Abstract
Background
Infection with Goose Reovirus (GRV) can cause serious economic losses in the goose breeding industry. In this study, the GRV allantoic fluid was concentrated and used as an antigen in a formalin-inactivated oil-emulsion vaccine.
Results
When 6 day-old geese were inoculated, antibodies against GRV became detectable at 6 days post-vaccination, their concentration peaked at 3 weeks. These antibodies were maintained for longer than 2 weeks. As the most susceptible age for GRV infection is birds under 2 weeks of age this vaccine should provide adequate cover for the most at risk birds. When geese were exposed to reovirus at different time intervals after immunization, the data revealed that the vaccine can provide a protection rate of 80%. The developed vaccine has good stability and could be stored at 4 °C for at least 12 months.
Conclusion
These results indicate that the developed GRV vaccine is safe, effectively absorbed, efficacious in inducing a rapid immune response, and effective in controlling GRV infection. Our results should be useful for the application of vaccines for controlling GRV in different goose flocks.
Similar content being viewed by others
Background
Avian reovirus (ARV) belongs to the genus Orthoreovirus within the family Reoviridae [1, 2]. Avian reovirus was first isolated from the respiratory tract of a chicken with chronic respiratory disease, liver necrosis, and arthritis by Fahey and Crawley in 1954 [3]. In 1972, Walke et al. confirmed the virus as a “reovirus” [4]. Researchers subsequently identified ARV infection in South Africa, France, Israel, Italy, China, and many other countries [5,6,7]. In 1997, the main Muscovy duck breeding region in China was affected by an outbreak of duck reovirus (DRV) disease, which resulted in white spots on the liver. Then, the duck reovirus was isolated from ducks in other breeding regions. Previous research has shown that the degree of identity at the sigma C protein level between the deduced amino acid sequence of the DRV and ARV was only 21–22% [8] and serological data showed that the DRV was antigenically different from the Avian Reoviruses present in chickens [9, 10]. All these above suggest that the DRV is very different from ARV and should be classified separately.
It has been reported that GRV can cause viral arthritis in geese, and infected geese often exhibited spleen inflammation, while those with subacute or chronic onset showed pericarditis, arthritis, and/or tenosynovitis [7]. The GRV induced clinical symptoms appeared at 2–3 weeks of age, and the disease course lasted 3–6 weeks [11]. GRV has been isolated from various organs/tissues of infected geese [7]. A previous experiment showed that the virus strains from ducks and geese shared high homology in the S1 genome segment and [12] physico-chemical, clinical and biological characteristics and molecular properties were also identical to each other [7]. Based on sequence data currently available and on criteria established for demarcation of reovirus species, previous reports [7] believe that GRVs and DRVs should be classified into a common species.
ARVs do not have a capsule membrane; instead, they have a double-capsid structure and spherical symmetry of 20 bodies. The virus particle diameter is approximately 60–80 nm. Its buoyant density in cesium chloride (CsCl) is 1.36 g/mL [13,14,15]. It does not aggregate red blood cells of the chicken, turkey, duck, goose, rat, or mouse [12, 16]. The virus also has the capacity to survive at low temperatures [17]. The viral genome is linear double-stranded RNA, which is composed of 10 genome segments. The genome segments were divided into three groups according to the different electrophoretic mobility of the fragments. There are three large gene segments (L1, L2, L3), three medium-sized gene segments (M1, M2, M3), and four small gene segments (S1, S2, S3, S4) [18,19,20,21]. The virus recombination rate is as high as 3%–5% [22].
GRV can replicate upon inoculation into goose embryos in the yolk sack, allantoic cavity, and allantoic membrane, the most effective option of which is inoculation in the yolk sac. Embryos inoculated with the virus generally die within 7 days [23,24,25]. The dead embryos show systemic bleeding, allantoic membrane thickening, and clear allantoic fluid [26, 27]. Those embryos that do survive more than seven days exhibit necrosis of the liver and spleen. Reovirus can cause significant inhibition of growth in birds, the age group of birds between one day and 9 weeks of age [28]. Reovirus transmission can be classified into two types: horizontal and vertical. The presence of the virus in the cloaca of injected hens has been confirmed, but the rate of vertical transmission in this context is low [29].
GRV disease has become common in many provinces of China. The reovirus can also occur as a co-infection with other pathogenic microorganisms, making the GRV infection more serious [20]. The most effective way to prevent the disease is vaccination. However, there are no vaccines available for GRV prevention. To bridge this gap, in this study, an oil emulsion inactivated vaccine for GRV was developed. The antibody responses in immunized geese were detected by an indirect ELISA method that was established in our laboratory. The results of challenge experiments demonstrated the efficacy of the inactivated GRV vaccine. The success of this study lays the foundations for the prevention and control of the disease, which should have major significance for the vitality of the goose industry.
Methods
Virus and animals
The SPF chicken embryos were purchased from the Poultry Research Institute of Shandong Academy of Agricultural Sciences. The geese and goose eggs were purchased from a company in Jinan, Shandong. The geese used in the study were allowed to acclimatize to their new surroundings and were healthy at the start of the experiment. Serum samples were collected from geese prior to challenge or immunization to confirm that these animals were free of GRV antibody via the ELISA assay.
Goose reovirus was isolated at our laboratory from the liver and spleen of infected geese from Jiangsu province in China, after several passages via the chorioallantoic membrane (CAM) of GRV antibody-negative goose eggs. The strain was named JS-01, the sigma C gene sequence of which has been deposited with the Genbank accession number MF139036. The virus was titrated on fertile SPF chicken eggs. The GRV liquid was subjected to a series of tenfold dilutions from 10−1 to 10−5, and each dilution was inoculated in 9-day-old SPF chick embryos. The egg lethal dose 50 (ELD50) was calculated by the method of Reed and Muench (1938).
Pathogenicity of the JS-01 strain
The experimental geese were housed in SPF animal isolators that were ventilated under negative pressure, and were provided with feed and water ad libitum. Inoculations of 6 day-old geese were performed intramuscularly with 0.2 ml JS-01 strain, which contained 10–3.75 ELD50. 20 geese were infected to investigate the pathogenicity of JS-01, and another 15 birds were left uninoculated as controls. Goslings in each group were inspected daily for signs of disease. Three birds per group were killed and necropsied on days 4, 6, 8,10 and 14 post injection.
Preparation of oil-adjuvant inactivated GRV vaccine
The GRV JS-01 strain was propagated via the CAM of GRV antibody-negative goose eggs. Eggs that died within 24 h were abandoned. Allantoic fluid of the remaining eggs was collected at 120 h post inoculation as the virus fluid. The allantoic fluid was inactivated by adding 0.2% formalin (v/v) and kept at 37 °C for 36 h, shaken every 8 h. The formalin-treated virus fluid was inoculated into SPF chicken embryos, and the harvested allantoic fluid, after three passages, was used to perform reverse transcriptase PCR (RT-PCR) for GRV detection to determine whether the virus was inactivated totally or not.
Firstly, 184 ml sterilized mineral, 12 ml span-80 (Sorbitan (Z)-mono-9-octadecenoate, ThermoFisher, USA) and 4 ml stearic acid aluminum were mixed uniformly as the oil phase. Then the oil phase and 200 ml inactivated virus fluid, which contains sterilized 4% tween-80 (Polysorbate-80) were churned at 3000 round per minute (rpm) for 30 min with homogenizer (voshin, Jiangsu China).
Purity of vaccine
Tests for microbial contaminants included mycoplasma examination and virus RT-PCR. The vaccine was innoculated onto Thiolglycollate medium (T.G) and Soybean-Casein Digest Agar Medium (TSA) cultivated at 37 °C and examined for microbial colony formation.
The vaccine was subcultured in modified Frey’s medium to perform the mycoplasma testing [30, 31].
RT-PCR assay was carried out to detect the presence of Avian Influenza virus and Newcastle disease Virus (Table 1).
Immunization and challenge procedures
Ninety geese were randomly divided into three groups (A, B and C) and kept in SPF animal isolators that were ventilated under negative pressure with consistent conditions (Table 2). Geese from group A and B were injected intramuscularly with GRV vaccine preparations at a volume of 0.2 (5.9276 × 10−5ELD50) or 0.5 (1.4819 × 10−4ELD50) mL, while group C were injected with 0.5 mL of PBS via the same route as challenge group. All geese were challenged intramuscularly with 0.2 ml of the JS-01 strain, which contained 10–3.75ELD50 at 7, 12, and 27 days after vaccination, five geese each time. After challenge, the birds were kept separately. Cloacal and oropharynx swabs were taken from each group on days 3, 7, and 12 days post injection, and viral shedding was tested by virus isolation and the RT-PCR assay. All geese including those died during the observation period were weighed twice a week and examined for gross lesions.
Sample collection, RNA extraction and nucleotide sequencing
Samples of liver and spleen were collected from the dead animals in each group and processed for the microscopic examination of lesions. Oropharyngeal and cloacal swabs were collected on days 3, 7, and 12 post-challenge (p.c.) for virus isolation and testing for viral shedding, and geese were observed for disease signs and death for 2 weeks p.c.. The swabs were immediately placed in 0.5 mL PBS (pH 7.2) and extensively vortexed. The swab fluids were freeze thawed three times, sterilized by passage through a 0.22 mm filter and stored at −20 °C for further virus isolation via injecting goose embryos. Viral RNA was extracted from the allantoic fluid harvested from the goose embryos used for virus isolation or homogenate using the Tranzol RNA Extraction Kit (TransGen Biotech, Beijing, China) according to the manufacturer’s instructions. The first-strand cDNA was generated from extracted RNA solution extracted using the Reverse Transcriptase M-MLV (Takara, Dalian, China) with Random Primer (pd(N)6, Takara, Dalian, China). The PCR mix containing 0.25 μL EX Taq polymerase (5 U/mL), 5 μl of 10× EX Taq Buffer (Mg2+ Plus), 4 μL of dNTP mixture (2.5 mM each dNTP) and 1 μL of each of the two primers (20 μM) was brought to 50 μL with RNase-free water. The reaction conditions were as follows: initial denaturation at 94 °C for 5 min, followed by 35 cycles of denaturation at 94 °C for 1 min, annealing at 54 °C for 30 s and extension at 72 °C for 2 min, with a final extension for 7 min at 72 °C. The RT-PCR products were purified, cloned into pMD18-T vector (TaKaRa, Dalian, China) and sequenced by BGI (Shenzhen, PR China).
A separate set of samples from tissues, such as liver and spleen were fixed in 10% neutral buffered formalin and embedded in paraffin wax, then cut into 4 mm thick sections. Sections were stained with hematoxylin and eosin for microscopic examination.
Before the challenge, sera were obtained from 10 geese from the control and vaccinated groups. After the challenge, five serum samples were also collected from the three groups. The serum samples were collected every three days in each week and evaluated for the development of an immune response against GRV using indirect ELISA. All serum samples were stored at −20 °C.
Alignment of JS-01 strain oC gene with selected reference sequences was performed using Clustal W method of MegAlign program of DNAStar software (version 7.10). A phylogenetic tree was assembled using the neighbor-joining method of the MEGA program (version 5.0) with absolute distances following 1000 bootstrap replicates [32].
The procedure for detecting GRV antibody by ELISA
A total of 100 μL of GRV fluid, harvested from goose eggs and concentrated by PEG20000, in 0.05 M carbonate buffer (pH 9.6) was coated onto the wells of an ELISA plate (Greiner Bio-One, Germany), which was incubated at 37 °C for 2 h. These plates were blocked by phosphate-buffered saline (PBS) containing 0.5% Polyoxyethy-lene(20)Sorbaitan Monolaurate (Tween-20, ThermoFisher, USA) and 5% powdered milk (solarbio, Beijing, China, w/v) and incubated at 37 °C for 1 h. After blocking, diluted goose serum (1: 10) was added into wells (200 μl/well) and incubated at 37 °C for 1 h. Afterward, the plates were washed three times with PBS and incubated with rabbit anti-goose IgG (1: 800) conjugated to horseradish peroxidase (HRP, Sigma-Aldrich) at 100 μl/well. The plates were, again, incubated at 37 °C for 1 h, washed three times with PBS, and 3′, 3′, 5′, 5′-tetramethylbenzidine substrate solution (TransGen, Beijing, China) was added into each well (100 μl/well). 50 μl of 3 M H2SO4 (Wuxi JINGKE Chemical CO., LTD.) was added to stop the reaction, and the optical density (OD) values were observed at 450 nm using an automated ELISA plate reader (Thermo, Waltham, MA, USA). The cut off value of the ELISA assay was an OD of 0.37 at 450 nm.
The rabbit anti-goose IgG was prepared by our lab. In order to isolate IgG from the goose egg yolk, we used the method of chloroform extraction, salt precipitation and DEAE chromatography. Sodium sulphate polyacrylamide gel electrophoresis indicates that we derived relative pure IgG. Rabbits were inoculated with the purified IgG 4 times in all. Serum from the rabbits was collected and purified IgG precipitated with saturated ammonium sulphate. Finally, the purified rabbit IgG was labeled with HRP.
Statistical evaluation
The data were analyzed by GraphPad Prism version 5.0 software (GraphPad Software Inc., San Diego, CA). To investigate the differences of the antibody titre among three groups, the data was analyzed using ANOVA via the method of Bonferroni. The p values <0.025 were considered to be statistically significant (SPSS Statistics 17.0, IBM, USA).
Results
Alignment and phylogenetic tree
The virus strain used in the study was sequenced for the σC gene, which shared homology of 94.8% ~ 96.5% with the waterfowl reovirus (Fig. 1). The σC gene of JS-01 (GenBank accession: MF139036) isolated in the study has been clustered, and it9 was in the same clade with other goose reovirus strains isolated from China. Among them, the YY10G strain (Genebank accession: KF729971.1) isolated in China shared the closest relationship with JS-01 strain (Fig. 2).
Pathogenicity of the JS-01 strain
The experimental infection of goslings resulted in mortality of 85% (17/20). Acute deaths occurred at the 2nd and 3rd day post injection. Those birds still alive showed general malaise and exhibited lameness during the whole period of observation. Splenomegaly and necrotic foci in the liver and spleen were obvious on post mortem. Virus was detected from the inoculated birds, dead and alive, from homogenate of liver and spleen via RT-PCR assay.
Response to challenge
To investigate the protective efficacy of the vaccine developed in the study, geese were challenged at different time points post vaccination. The results of these experiments are summarized in Table 3. At post-mortem examination, geese that died in the period of observation showed swelling of the kidneys, liver and spleen; necrotic foci were present in the last two organs (Fig. 3 and Fig. 4).
Necropsy of geese infected with JS-01 strain. a and b Liver hemorrhage and the appearance of white necrotic spots. d and e Spleen sclerosis, with a large number of necrotic spots appearing in the spleen. c liver of healthy a goose from vaccinated group. f: spleen of a healthy goose from vaccinated group
The histopathological changes of geese infected with JS-01 strain. a Punctate hemorrhage of liver cells, atrophy of hepatic acini, and necrosis. Inflammatory cell infiltration. b Necrosis of liver cells and vacuolization. c Intact structures of hepatic lobules, no degeneration and infiltration of lymphocytes. d Spleen cell necrosis and cellular hyperplasia. e Spleen cell hemorrhage. f Arranged densely lymphocytes, no necrosis
Antibody response of vaccinated geese
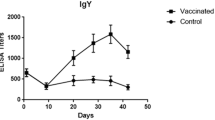
Before the vaccination, GRV antibody was measured by the ELISA assay. The antibody level began to rise markedly at 6 d.p.v., and peaked after 3 weeks (Fig. 3). During the next two weeks, the ELISA values fell slowly, but still remained in the positive range and were much higher than the cut-off value (Fig. 5). The age of the highest susceptibility to GRV infection is from 1 to 4 weeks old, therefore the results demonstrate that the vaccine can provide protection rate over 80% in susceptible birds.
The dynamic changes in antibodies induced by the inactivated vaccine in geese. One-week-old geese were injected intramuscularly (i.m.) with 0.2 or 0.5 mL of formalin-inactivated vaccine, and sera were collected randomly from six geese every three days for GRV antibody detection. The bars indicate the standard deviation
The results showed that, the group immunized with 0.5 ml of vaccine acquired protection rate of 80% against GRV (Table 4). Taking the titer of antibody into consideration, (Table 4), when the ELISA values of serum antibody increased to 0.60 or higher, the geese were completely protected. These results also demonstrate that geese were protected from the viral challenge for at least 1 month after inoculation, which is sufficient to cover the whole of the highly infectious period.
Viral shedding and virus isolation after challenge
To test for viral shedding, oropharyngeal and cloacal swabs were collected for virus isolation via goose embryos. Harvested allantoic fluid was detected by RT-PCR. Positive results in the RT-PCR indicated that the geese were shedding virus. (dates are shown in Table 4).
Discussion
In this experiment, we developed a GRV inactivated vaccine and evaluated its safety and efficacy in protecting goslings from GRV infection. The ELISA results showed that the antibody level in innoculated birds increased to OD0.6 or higher, and the vaccine exhibited a strong protective effect in geese.
When inoculated with 0.5 ml vaccine at 7 dpi, it can provide 80% protection. However, innoculation at 12 dpi with 0.5 ml vaccine can providing 100% protection. (Table 4).
Inactivated vaccines are used to control many infectious diseases in the poultry, due to their readily availability. In addition, inactivated whole-virus vaccines has been confirmed to be safe and high efficacy [33, 34]. However there has been little research into the protective immune response to GRV vaccines, possibly as the disease is newly emergent and largely present in China.
Compared with the live virus vaccines, the inactivated vaccine preparation does not appear to be toxic to goslings and produces a protective antibody response in inoculated birds for a length of time that would protect from natural disease. Selection of the inactivation agent is the most important factor in the production of an inactivated vaccine. Ideally, inactivation involves the virus completely losing its activity while maintaining good immunogenicity; therefore, there is a strong relationship between the effect of inactivation and the choice of the inactivated agent. Formaldehyde is the most commonly used inactivation agent for RNA viruses. Considering that ARV is resistant to low temperatures and a low concentration of formaldehyde solution, in this study, we chose reaction conditions of 0.2% formaldehyde and incubation at 37 °C.
An indirect ELISA was developed using concentrated virus as the antigen. Serological responses following vaccination, as measured by this ELISA, were effective in most birds and correlate closely with protection. Over the whole observation after vaccination, the antibody titre of the control group against GRV was significantly lower when compared with vaccinated groups (p value <0.025, Fig. 6).
Previous study has confirmed the efficacy of oil-emulsion inactivated vaccines for protecting ducks from DRV [35]. Efficacy evaluations have been based on a challenge study performed a few weeks after vaccination, when the antibody titers reach or are around their peak. Our study has indicated that the vaccinated geese could be completely protected from GRV challenge when the antibody titers to the challenge virus equaled or were greater than OD0.6, a titer that can be easily induced by inactivated vaccine immunization.
Horizontal transmission is a major route of infection for GRV. The elimination time of GRV from the intestinal and respiratory tracts is long, especially in the intestines, which means that fecal pollution is a major source of infection [36, 37]. Specifically, infection can quickly spread through feces in the same house. In this study, young geese immunized by the inactivated vaccine showed a high protection rate, which is over 80%, against viral challenging. Thus, we believe that the vaccine can aid in preventing the disease from spreading.
The data indicate that the inactivated GRV vaccine may represent an effective method for controlling GRV infection in the goose industry. However, vertical transmission is also an important route of GRV infection. Vertically transmitted GRV causes great economic losses due to mortality of the affected offspring, although infected breeding geese do not show clinical signs. It could thus have significant economic benefits for the industry if immunizing laying breeders could prevent vertical transmission [38]. In chickens the immune effect is generally better for breeding hens if inoculation with a living vaccine is performed before that with an inactivated vaccine [29]. We plan to study the immune effect of this vaccine in breeding geese in future work.
In this context, it has yet to be studied whether our vaccine would interfere with other vaccines for goose diseases, but this is worthy of investigation in future studies. We also need to develop and improve immunization programs for geese field use. Nonetheless, this study has provided a useful foundation by showing that the inactivated GRV vaccine could control and prevent infection.
Conclusion
The present work demonstrated that immunization with the new inactivated vaccine provides protective immunity to goslings, thereby reducing mortality, morbidity, and fecal shedding through challenge-exposure with virulent GRV. Since the advent of new, effective, and safe GRV vaccines on the market is in urgent in the near future to combat GRV. We should bear in mind that full biosecurity and husbandry management in the field can maximize vaccine effectiveness for prevention and control of GRV.
Abbreviations
- ANOVA:
-
Analysis of variance
- ARV:
-
Avian reovirus
- CAM:
-
Chorioallantoic membrane
- DRV:
-
Duck reovirus
- ELD50:
-
Egg lethal dose 50
- ELISA:
-
Enzyme linked immunosorbent assay
- GRV:
-
Goose reovirus
- HRP:
-
Horseradish peroxidase
- OD:
-
Optical density
- p.c.:
-
Post-challenge
- PBS:
-
Phosphate buffered saline
- PCR:
-
Polymerase chain reaction
- PEG:
-
Polyethylene glyco
- RNA:
-
Ribonucleic acid
- RT-PCR:
-
Reverse transcriptase PCR
- SPF:
-
Specific pathogen free
- T.G:
-
Thiolglycollate medium
- TSA:
-
Soybean-Casein Digest Agar Medium
References
Kawamura H, Tsubahara H. Common antigenicity of avian reoviruses. Natl Inst Anim Health Q (Tokyo). 1966;6(4):187–93.
van der Heide L. The history of avian reovirus. Avian Dis. 2000;44(3):638–41.
Fahey JE, Crawley JF. Studies on chronic respiratory disease of chickens II. Isolation of a virus. Can J Comp Med Vet Sci. 1954;18(1):13–21.
Walker ER, Friedman MH, Olson NO. Electron microscopic study of an avian reovirus that causes arthritis. J Ultrastruct Res. 1972;41(1):67–79.
Adair BM, Burns K, McKillop ER. Serological studies with reoviruses in chickens, turkeys and ducks. J Comp Pathol. 1987;97(5):495–501.
Dandar E, et al. The complete genome sequence of a European goose reovirus strain. Arch Virol. 2014;159(8):2165–9.
Palya V, et al. Reovirus identified as cause of disease in young geese. Avian Pathol. 2003;32(2):129–38.
Zhang Y, et al. Characterization of the sigmaB-encoding genes of muscovy duck reovirus: sigmaC-sigmaB-ELISA for antibodies against duck reovirus in ducks. Vet Microbiol. 2007;121(3–4):231–41.
Kuntz-Simon G, et al. Muscovy duck reovirus sigmaC protein is atypically encoded by the smallest genome segment. J Gen Virol. 2002;83(Pt 5):1189–200.
Bassel-Duby R, et al. Sequence of reovirus haemagglutinin predicts a coiled-coil structure. Nature. 1985;315(6018):421–3.
Gouvea V, Schnitzer TJ. Pathogenicity of avian reoviruses: examination of six isolates and a vaccine strain. Infect Immun. 1982;38(2):731–8.
Banyai K, et al. The goose reovirus genome segment encoding the minor outer capsid protein, sigma1/sigmaC, is bicistronic and shares structural similarities with its counterpart in Muscovy duck reovirus. Virus Genes. 2005;31(3):285–91.
Glass SE, et al. Isolation and characterization of a virus associated with arthritis of chickens. Avian Dis. 1973;17(2):415–24.
Schnitzer TJ, Ramos T, Gouvea V. Avian reovirus polypeptides: analysis of intracellular virus-specified products, virions, top component, and cores. J Virol. 1982;43(3):1006–14.
Spandidos DA, Graham AF. Physical and chemical characterization of an avian reovirus. J Virol. 1976;19(3):968–76.
Kawamura H, Shimizu F, Tsubahara H. Avian Adenovirus: Its Properties and Serological Classification. Natl Inst Anim Health Q (Tokyo). 1964;4:183–93.
Gouvea, V.S. and Schnitzer TJ, Polymorphism of the genomic RNAs among the avian reoviruses. J Gen Virol, 1982. 61 (Pt l): p. 87–91.
Schwartz LD, et al. Infectious tenosynovitis in commercial white leghorn chickens. Avian Dis. 1976;20(4):769–73.
Varela R, Benavente J. Protein coding assignment of avian reovirus strain S1133. J Virol. 1994;68(10):6775–7.
Cook ME, et al. Severity of tenosynovitis in reovirus-infected chickens fed various dietary levels of choline, folic acid, manganese, biotin, or niacin. Avian Dis. 1984;28(3):562–73.
Benavente J, Martinez-Costas J. Avian reovirus: structure and biology. Virus Res. 2007;123(2):105–19.
Xu, Y., Molecular virology. 2000: Hubei Science & Technology Press.
Jones RC, Jordan TW, Lioupis S. Characteristics of reovirus isolated from ruptured gastrocnemius tendons of chickens. Vet Rec. 1975;96(7):153–4.
Guneratne JR, Jones RC, Georgiou K. Some observations on the isolation and cultivation of avian reoviruses. Avian Pathol. 1982;11(3):453–62.
Barta V, Springer WT, Millar DL. A comparison of avian and mammalian cell cultures for the propagation of avian reovirus WVU 2937. Avian Dis. 1984;28(1):216–23.
Van Der Heide L. Viral arthritis/tenosynovitis: a review. Avian Pathol. 1977;6(4):271–84.
Al-Muffarej SI, Savage CE, Jones RC. Egg transmission of avian reoviruses in chickens: comparison of a trypsin-sensitive and a trypsin-resistant strain. Avian Pathol. 1996;25(3):469–80.
van Loon AA, et al. Isolation of a new serotype of avian reovirus associated with malabsorption syndrome in chickens. Vet Q. 2001;23(3):129–33.
Wood GW, Muskett JC, Thornton DH. Observations on the ability of avian reovirus vaccination of hens to protect their progeny against the effects of challenge with homologous and heterologous strains. J Comp Pathol. 1986;96(2):125–9.
Ansari AA, Taylor RF, Chang TS. Application of enzyme-linked immunosorbent assay for detecting antibody to mycoplasma gallisepticum infections in poultry. Avian Dis. 1983;27(1):21–35.
Frey ML, Hanson RP, Andrson DP. A medium for the isolation of avian mycoplasmas. Am J Vet Res. 1968;29(11):2163–71.
Felsenstein J. Confidence limits on phylogenies: an approach using the bootstrap. Evolution. 1985:783–91.
Couch RB, et al. A randomized clinical trial of an inactivated avian influenza a (H7N7) vaccine. PLoS One. 2012;7(12):e49704.
Liu L, et al. Study of the integrated immune response induced by an inactivated EV71 vaccine. PLoS One. 2013;8(1):e54451.
Liu SJ, Wang FY, Cheng ZQ, et al. Development of the proplis-adjuvant inactivated vaccine of reovirus disease in muscovy duck. Chinese Journal of Preventive Veterinary Medicine. 2006;2:027.
Jones RC, Onunkwo O. Studies on experimental tenosynovitis in light hybrid chickens. Avian Pathol. 1978;7(1):171–81.
Macdonald JW, et al. Observations on viral tenosynovitis (viral arthritis) in Scotland. Avian Pathol. 1978;7(4):471–82.
Dobson KN, Glisson JR. Economic impact of a documented case of reovirus infection in broiler breeders. Avian Dis. 1992;36(3):788–91.
Acknowledgements
The work was done at Research Institute of Poultry Disease, Agricultural University of Shan Dong Province.
Funding
This study was supported by the China Agriculture Research System (grant number: CARS-43-10) and the Shandong Science and Technology Development Program (grant number: 2014GNC111023).
Availability of data and materials
The datasets generated and analyzed during the current study are available from the corresponding author, on reasonable request. Sequences derived from this study are publicly available on Genbank®. The phylogenetic data was deposited into TreeBase (Study Accession URL: http://purl.org/phylo/treebase/phylows/study/TB2:S21245, and the reviewer access URL: http://purl.org/phylo/treebase/phylows/study/TB2:S21245?x-access-code=10b4b4d9cfdea50bbb01f83be0d04801&format=html).The alignment of sequence is presented in the Additional file 1: alignment data.
Author information
Authors and Affiliations
Contributions
YD and YT made substantial contributions to conception and design, and had the initial idea, was partially guiding the experiments and contributed substantially to the manuscript. XN performed the experiment and statistical analysis and drafted the manuscript. BZ made substantial contributions to conception and design, was actively involved in the planning and conduction of the experiment, and revised the manuscript critically. XY, YD and XZ performed measurements in the interpretation of the results as well as in the statistics. All authors have read and approved the final version of the manuscript.
Corresponding authors
Ethics declarations
Ethics approval
This study was approved by the Committee on the Ethics of Animal of Shandong (permit number20,156,681). And all applicable international, national, and institutional guidelines for the care anduse of animals were followed.
Consent for publication
Not applicable.
Competing of interest
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Additional file
Additional file 1:
Alignment data of the sequence among JS-01 strain and other reovirus strains. (TXT 61 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Niu, X., Zhang, B., Yu, X. et al. Preparation and evaluation of goose reovirus inactivated vaccine. BMC Vet Res 13, 214 (2017). https://doi.org/10.1186/s12917-017-1134-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12917-017-1134-0