. It is most commonly produced by yeast, namely Torula corallina [4, 5], Candida magnoliae [6, 7], Pseudozyma tsukubaensis [8], and Trichosporonoides megachiliensis [9] with YP/S conversion yield ranging from 0.43 to 0.61 g/g. In yeast, erythritol is synthesized via the pentose phosphate pathway (PPP, [10, 11]) as an osmoprotectant in response to osmotic stress [1].
Recently, the yeast Yarrowia lipolytica has also been found to be an efficient erythritol producer [2]. Several processes based on wild-type strains have been developed [12,13,14], but the most promising processes are based on metabolically engineered strains. Overexpression of genes involved in PPP, namely transketolase (TKL1, YALI0E06479g), transaldolase (TAL1, YALI0F15587g) and erythrose reductase (ylER, YALI0F18590g), allowed the erythritol productivity to increase with various magnitudes (from 16 to 200%) [11, 15, 16]. Other engineering strategies based on improved carbon source metabolism [10, 16] or co-factor engineering [11] have also been employed with success to improve erythritol production.
The final step of erythritol synthesis is the reduction of erythrose from the PPP by erythrose reductase (ER) with concurrent NAD(P)H oxidation [7, 17]. Several studies have been conducted on ER from the yeast. One ER has been purified and characterized from Candida magnoliae [7], while three ER isozymes were found in Trichosporonoides megachiliensis (ER-I, ER-II and ER-III isozymes [18], and two in Moniliella sp. (MsER1 and MsER2 isozymes, [19]). However, the exact biological activity and properties of these isozymes in erythrose reduction remain to be characterized. Recently, gene YALI0F18590g was reported as encoding an ER in Y. lipolytica [15]. Overexpression of the latter in strain Y. lipolytica MK1 yielded an erythritol titer of 44.4 g/L and productivity of 0.77 g/L h.
In order to get more insights on ER in Y. lipolytica and with the aim to further increase the erythritol production, we identified and characterized two additional ER (namely ER10 and ER27) in Y. lipolytica strain CGMCC7326, a strain able to produce erythritol with very high titer (more than 150 g/L, [14, 20]). Overexpression of those ER encoding genes together with ZWF1 (YALI0E22649g) and GND1 (YALI0B15598g) that allow NADPH to be replenished, yielded significantly improved erythritol productivity.
Results and discussion
Identification of erythrose reductase-encoding genes in Y. lipolytica CGMCC7326
In erythritol producing yeast, the final step of erythritol synthesis consists in the reduction of erythrose by specific erythrose reductase [10, 11]. Recently, Janek et al. [15] identified an ylER enzyme (YALI0F18590p) in Y. lipolytica based on sequence similarity with erythrose reductase from Candida magnoliae [7]. These ER enzymes, belonging to the aldose reductase family (ALR), have also been reported mainly dependent on NADPH as a redox co-factor [5, 18, 21, 22]. Different ER isozymes have been reported in T. megachiliensis [18] and Moniliella sp. [19], and these erythrose reductases are strictly dependent on NADPH as a redox co-factor [18, 21, 22]. We searched the gene function from the genome annotation of Y. lipolytica strain CGMCC 7326 for enzymes with reductase and NADPH as keywords. Then only enzymes with NADPH dependent reductase were obtained from the genome annotation.
This led to the identification of 12 putative genes, including g141.t1 (ER10), g3023.t1 (ER25) and g801.t1 (ER27) (Table 1). In order to confirm the predicted catalytic activity, these twelve genes were PCR amplified from the genomic DNA of strain CGMCC7326 using primers listed in Additional file 1: Table S1, cloned into a pET28a vector and expressed in E. coli BL21(DE3). Assay for reductase activity on cell extract of IPTG-induced cells using d-erythrose as a substrate and NADPH as a co-factor led to the identification of strain HCE102 (gene g141.t1, ER10), HCE110 (gene g3023.t1, ER25) and HCE111 (gene g801.t1, ER27) with NADPH-dependent erythrose reductase activity. Indeed, reduction of d-erythrose into erythritol could be detected with the above-mentioned cell extract as shown in Fig. 1. No reductase activity could be found in the crude extract of non-transformed E. coli in those experimental conditions.
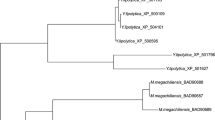
Protein BLAST search using a translated sequence of genes g141.t1, g3023.t1, and g801.t1 as a query, highlighted in Y. lipolytica strain CLIB122 genes YALI0D07634g, YALI0C13508g, and YALI0F18590g, respectively, with 100% identity. Therefore, gene g801.t1 corresponds to the gene YALI0F18590g identified as encoding ylER in Y. lipolytica strain MK1 [15]. Amino acid sequence identity between ER10, ER25, and ER27 ranged from 26.8 to 31.8%, while identity ranged from 24.0 to 38.0% for C. magnoliae ER (ACT78580.1), T. megachiliensis ER1 (BAD90687), Tilletiaria anomala ER3 (XP_013243550.1) and Moniliella sp. ER3 (AGB07593.1) (Additional file 1: Table S2). To verify Blast analysis, a phylogenetic tree was also constructed with a full-length amino acid sequence of ER10, ER25, ER27, other selected ER and NADPH-dependent aldose reductases using the MEGA7 software. The most striking point shown in Fig. 2 is that Y. lipolytica ER10 is closely related to l-arabinose reductase from the fungus Magnaporthe grisea (54.27% identity) and is relatively distant from Y. lipolytica ER25 and ER27 (26.8% and 31% of identity, respectively). This in silico analysis suggests that ER10 has evolved differently from ER25 and ER27 in the ALR family.
Phylogenetic analysis of the three erythrose reductases (ER10, ER25, and ER27, underlined in blue) from Y. lipolytica and other selected reductases (ADR aldose reductase, LAR l-arabinose reductase, XR xylose reductase). The phylogenetic tree was constructed based on the alignment of full amino acid sequences. All the analyzed sequences of aldose reductase enzymes were retrieved from GenBank and SWISS-PROT databases
Disruption of three ERs gene and its effects on erythritol synthesis
To confirm that the three putative identified ERs are indeed involved in erythritol biosynthesis, the corresponding encoding gene (i.e. ER10, ER25 and ER27), were disrupted in Y. lipolytica strain CGMCC7326. Disruption cassettes, namely pINA-UPER10-DWER10, pINA-UPER25-DWER25, pINA-UPER27-DWER27, were constructed as described in “Methods”, and used to disrupt the corresponding gene separately or simultaneously. The disrupted mutants were grown in EPMG medium and erythritol was quantified directly after glucose depletion in the medium. As shown in Table 2, the specific growth rate of the disrupted mutants were significantly decreased from that of the parental strain. Compared to the parental strain CGMCC7326, disruption of genes g3023.t1 (ER25, strain HCY101) and g801.t1 (ER27, strain HCY102) resulted in a lower erythritol production (11.1% and 17.1% decrease, respectively), a lower productivity (30% and 39% decrease, respectively) and a lower yield (11.7% and 17.6% decrease, respectively) (Table 2). Unexpectedly, the disruption of gene g141.t1 (ER10, strain HCY100) led to an increase in erythritol titer and yield (10% and 9%, respectively). Disruption of the three ER encoding genes in strain HCY103 led to a lower erythritol production (decreased by 36.8%), a lower productivity (decreased by 61.2%) and a lower yield (decreased by 37.2%). From this, it could be concluded that the three identified genes encode proteins involved in the erythritol biosynthetic pathway in Y. lipolytica CGMCC7326, deletion of ER10 gene increased erythritol biosynthetic capacity, while deletion of ER25 and ER27 gene decreased erythritol biosynthetic capacity. However, the ability of the multiple disrupted strain HCY103 to synthesize erythritol highlights that alternative biochemical pathway for erythritol synthesis or other unknown ER gene(s) remain to be identified.
Purification of erythrose reductases
To further characterize the catalytic activity of ER10, ER25, and ER27, the enzymes were separately produced using E. coli and purified. After 10 h of induction of the corresponding E. coli BL21(DE3) strains, namely HCE102, HCE110 and HCE111, cells were collected and proteins were purified by Ni–NTA and gel filtration chromatography (see “Methods” for details). The apparent molecular weights of ER10, ER25 and ER27 deduced from SDS-PAGE corresponded to the one calculated from the amino acid sequence (35.8 kDa, 35.0 kDa and 36.2 kDa, respectively; Additional file 1: Figure S1). The purity of the three purified ER, estimated by scanning densitometry of the SDS-PAGE gel after protein staining with Coomassie brilliant blue G250 was higher than 98% for ER10, ER25 and ER27.
Determination of the kinetic parameters of erythrose reductases
The catalytic constants were deduced from the non-linear regression curves (Table 3 and Additional file 1: Figure S2). All three of the enzymes were found able to convert erythrose into erythritol. ER25 showed the highest values of Kcat (19.7 s−1) and Kcat/Km (1.09 mM−1 s−1) demonstrating a higher ability to catalyze the reduction of erythrose into erythritol than ER10 (11.43 s−1 and 0.98 mM−1 s−1, respectively) and ER27 (15.8 s−1 and 0.61 mM−1 s−1) respectively.
We also assessed the ability of the purified ER to catalyze the backward reaction (i.e. the oxidation of erythritol) at pH8.0. For this purpose, erythritol was used as a substrate at different concentrations (from 20 to 1500 mM) and NADP+ was used as the cofactor (2 mM). As shown in Table 3, ER10 was found able to oxidize erythritol in the presence of NADP+, however with a lower efficiency than for the forward reaction (0.86 s−1 and 0.005 mM−1 s−1, respectively). Surprisingly, the reaction product, analyzed by high-performance liquid chromatography, was found to correspond to erythrulose and not to erythrose (Fig. 3). It has been reported recently in Y. lipolytica, that erythrulose, the first intermediate of erythritol catabolism, is obtained from an oxidation reaction catalyzed by an erythritol dehydrogenase, namely EYD1 (gene YALI0F01650g, [23]). However, such an oxidative activity on erythritol has been also reported for the ER from C. magnoliae KFCC11023 but only at a low rate and in alkaline conditions [7]. Based on kinetic parameters, the reaction catalyzed by ER10 in cells is more likely the reduction of erythrose to erythritol than its oxidation of erythritol to l-erythrulose. As described in the above section, the erythritol titer of strain HCY100 increased by 10% than that of the wild-type strain CGMCC7326, when ER10 gene was disrupted (Table 2). The utilization of erythritol by Y. lipolytica slowed down when ER10 gene was disrupted, and erythritol synthesis can be compensated by ER25, ER27 or other unidentified aldose reductases with erythrose reductase activity. For ER25 and ER 27, the oxidation of erythritol could not be detected in the experimental conditions tested (data not shown).
Biochemical properties of purified ER
In Y. lipolytica strain MK1, the erythrose reductase encoded by gene YALI0F18590g (namely ylER) displays the highest activity at pH 3 in the presence of 0.25 mM of Zn2+ [15]. Similarly, the effect of pH and divalent metal ions on the catalytic activity was determined for ER10 and ER25. The erythrose reductase activity was tested at pH values ranging from 3 to 8. As shown in Fig. 4, the optimal pH for ER10 reductase activity is 6.0; the activity was reduced by 10% at pH 5.0 and 7.0. At pH 3.0 and 8.0, the enzymatic activity was reduced by 37% and 32%, respectively. The optimal pH for ER25 reductase activity is 4, and activity significantly decreases at pH 3 and 8 (26% and 40%, respectively). The optimal pH value of ER10 (pH 6.0) and ER25 (pH 4.0) are somewhat higher than the one reported for ylER (pH 3.0) by Janek et al. [15]. These results showed that the three ERs characterized so far in Y. lipolytica prefer acidic conditions for maximal activity, which is a typical characteristic of aldo–ketoreducatse (AKR, [24]).
Effect of pH on the specific activity of ER10 and ER25 reductases. Purified enzymes were mixed with Mcilvaine’s buffer and assayed at various pH values ranging from 3.0 to 8.0. The values provided are the means of three independent replicates; the standard deviations represented less than 10% of the means
Similarly, to ylER from Y. lipolytica strain MK1, the addition of Zn2+ increases the activity of both ER10 and ER25 (Additional file 1: Table S3). Ni2+ also seems to enhance ER25 activity at concentrations ranging from 0.5 to 1 mM but reduced significantly the activity of ER10. Other divalent ions, such as Cu2+, Mn2+, or Ca2+ also inhibit the activity of both enzymes (Additional file 1: Table S3).
Substrate and cofactor specificity of ER10 and ER25
The ylER from Y. lipolytica MK1 was also shown to have a high specific activity for d-erythrose as well as for d-arabinose, d-fructose, d-galactose, and d-glucose [15]. Similar experiments showed that ER10 and ER25 present the highest specific activity for d-erythrose (8.2 and 14.6 U/mg, respectively; Table 4). In contrast to ER25, ER10 is able to use l-arabinose, d-xylose, d-ribose, and d-xylulose as substrate, however with a lower specificity (4.3, 2.4, 3.4, 1.3 U/mg, respectively; Table 4). Both ER10 and ER25 were found unable to reduce d-fructose, d-galactose and d-glucose in contrast to the ylER from strain MK1 (data not shown). The substrate specificity of ER10 was consistent with the erythrose reductase from C. magnoliae, which also has enzymatic activity against d-erythrose, d-xylose, and d-ribose [21].
Yeast ALRs can be classified into two groups according to their strict dependency to NADPH or their dual coenzyme (i.e. NADPH and NADH) specificity [25, 26]. Janek et al. [15] has already demonstrated that ylER is dependent on NADH as a co-factor. To investigate the cofactor preference of ER10 and ER25, reduction of d-erythrose was measured in the presence of both NADPH and NADH. Reduction of erythrose was only detected when NADPH was used as a cofactor (8.2 U/mg protein for ER10, and 14.6 U/mg protein for ER25). Erythrose reduction could not be measured in the presence of NADH even at high concentration (10 mM). Similarly, oxidation of erythritol by ER10 could only be detected in the presence of NADP (0.65 U/mg of protein). The specific motif IPKSXXXR has been reported as a common catalytic feature found in both aldo–keto reductase (AKR) and short-chain dehydrogenase/reductase (SDR) implicated in cofactor binding. Indeed, it has been demonstrated that the lysine residue binds the 2′-phosphate of NADPH [27, 28]. Protein sequence analysis highlighted such a motif for ER25 at position 266 (i.e. IPKSNNVER) and for ER10 at position 258 despite the serine residue is replaced by a threonine (i.e. IPKSTRSVER) (Additional file 1: Figure S3). In contrast, this motif could not be detected in ER27. This confirms that both ER10 and ER25 are NADPH dependent reductase.
Overexpression of erythrose reductase
With the goal to increase erythritol productivity, gene encoding ER10 (g141.t1), ER25 (g3023.t1) and ER27 (g801.t1) were overexpressed under the control of the strong constitutive promoter hp4d, either separately or simultaneously, in the Y. lipolytica strain CGMCC7326. Gene overexpression was confirmed by qPCR (12-fold increased expression in average as compared to the wild-type strain, data not shown), demonstrating that the expression cassettes were functional in transformed strains. The engineered strains were grown in EPMG medium until the carbon source (glucose 300 g/L) was totally consumed. As shown in Table 5, the specific growth rates of the ER overproducing strains are not significantly different of that of the parental strain. Strains HCY104 (php4d-ER10), HCY105 (php4d-ER25) and HCY106 (php4d-ER27) all exhibited an increased erythritol titer as compared to the parental strain CGMCC7326 (12%, 13% and 16%, respectively; Table 5). Engineered strain AJD pAD-ylER overexpressed YALI0F18590g (ER27 gene in this study) in the starting strain Y. lipolytica MK1 resulted in an enhanced erythritol production of 44.44 g/L, 20% higher than that of the control strain Y. lipolytica MK1, and 4% higher than the strain HCY106 (13% higher than that of the control strain CGMCC7326). However, the productivity of the HCY106 strain was higher relative to the engineered strain AJD pAD-ylER (2.2 g/L h vs 0.77 g/L h). The time for glucose consumption was also reduced by 13% for strain overexpressing ERs encoding gene as compared to the parental strain. Overexpressing strains also exhibited a slightly higher glucose uptake rate (Table 5). Glucose was totally depleted after 96 h for the control strain, while only 84 h were needed for all engineered strains.
Next, we overexpressed the gene coding for ER10, ER25, and ER27 simultaneously in strain CGMCC7326 with the aim to further increase the erythritol productivity. In the resulting HCY107 strain, expression of genes YALI0D07634g (ER10), YALI0C13508g (ER25), and YALI0F18590g (ER27) were increased by 5.3, 15.7 and 16.4-fold, respectively, as compared to the parental strain (data not shown). However in contrast to our expectation, erythritol productivity and yield for strain HCY107 were not further increased as compared to that of strain HCY104, HCY105, and HCY106, respectively (Table 5). To explain this observation, we hypothesized that the intracellular pool of NADPH was not sufficient to sustain the ER activity in strain HCY107.
Engineering of cofactor metabolism
As demonstrated above, the reduction of erythrose by ER needs NADPH as a cofactor. Therefore, regenerating this cofactor from NADP is a key factor to increase erythritol productivity in strain HCY107. The first reaction of the PPP pathway, catalyzed by the glucose-6P dehydrogenase (G6PD), generates NADPH from glucose-6P and NADP+. In yeast, it has been suggested that G6PD has a major role in NADPH production [29]. The 6-phosphogluconate dehydrogenase, that catalyzes the third reaction of the PPP pathway, also generates NADPH using 6-phosphogluconate as substrate. In Y. lipolytica, these two enzymes are encoded by gene ZWF1 (YALI0E22649g) and GND1 (YALI0B15598g), respectively [11]. Therefore, overexpressing these two genes in strain HCY107 will regenerate the NADPH consumed by ER. Moreover, the flux of carbon (glucose-6P from glycolysis) will be redirected toward the PPP pathway, and thus the synthesis of erythrose, the substrate of ER. In the resulting strain HCY108 (php4d-ER10-25-27-pTEF-ZWF1-GND1), erythritol yield and productivity were further improved by 6.7% and 14.3%, respectively, as compared to strain HCY107, and 23.5% higher erythritol yield and 50% higher productivity compared to the wild-type strain CGMCC7326 (Table 5). Though the erythritol titer was only average 6–7% higher for the strain HCY108 (overexpression of the three ER genes plus zwf1 and gnd1 genes) than that of HCY107 (overexpression of the three ER genes), the average 6–7% improvement was also important to increase its economy. The titer could be further improved by overexpression of other key genes related to erythritol synthesis such as transketolase or ribose 5-phosphate epimerase genes. This demonstrates that overexpression of genes ZWF1 and GND1 have some positive impact on erythritol synthesis.
In contrast to other reports [11], the byproducts such as mannitol and arabitol were not increased in all engineered strains, as compared to the wild-type, while erythritol production increased significantly for all engineered strains (Additional file 1: Figure S4). The mannitol and arabitol dehydrogenase activities which are responsible for byproducts mannitol and arabitol synthesis are very low, 0.021 U/ml and 0.015 U/mL, almost the same to the wild type. In contrast to the control strain CGMCC7326, the engineered strain HCY108 produced less biomass and citrate (6.3 g/L citrate for HCY108 and 13.4 g/L for the control strain CGMCC7326), when fermented at starting pH 6.5, resulting in higher pH than the control strain CGMCC7326 (Additional file 1: Figure S5). The results indicated that overexpression of the three ER genes plus ZWF1 and GND1 could push glucose metabolism into phosphate pentose pathway leading to synthesize more erythritol (Table 5).
Conclusions
We have isolated, purified and characterized two novel ER enzymes of Y. lipolytica. They were found able to reduce erythrose to erythritol specifically in the presence of NADPH as a cofactor. In contrast to previously reported ER in the yeast Y. lipolytica, ER10 and ER25 have an optimal catalytic activity at higher pH than that of YALI0F18590p (ER27 in this study). Overexpression of genes encoding these ER together with the engineering of NADPH metabolism allowed significantly increased erythritol production titers.
Methods
Chemicals
d-erythrose, l-erythrulose, d-erythritol, d-arabitol, l-arabinose, d-xylose, d-ribose, and d-xylulose were obtained from Sigma-Aldrich (St. Louis, United States). Coomassie brilliant blue R-250, cofactor NADP, NAD, NADH, NADPH, and antibiotics were purchased from Sangon Biotech (Shanghai, China). The chromatographic media were procured from GE Healthcare Life Sciences (Sweden). All other chemicals were of analytical grade and used as such without any purification.
Strains, media, and culture conditions
The Escherichia coli and Y. lipolytica strains used in this study are listed in Additional file 1: Table S1. The E. coli strains were grown at 37 °C in Luria–Bertani medium supplemented with kanamycin sulfate (50 mg/L). The Y. lipolytica strains were grown at 28 °C in YPD (10 g/L yeast extrac, 5 g/L tryptone, and 10 g/L dextrose) or YNB medium (10 g/L yeast nitrogen base without amino acids, 5 g/L ammonia sulfate) supplemented sucrose (20 g/L, YNBS), lactose (20 g/L, YNBL) or xylitol (20 g/L, YNBX). Hygromycin (200 μg/mL) was added in YPD when necessary to screen transformants. For erythritol synthesis, the EPMG medium was employed (per L): 300 g glucose, 8 g yeast extract, 8 g corn syrup, 2 g ammonium citrate, 0.05 g MnSO4·H2O, 0.05 g ZnSO4·7H2O and 0.01 g CuSO4·5H2O, initial pH 6.5. For solid media, agar (15 g/L) was added. Shake-flask cultures for erythritol production were performed in triplicate using 2 L baffled flasks containing 500 mL EPMG medium, at 30 °C and 200 rpm. Cultures were performed until glucose was depleted. All the baffled Erlenmeyer flasks containing culture medium were weighed both at the start of fermentation and then prior to taking samples aliquots for analysis. Sterile water was supplemented to compensate for the medium evaporation during fermentation. At the time of sampling, the resulting reduced weight was replenished by the addition of same weight of distilled water. At the end of the fermentation, the final weight was essentially the same as the initial weight.
Analytical methods and calculation of erythritol production parameters
Erythritol, erythrose, l-erythrulose, mannitol, arabitol, arabinose, arabitol, xylose, xylitol, ribose, ribitol, and glucose were quantified by HPLC using a refractive index detector (Shodex RI101) and a Shodex SP0810 ion exclusion column (300 × 8 mm). Elution was performed at 70 °C using pure water at a flow rate of 1 mL/min. The concentrations of eluted compounds were calculated by internal standard methods [30]. The citrate concentration was determined by HPLC using column SH1011 (Shodex. Japan), eluent was 5 mM HCLO4, flow rate 1 mL/min, detector was VIS at 430 nm.
The mass yield of erythritol (YERY) was expressed in g/g from glucose and was calculated from the equation YERY = P/S. The volumetric productivity (QERY) was expressed in g/L h and was calculated from QERY= P/V·t, where P is the amount of erythritol in the culture liquid at the end of fermentation (g); S is the total amount of glucose consumed (g); V is the initial volume of culture liquid (L), and t is the culture time (h). Glucose consumption rate (rGLU) was calculated as the amount of glucose consumed per hour and per liter of culture medium.
General molecular biological techniques
Standard media and techniques were used for E. coli [31], and the media and techniques used for Y. lipolytica have been described elsewhere [32]. The restriction enzymes, DNA polymerases, and ligase were supplied by Thermo Fisher Scientific. Genomic DNA from Y. lipolytica was prepared in accordance with Cheng et al. [33]. PCR was performed using the primers listed in Additional file 1: Table S1. Dream Taq DNA polymerase (Thermo Scientific) was used for cloning, and ExTaq DNA polymerase (Takara) was used to verify the gene integration in the genome. The PCR fragments were purified from the agarose gels using a GeneJet Gel Extraction Kit (Thermo Scientific). DNA sequencing and primers synthesis were performed by Sangon Biotech (https://www.sangon.com). Yeast transformation was as described in An et al. [34].
Sequence analysis
Protein BLAST searches were performed on National Center for Biotechnology Information web server (http://blast.ncbi.nlm.nih.gov/Blast). The phylogenetic tree of putative ER genes was constructed with MEGA7 software [35] using the neighbor-joining method [36]. Bootstrap analysis was used with 1000 replicates to test the relative support for the branches produced by the neighbor-joining analysis. The evolutionary distances were computed using the JTT matrix-based method [37]. The rate variation among sites was modeled with a gamma distribution (shape parameter of 1). Evolutionary analyses were performed based on the alignment of the full-length amino acids sequences. All the analyzed sequences of aldose reductase enzymes were retrieved from GenBank and SWISS-PROT databases.
Cloning of ER encoding genes
Putative ER genes (Table 1) were PCR amplified for genomic DNA of strain CGMCC7326 using primer listed in Additional file 1: Table S1. Forward (F) and reverse (R) primers (name according to gene nomenclature) were designed to introduce a NdeI and XhoI site in the resulting amplicons, respectively. Amplicons were digested by NdeI and XhoI and cloned into pET28a expression vector at the corresponding sites. Strain E. coli BL21(DE3) was then transformed with the different constructs. The correctness of the resulting vectors was verified by DNA sequencing using a T7 primer (Additional file 1: Table S1).
Disruption of ER encoding genes
To construct the ER10 disruption cassette, 2 kb DNA fragment located upstream (P) and downstream (T) of gene g141.t1 were PCR amplified using genomic DNA of strain CGMCC7326 as a template, and primers pairs ER10-F1/ER10-R1 and ER10-F2/ER10-R2, respectively. After purification, P fragment was digested with NotI and SalI and cloned at corresponding sites of plasmid pINA-Pir1-A.oryFTase [38], to yield plasmid pINA-UPER10 (Additional file 1: Table S1). The purified T fragment was then digested with EcoRI and NotI, and cloned at corresponding sites of plasmid pINA-UPER10, to yield plasmid pINA-UPER10-DWER10. Similarly, to construct ER25 disruption cassette, P and T fragments of gene g3023.t1 were amplified using primer pairs ER25-F1/ER25-R1 and ER25-F2/ER25-R2, respectively. Purified P and T fragments were then digested with NotI-SalI and EcoRI-NotI, respectively, and cloned in two steps in plasmid pHyg-Pir1-PdSIase [39] to yield final plasmid pINA-UPER25-DWER25. The ER27 gene (g801.t1) disruption plasmid pINA-UPER27-DWER27 was constructed similarly with primers pairs ER27-F1/ER27-R1 and ER27-F2/ER27-R2. P and T fragments were digested with NotI-SalI and EcoRI-NotI, respectively, and cloned in two steps in plasmid pHP4d-Pir1-A.oryGal [34]. The above plasmids were then NotI digested and the purified ER disruption cassettes were used to transform Y. lipolytica strain CGMCC7326. Transformants were selected using sucrose, lactose or hygromycin B as a selectable marker. The correctness of genes disruption in the resulting strains HCY100, HCY101, and HCY102 was verified by analytical PCR using primer pair ER10-F/ER10-R, ER25-F/ER25-R and ER27-F/ER27-R (Additional file 1: Table S1). To construct strain disrupted for ER10, ER25 and ER27, the disruption cassettes released from plasmid pINA-UPER10-DWER10 and pINA-UPER27-DWER27 after NotI digestion were used to transform sequentially strain HCY101. After selection of transformants on medium containing sucrose (YNBS) and lactose (YNBL), the correctness of ER10 and ER27 gene disruption was verified by analytical PCR using primer pairs ER10-F/ER10-R and ER27-F/ER27-R, respectively. The strain deleted for the three ER encoding genes was named as HCY103 (Additional file 1: Table S1).
Overexpression of ER encoding genes
Genes g141.t1 (ER10), g3023.t1 (ER25) and g801.t1 (ER27) were amplified with primer pairs ER10-F11/ER10-R11, ER25-F11/ER25-R11, and ER27-F11/ER27-R11, respectively, and genomic DNA of strain CGMCC7326 as a template. Primers F and R were designed to introduce a HindIII and BamHI site at, respectively, 5′ and 3′ end of each ER encoding gene (Additional file 1: Table S1). Purified amplicons were then digested using HindIII and BamHI, and cloned at the corresponding sites of plasmid pHyg-Pir1-PdSIase [39], a derivative of plasmid pINA1313 [40] that allow random integrations into the yeast genome. The resulting constructs were named pINA-ER10, pINA-ER25, and pINA-ER27, respectively (Additional file 1: Table S1). The correctness of the resulting constructs was verified by DNA sequencing. Expression cassettes for genes g141.t1 (ER10), g3023.t1 (ER25) and g801.t1 (ER27) were rescued from the corresponding vectors by NotI digestion. They were then purified from the agarose gel and used to transform Y. lipolytica strain CGMCC7326. Transformants were selected on YPD medium supplemented with hygromycin B (200 μg/mL). The resulting strains were named HCY104 (php4d-ER10), HCY105 (php4d-ER25), HCY106 (php4d-ER27). To construct strain HCY107 (Additional file 1: Table S1), that overexpress the three ER genes (ER10 + ER25 + ER27), the hygromycin concentration was increased to allow additional integration of the ER overexpression cassette.
We started from a strain overexpressing ER10 (HCY104) and we transformed it with ER25 expression cassette. We then selected the corresponding transformant (genotype ER10-ER25) on medium containing 800 μg/mL of hygromycin. This strain was then transformed with ER27 expression cassette and transformants were selected and 2000 μg/mL of hygromycin. In brief, transformants were selected on YPD supplemented with hydromycin B at a concentration of 200 μg/mL of hygromycin for mono-integration, 800 μg/mL for double-integration and 2000 μg/mL for triple-integration.
Overexpression of gene ZWF1 and GND1
Genes ZWF1 (YALI0E22649g) and GND1 (YALI0B15598g) from Y. lipolytica were also overexpressed in the strain HCY107. A DNA fragment containing genes ZWF1 and GND1 under the control of the constitutive hp8d promoter and XDH gene from Scheffersomyces stipitis CBS 6054 used as a selectable marker was synthesized by GENEWIZ (Suzhou, China), and cloned at EcoRI site of pUC57 to yield pUC57-zwf-gnd (see Additional file 1: Figure S6 for details). The ZWF1 and GND expression cassette rescued from pUC57-zwf-gnd by using EcoRI were then used to transform strain HCY107. Transformants were selected using xylitol as a selectable marker (YNBX). The final strain that overexpresses genes ER10, ER25, ER27, ZWF1 and GND1 was designated as HCY108.
RNA isolation and transcript quantification
Shake-flask cultures were grown in EPMG medium. Cells were collected at an OD600 of 2.0 and stored at − 80 °C in Trizol solution. Total RNAs were extracted using the TRNzol kit from TianGen Biotech (Beijing, China), and cDNA was obtained using PrimeScript™ RT reagent Kit with gDNA Eraser (Takara, Dalian, China). The qRT-PCRs were performed using SYBR® Premix ExTaq™ II (TliRNaseH Plus), Takara, Dalian, China) and ABI7500 Real-Time PCR system (Applied Biosystems). Primer pairs for qRT-PCRs were, ER10-Ffluo/ER10-Rfluo, ER25-Ffluo/ER25-Rfluo, ER27-Ffluo/ER27-Rfluo for gene ER10, ER25 and ER27, respectively (Additional file 1: Table S1). Gene expressions were normalized to that of the actin gene (primers β-actin-up/β-actin-down; ∆CT method). The fold differences in ER gene expression between the transformants and the control strains CGMCC7326 were calculated as 2−ΔΔCT [41]. All samples were analyzed in triplicate.
Protein production and purification
A single colony of recombinant E. coli BL21 (DE3) was grown in 5 mL of Luria–Bertani medium (LB) with 100 μg/mL kanamycin in 30-mL universal tubes at 37 °C for 3 h with shaking at 220 rpm. Thereafter, the 5 mL of cells were transferred to 495 mL of LB medium with 100 μg/mL kanamycin in a 2-L Erlenmeyer flask. The cells were incubated at 37 °C with shaking at 220 rpm until the OD600 reached 1.0. Then, isopropyl-β-d-thiogalactopyranoside (IPTG) was added at a final concentration of 1 mM and cultures were further incubated at 25 °C for 10 h (until OD600 reached 4.5). Two hundred and fifty milliliters of cells were harvested by centrifugation at 12,000g and 4 °C for 10 min, before being resuspended in 100 mL binding buffer (20 mM Tris–HCl pH 8.0, 200 mM NaCl, 1 mM PMSF, and 2 mM β-mercaptoethanol). Cells were sonicated at 4 °C for twenty cycles of 5 s at 5 s interval. Cell debris was removed by centrifugation at 10,000g for 10 min at 4 °C. The cell extract was finally filtered through a 0.45 μm membrane filter (Millipore).
Erythrose reductase from cell lysates was purified using Ni–nitrilotriacetate agarose (Ni–NTA) according to the manufacturer recommendation. Proteins were eluted with a buffer containing 25 mM NaH2PO4-150 mM NaCl-200 mM imidazole (pH 8.0). The Ni–NTA purified erythrose reductases were further applied to gel filtration on Sephadex G-25 and eluted with 50 mM Tris–Cl, 200 mM NaCl, pH 8.0 following the manufacturer’s instructions. SDS-PAGE was performed in a 12% (w/v) acrylamide gel at a constant current of 200 mA. Low molecular weight protein standard was used as size marker (GenScript, NJ, USA). After electrophoresis, gels were stained with Coomassie brilliant blue G-250 via standard procedures. Scanning densitometry of SDS-PAGE gel was performed using Phoretics 1D Non-linear Dynamics (Newcastle, UK) software.
Enzyme assay
The accurate assays of erythrose reductase activity or its Km values were problematic as commercial d-erythrose (Sigma Catalog No. E7625-250 mg) tested were only 75% purity. In order to evaluate more accurately its activity and Km values, we purified it by semi-preparative HPLC to obtain d-erythrose with 95% purity.
Erythrose reductase activity was determined by monitoring the reduction or oxidation of cofactor NADP(H) by absorbance measurement at 340 nm [5, 7]. The assay mixture (1.5 mL) contained 50 mM phosphate buffer (pH 6), 2 mM of co-factor (NADP or NADPH), 10 mM of substrate unless stated otherwise (erythrose or erythritol), and 0.2 mL of enzyme solution (cell extract or purified ER). This reaction mixture was allowed to stand for one min to eliminate any endogenous oxidation of cofactor before adding the substrate. The arabitol and mannitol dehydrogenase activity were tested according to the method described by Cheng et al. [42]. One unit of enzyme activity corresponds to 1 μmol of cofactor consumed or generated per minute at 30 °C. Activities were expressed as units/mg of protein and the results correspond to mean value of triplicate assays. The value used for ε of NADPH and NAD was as 6.22 mM/cm.
Kinetic parameters
The kinetic parameters of the purified ER enzymes were calculated. For this purpose, initial velocity studies were performed with erythrose as a variable substrate concentration ranged from 2 to 200 mM in the presence of a fixed concentration of cofactor (NADPH, 2 mM). When using erythritol as a substrate, the concentration varied from 20 to 1500 mM in the presence of NADP+ (2 mM). Kinetic parameters were calculated by non-linear regression of velocities against substrate concentrations, and processed using OriginPro 8.0 software.
Effect of pH and metal ions on ER enzyme relative activity
The influence of pH on the activity of ERs was carried out at 30 °C in the Mcilvaine’s buffer (from pH 3.0 to 8.0), containing 10 mM erythrose and 2 mM NADPH and 50 μL of purified ER enzymes (1 mg/mL). The assays were performed in triplicate at each pH point. The effect of metal ions on ER activity was studied by assaying different ZnSO4, CuSO4, MnSO4, NiSO4 and CaCl2 concentrations (from 0.1 to 5 mM) at 30 °C in 25 mM Mcilvaine’s buffer (pH 6.0) using erythrose as a substrate.
Substrate specificity of ER enzymes
The substrate specificity assays were performed using erythritol, d-erythrose, d-fructose, d-glucose, d-galactose, d-arabinose, l-arabinose, xylose, ribose, xylulose as substrate. The reaction mixture contained (1.5 mL final volume) 50 mM substrate, 2 mM NADPH, 50 μL of purified ER enzymes and 25 mM potassium phosphate buffer (pH 6.0). Specificity was expressed as U/mg of protein and normalized to that obtained for d-erythrose. For cofactor specificity, ER activity on erythrose (10 mM) was determined as described above using cofactor (NADH or NADPH) at a concentration of 2 mM and 10 mM. Oxidation of erythritol was measured in the same conditions in the presence of NADP or NAD.
References
Carly F, Fickers P. Erythritol production by yeasts: a snapshot of current knowledge. Yeast. 2018;35:455–63.
Regnat K, Mach RL, Mach-Aigner AR. Erythritol as sweetener-where from and where to? Appl Microbiol Biotechnol. 2018;102:587–95.
Rzechonek DA, Dobrowolski A, Rymowicz W, Mirończuk AM. Recent advances in biological production of erythritol. Crit Rev Biotechnol. 2017;27:1–14.
Lee JK, Koo BS, Kim SY. Fumarate-mediated inhibition of erythrose reductase, a key enzyme for erythritol production by Torula corallina. Appl Environ Microbiol. 2002;68:4534–8.
Lee JK, Hong KW, Kim SY. Purification and properties of a NADPH-dependent erythrose reductase from the newly isolated Torula corallina. Biotechnol Prog. 2003;19:495–500.
Koh ES, Lee TH, Lee DY, Kim HJ, Ryu YW, Seo JH. Scale-up of erythritol production by an osmophilic mutant of Candida magnoliae. Biotechnol Lett. 2003;25:2103–5.
Lee JK, Kim SY, Ryu YW, Seo JH, Kim JH. Purification and characterization of a novel erythrose reductase from Candida magnoliae. Appl Environ Microbiol. 2003;69:3710–8.
Jeya M, Lee KM, Tiwari MK, Kim JS, Gunasekaran P, Kim SY, Kim IW, Lee JK. Isolation of a novel high erythritol-producing Pseudozyma tsukubaensis and scale-up of erythritol fermentation to industrial level. Appl Microbiol Biotechnol. 2009;83:225–31.
Sawada K, Taki A, Yamakawa T, Seki M. Key role for transketolase activity in erythritol production by Trichosporonoides megachiliensis SN-G42. J Biosci Bioeng. 2009;108:385–90.
Mirończuk AM, Rakicka M, Biegalska A, Rymowicz W, Dobrowolski A. A two-stage fermentation process of erythritol production by yeast Yarrowia lipolytica from molasses and glycerol. Bioresour Technol. 2015;198:445–55.
Mirończuk AM, Biegalska A, Dobrowolski A. Functional overexpression of genes involved in erythritol synthesis in the yeast Yarrowia lipolytica. Biotechnol Biofuels. 2017;10:77.
Rymowicz W, Rywińska A, Marcinkiewicz M. High-yield production of erythritol from raw glycerol in fed-batch cultures of Yarrowia lipolytica. Biotechnol Lett. 2009;31:377–80.
Tomaszewska L, Rywińska A, Gładkowski W. Production of erythritol and mannitol by Yarrowia lipolytica yeast in media containing glycerol. J Ind Microbiol Biotechnol. 2012;39:1333–43.
Wang S, Wang H, Lv J, Deng Z, Cheng H. Highly efficient erythritol recovery from waste erythritol mother liquor by a yeast-mediated biorefinery process. J Agric Food Chem. 2017;65:11020–8.
Janek T, Dobrowolski A, Biegalska A, Mirończuk AM. Characterization of erythrose reductase from Yarrowia lipolytica and its influence on erythritol synthesis. Microb Cell Fact. 2017;16:118.
Carly F, Vandermies M, Telek S, Steels S, Thomas S, Nicaud JM, Fickers P. Enhancing erythritol productivity in Yarrowia lipolytica using metabolic engineering. Metab Eng. 2017;42:19–24.
Moon HJ, Jeya M, Kim IW. Biotechnological production of erythritol and its application. Appl Microbiol Biotechnol. 2010;86:1017–25.
Ookura T, Azuma K, Isshiki K, Taniguchi H, Kasumi T, Kawamura Y. Primary structure analysis and functional expression of erythrose reductases from erythritol-producing fungi (Trichosporonoides megachiliensis SNG-42). Biosci Biotechnol Biochem. 2005;69:944–51.
Deng H, Han Y, Liu Y, Jia W, Zhou Z. Identification of a newly isolated erythritol-producing yeast and cloning of its erythrose [corrected] reductase genes. J Ind Microbiol Biotechnol. 2012;39:1663–72.
Cheng, H, Lv JY, Wang B, Li DC, Deng ZX. Yarrowia lipolytica and its use in erythritol production. Chinese patent 2013; 201310282059.X.
Lee DH, Lee YJ, Ryu YW, Seo JH. Molecular cloning and biochemical characterization of a novel erythrose reductase from Candida magnoliae JH110. Microb Cell Fact. 2010;9:43.
van der Woude AD, Perez Gallego R, Vreugdenhil A, Puthan Veetil V, Chroumpi T, Hellingwerf KJ. Genetic engineering of Synechocystis PCC6803 for the photoautotrophic production of the sweetener erythritol. Microb Cell Fact. 2016;15:60.
Carly F, Steels S, Telek S, Vandermies M, Nicaud JM, Fickers P. Identification and characterization of EYD1, encoding an erythritol dehydrogenase in Yarrowia lipolytica and its application to bioconvert erythritol into erythrulose. Bioresour Technol. 2017;247:963–9.
Carper DA, Wistow G, Nishimura C, Graham C, Watanabe K, Fujii Y, Hayashi H, Hayaishi O. A superfamily of NADPH-dependent reductases in eukaryotes and prokaryotes. Exp Eye Res. 1989;49:377–88.
Mishra P, Singh A. Microbial pentose utilization. Adv Appl Microbiol. 1993;39:91–152.
Jez JM, Bennett MJ, Schlegel BP, Lewis M, Penning TM. Comparative anatomy of the aldo-keto reductase superfamily. Biochem J. 1997;326:625–36.
Lee JK, Koo BS, Kim SY. Cloning and characterization of the xyl1 gene, encoding an NADH-preferring xylose reductase from Candida parapsilosis, and its functional expression in Candida tropicalis. Appl Environ Microbiol. 2003;69:6179–88.
Gururajan VT, Pretorius IS, Otero RRC. Molecular cloning and functional expression of a novel Neurospora crassa xylose reductase in Saccharomyces cerevisiae in the development of a xylose fermenting strain. Annals Microbiol. 2007;57:223–31.
Nogae I, Johnston M. Isolation and characterization of the ZWF1 gene of Saccharomyces cerevisiae, encoding glucose-6-phosphate dehydrogenase. Gene. 1990;96:161–9.
Usher KM, Hansen SW, Amoo JS, Bernstein AP, McNally MPLCGC. Precision of internal standard and external standard methods in high performance liquid chromatography. Spectroscopy. 2015;33:40–6.
Sambrook J, Russell DW. Molecular cloning: a laboratory manual. 3rd ed. Cold Spring Harbor: Cold Spring Harbor laboratory press; 2001.
Barth G, Gaillardin C. Physiology and genetics of the dimorphlic fungus Yarrowia lipolytica. FEMS Microbiol Rev. 1997;19:219–37.
Cheng H, Jiang N. Extremely rapid extraction of DNA from bacteria and yeasts. Biotechnol Lett. 2006;28:55–9.
An J, Zhang L, Li L, Liu D, Cheng H, Wang H, Nawaz MZ, Cheng HR, Deng Z. An alternative approach to synthesizing galactooligosaccharides by cell-surface display of β-galactosidase on Yarrowia lipolytica. J Agric Food Chem. 2016;64:3819–27.
Kumar S, Stecher G, Tamura K. MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol Biol Evol. 2016;33:1870–4.
Saitou N, Nei M. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol. 1987;4:406–25.
Jones DT, Taylor WR, Thornton JM. The rapid generation of mutation data matrices from protein sequences. Bioinformatics. 1992;8:275–82.
Zhang L, An J, Li L, Wang H, Liu D, Li N, Cheng H, Deng Z. Highly efficient fructooligosaccharides production by an erythritol-producing yeast Yarrowia lipolytica displaying fructosyltransferase. J Agric Food Chem. 2016;64:3828–37.
Li L, Wang H, Cheng H, Deng Z. Isomaltulose production by yeast surface display of sucrose isomerase from Pantoea dispersa on Yarrowia lipolytica. J Funct Foods. 2017;32:208–17.
Nicaud JM, Madzak C, van den Broek P, Gysler C, Duboc P, Niederberger P, Gaillardin C. Protein expression and secretion in the yeast Yarrowia lipolytica. FEMS Yeast Res. 2002;2:371–9.
Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods. 2001;25:402–8.
Cheng H, Li Z, Jiang N, Deng Z. Cloning, purification and characterization of an NAD-dependent d-Arabitol dehydrogenase from acetic acid bacterium, Acetobacter suboxydans. Protein J. 2009;28:263–72.
Authors’ contributions
HlC, SW, XG and CZ performed the experiments. PF and HC wrote the manuscript. MB was involved in gene identification. HC supervised the whole work. All authors read and approved the final manuscript.
Acknowledgements
The authors wish to thank Dr. Chis Theron for editing the English version of the manuscript.
Competing interests
The authors declare that they have no competing interests.
Availability of data and materials
The datasets supporting the conclusions of this article are included within the article and its Additional files.
Consent for publication
Not applicable
Ethics approval and consent to participate
Not applicable.
Funding
This research was supported by the National Natural Science Foundation of China (No. 21877078).
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Author information
Authors and Affiliations
Corresponding authors
Additional file
Additional file 1: Table S1.
Primers, gene cassettes, and strains used in this study. Table S2. Identity of YALI0D07634p (ER10), YALI0C13508p (ER25), YALI0F18590p (ER27) with ER from Candida magnoliae ER (ACT78580.1), Trichosporonoides megachiliensis ER1 (BAD90687), Tilletiaria anomala ER3 (XP_013243550.1) and Moniliella sp. ER3 (AGB07593.1). Table S3. Effect of divalent metal ions on the activities of ER10 and ER25. Figure S1. SDS-PAGE analysis of the ER10, ER25, and ER27 overexpressed in E. coli BL21(DE3). Lane M: protein standards; lane 1: crude extract of non-induced cells; lane 2: crude extract of IPTG-induced cells; lane 3: purified ER protein by Ni2+ affinity resin; lane 4: purified ER protein by gel-filtration. Figure S2. The non-linear regression plots of initial-velocity against D-erythrose (A, C, D) or erythritol (B) using ER10 (A, B), ER25 (C) and ER27 (D) as enzymes. Figure S3. Amino acid sequences of ER27, ER25, and ER10. The specific motif IPKSXXXXR is highlighted in bold. Figure S4. Comparison of HPLC spectrum of culture supernatant of the Y. lipolytica wild-type strain CGMCC7326 and engineered strains, namely HCY104 (php4d-ER10), HCY105 (php4d-ER25), HCY106 (php4d-ER27), HCY108 php4d-ER10-25-27, php8d-ZWF1-GND1). Figure S5. pH or OD600 change during fermentation of strains CGMCC7326, HCY104, HCY107, HCY108, at the starting pH 6.5 (A, C), and pH 3.0 (B, D). For the control strain CGMCC7326, pH was decreased to 3.2 in 48 h and retained around 2.9±0.2 during fermentation until the depletion of glucose, and pH increased to 3.4 after glucose was completely exhausted, when the starting pH was 6.5 (A); For the engineered strains (HCY104, HCY107, and HCY108), pH was decreased to 4.6±0.05 in 24 h and maintained around 4.2±0.2 during fermentation until the depletion of glucose, and pH increased to 4.6±0.1 after glucose was depleted, when the starting pH was 6.5 (A); For the control strain CGMCC7326, the pH was maintained around 2.9±0.1 when fermented at starting pH 3 buffered with citrate until glucose was depleted (B). For the engineered strain, the pH was maintained around 3.2±0.2 when fermented at starting pH 3 buffered with citrate until glucose was depleted (B). The control strain produced more cell biomass and citrate than the engineered strains whenever at starting pH 6.5 or 3.0 (C, D). Figure S6. Schematic representation of the DNA fragment used to overexpress ZWF1 and GND1 genes in strain HCY108.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Cheng, H., Wang, S., Bilal, M. et al. Identification, characterization of two NADPH-dependent erythrose reductases in the yeast Yarrowia lipolytica and improvement of erythritol productivity using metabolic engineering. Microb Cell Fact 17, 133 (2018). https://doi.org/10.1186/s12934-018-0982-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12934-018-0982-z