Abstract
Background
To assess whether application of a hyaluronic acid-carboxymethyl cellulose membrane (HA/CMC) to the prostate bed and neurovascular plate facilitated early return of continence after nerve-sparing robot-assisted radical prostatectomy (RARP).
Methods
The subjects were 183 consecutive patients with organ-confined prostate cancer who underwent unilateral or bilateral nerve-sparing RARP. After vesicourethral anastomosis, HA/CMC was placed to cover Denonvilliers’ fascia (behind the anastomotic suture) and the preserved neurovascular plate. The time until complete continence after RARP and perioperative complications were compared between patients with or without HA/CMC.
Results
HA/CMC was applied in 13/46 patients (28.3%) receiving bilateral nerve-sparing surgery and 40/137 patients (29.2%) receiving unilateral nerve-sparing surgery. After bilateral nerve-sparing RARP, the median time until continence was significantly shorter in patients with HA/CMC than in those without HA/CMC (3.2 vs. 9.3 months, respectively, p < 0.01). After unilateral nerve-sparing RARP, the median time until continence was also significantly shorter in patients with HA/CMC than in those without HA/CMC (3.2 vs. 12.0 months, respectively, p < 0.01). Multivariate Cox proportional hazards regression analysis showed that an age < 70 years (hazard ratio [HR]: 1.74, 95% confidence interval [CI]: 1.12–2.80), institutional caseload > 200, (HR: 1.64, 95%CI: 1.10–2.47), and use of HA/CMC (HR: 1.84, 95%CI: 1.22–2.76) were independent predictors of early postoperative continence. Complication rates, including urinary leakage, did not differ significantly between patients with or without HA/CMC.
Conclusion
Application of HA/CMC to the prostate bed and neurovascular plate resulted in significantly faster postoperative return of continence after both unilateral and bilateral nerve-sparing RARP.
Similar content being viewed by others
Background
In men who undergo radical prostatectomy, urinary incontinence is one of the most unwanted postoperative complications [1], and it is well known to have multiple causes [2]. Some studies have shown that fibrosis of periurethral tissue is a risk factor for postoperative incontinence after radical prostatectomy [3, 4].
Elasticity is critical to the function of organs that undergo repeated expansion and contraction [5], and loss of elasticity due to elastic fiber damage is a major contributor to degeneration of connective tissue [6]. Hyaluronic acid (HA) is an important component of the extracellular matrix that coexists with elastic fibers and cooperates functionally with these fibers in various organs/tissues of the human body [7, 8]. HA can increase the retention of water around dehydrated elastic fibers, and hydration is enhanced by self-aggregation of HA [9], leading to further accumulation of water near elastic fibers. This improves elastic fiber recoil by increasing the availability of water molecules for hydrophobic groups in the elastin polypeptide chains. These chains become more disordered with increased hydration, allowing them to store more elastic potential energy during distention [10, 11]. We previously demonstrated the coexistence of elastic fibers and HA in the urethral submucosa and smooth muscle sphincter in male human cadavers, and we also reported that HA is distributed throughout the periurethral nerves [12].
Application of a hyaluronic acid/carboxycellulose membrane (HA/CMC) has been used to prevent postoperative visceral adhesions resulting from inflammation and fibrosis [13]. Elastic fibers and HA coexist in the smooth and striated urethral sphincter muscles, which could be a target for prevention and treatment of urethral sphincter failure. Focusing on the efficacy of HA/CMC for preventing postoperative fibrosis and scarring, we hypothesized that application of HA/CMC could improve postoperative continence after radical prostatectomy. Accordingly, the aim of the present study was to investigate whether intraoperative application of HA/CMC to the prostatic bed could maintain the elasticity of periurethral connective tissue and preserve nerves after surgical invasion, thus facilitating postoperative return of continence after radical prostatectomy.
Methods
Use of an HA/CMC adhesion barrier for prevention of adhesions during abdominopelvic surgery is approved by the US Food and Drug Administration. After receiving approval from our institutional review board (approval No.170108), we conducted a retrospective study by reviewing the Kobe University Hospital database between October 2012 and June 2015. The study population was a cohort of 183 consecutive patients with a diagnosis of organ-confined prostate cancer (clinical T1c-2N0M0) who underwent robot-assisted radical prostatectomy (RARP) using unilateral or bilateral nerve-sparing techniques. Nerve-sparing surgery was defined as use of intrafascial or interfascial nerve-sparing techniques, as described previously [14]. Seprafilm® (Genzyme Corporation, Cambridge, MA) was used as HA/CMC in the present study (Fig. 1). HA/CMC turns into a gel within 24–48 h after placement and stays in place for up to 7 days. By day 7, HA/CMC is resorbed and it is excreted from the body by day 28 [15]. After the posterior reconstruction (“Rocco” stitch) [16], 6.35 cm × 7.35 cm sized HA/CMC was placed to cover Denonvilliers’ fascia behind the anastomotic suture and to cover the preserved neurovascular plate. Then the vesicourethral anastomosis was performed. After the vesicourethral anastomosis, HA/CMC was placed on the ventral side of the anastomosis. The 183 patients were divided into three groups based on their treatment during the study period, since the first 42 patients underwent RARP without HA/CMC, the subsequent 53 patients underwent RARP with HA/CMC, and the final 42 patients received RARP without HA/CMC.
The four surgeons who carried out RARP in this series had all performed more than 40 RARP procedures before the study period. The nerve-sparing technique was applied if the tumor was not located ipsilaterally in the prostate on MRI. We excluded patients who had a history of urinary retention or preoperative incontinence. Demographic information was collected, including the age, body mass index, Charlson comorbidity index, pretreatment prostate specific antigen (PSA), prostate volume, hospital patient number, and clinical stage at enrollment. Perioperative and oncological outcomes were also recorded, including the console time, estimated blood loss, use of HA/CMC, perioperative complications categorized by the Clavien-Dindo classification, final pathologic diagnosis, and biochemical recurrence.
The urethral catheter was routinely removed on postoperative day 7. Continence was defined as freedom from pads and the date when each patient achieved pad-free status was determined by interview. Expanded Prostate Cancer Index Composite (EPIC) questionnaires were mailed to the patients every 3 months postoperatively. Biochemical recurrence was defined as a serum PSA level > 0.2 ng/ml on two separate occasions with an interval of at least 6 weeks.
Statistical analysis
The clinical and pathological descriptive data are presented as percentages, while continuous variables are presented as the median and interquartile range (IQR). Comparison of categorical valuables was performed by the unpaired t-test or Pearson’s chi-square test, as appropriate. The Kaplan-Meier method was used to estimate the continence rate and curves were compared by employing the log-rank test. Cox proportional hazards regression analysis was performed to assess the prognostic significance of target covariates. All statistical analyses were done with JMP software (version 13.0; SAS Institute, Cary, NC, USA), and P < 0.05 was taken to indicate statistical significance.
Results
Among the 183 consecutive patients who underwent nerve-sparing RARP, none of them were excluded due to a history of urinary retention or incontinence. Consequently, all 183 patients were assessed in this study, including 46 patients who underwent bilateral nerve-sparing surgery and 137 patients who received unilateral nerve-sparing surgery. HA/CMC was used in 13/46 patients (28.3%) undergoing bilateral nerve-sparing RARP and 40/137 patients (29.2%) receiving unilateral nerve-sparing RARP.
The demographic and clinical characteristics of the bilateral and unilateral nerve-sparing RARP groups are shown in Table 1. In both groups, there were no statistically significant differences with respect to age, pretreatment PSA, prostate volume, clinical stage, Gleason score at prostate biopsy, Charlson comorbidity index, institutional caseload at surgery, and follow-up period between the patients with or without HA/CMC. Table 2 summarizes the perioperative, histological, and oncological outcomes. In both the bilateral nerve-sparing and unilateral nerve-sparing RARP groups, the operating time and complication rate did not differ significantly between patients with and without HA/CMC. There was also no significant difference of HA/CMC use between the unilateral and bilateral nerve-sparing RARP groups. Moreover, the final pathological and oncological outcomes showed no significant differences between patients with and without HA/CMC in both groups.
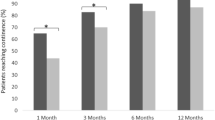
Among the 46 patients who received bilateral nerve-sparing RARP, the median time to achieve continence was significantly shorter in patients with HA/CMC than in those without HA/CMC (3.2 months vs. 9.3 months, respectively, p < 0.01) (Fig. 2a). Among the 137 patients who had unilateral nerve-sparing RARP, the median time to continence was also significantly shorter in patients with HA/CMC than in those without HA/CMC (3.2 months vs. 12.0 months, respectively, p < 0.01) (Fig. 2b).
Potential predictors of the time until complete postoperative continence are evaluated in Table 3. Univariate analysis showed that the age, institutional caseload, and use of HA/CMC were the predictors of achieving pad-free status. Multivariate Cox analysis indicated that an age < 70 years (hazard ratio [HR]: 1.74, 95% confidence interval [CI]: 1.12–2.80), institutional caseload > 200, (HR: 1.64, 95%CI: 1.10–2.47), and use of HA/CMC (HR: 1.84, 95%CI: 1.22–2.76) were independent predictors of the early return of continence.
Discussion
We investigated the influence of HA/CMC on early return of continence after nerve-sparing RARP and demonstrated that use of HA/CMC during nerve-sparing RARP significantly shortened the duration of postoperative incontinence. This difference was not only seen in patients undergoing bilateral nerve-sparing surgery, but also in those having unilateral nerve-sparing surgery. Multivariate analysis confirmed that use of HA/CMC was an independent predictor of postoperative recovery of continence.
Periurethral fibrosis and scarring have been reported as factors that predict postoperative incontinence after radical prostatectomy [3, 4]. Technical factors such as the amount of postoperative oozing or a prolonged operating time might contribute to fibrotic change. These factors can be improved by increasing the surgeon’s or institution’s experience, but complete prevention of inflammation or fibrosis after surgical invasion is theoretically impossible, and no method of preventing fibrosis or scarring after radical prostatectomy has been reported to our knowledge.
One of the main causes of postoperative incontinence after radical prostatectomy could be periurethral scarring, which can lead to chronic compression and ischemia of the periurethral tissues as well as sphincteric nerves. HA is a major component of the extracellular matrix and it plays an important role in early wound healing together with fibrin [17]. HA stimulates the production of interleukin-1 (IL-1) and IL-1 promotes fibroblast proliferation and collagenase production [18]. HA is also involved in regulating leukocyte motility, adhesion, and phagocytosis, and it acts to suppress scar formation caused by infiltration of inflammatory cells into damaged tissues [19]. HA/CMC is a surgical material with a 66.6% content of HA that is widely been used to prevent visceral adhesion by inhibiting postoperative local inflammation and fibrosis [20]. The improvement of early postoperative continence after RARP observed in the present study could have been due to suppression of periurethral scarring by intraoperative application of HA/CMC. Because HA/CMC is available worldwide, easy to use, and low cost, it seems to be a reasonable option for prevention of scarring of the urethral sphincter and periurethral tissue after radical prostatectomy.
HA also has an important role in maintaining tissue elasticity. HA increases hydration of the extracellular matrix and traps water molecules around elastic fibers, thus improving their function. Elastic fibers are a major insoluble component of the extracellular matrix that permit deformation and passive recoil of organs or tissues [9, 10]. We previously demonstrated co-localization of elastic fibers and HA in the submucosa and smooth muscle of the membranous urethra [12]. Surgical invasion can reduce tissue elasticity derived from water captured by a matrix of HA and elastic fibers, so the elasticity of periurethral tissue could be impaired after radical prostatectomy. Loss of elasticity was reported to lead to the degeneration of connective tissue [6]. In the present study, faster recovery of continence after RARP was confirmed in patients with HA/CMC than in those without it. Although direct measurement of the urethral elasticity is impossible, this difference might have been related to maintenance of urethral elasticity by application of HA/CMC.
HA can also prevent cicatrization of peripheral nerves and promote nerve regeneration. For example, rats showed faster functional recovery after sciatic nerve dissection when HA was applied to the dissected nerve [21]. Furthermore, HA creates a hydrated open lattice in the extracellular matrix that facilitates migration of regenerating axons [22]. We previously reported that peripheral nerves running through the hiatus between the bilateral levator ani slings were positive for HA in human male cadavers [12]. An association between sparing the cavernous nerves and early postoperative return of urinary continence has already been described [23]. Repeated injection of HA into nerve conduits was reported to enhance regeneration of sciatic nerve defects in rats [22]. In another study, HA reduced perineural adhesions at 4 weeks and 12 weeks after neurorrhaphy, and also enhanced peripheral nerve regeneration at 12 weeks postoperatively [21]. Furthermore, HA/CMC has been reported to reduce extraneural adhesions and promote nerve regeneration at 3 months after sciatic nerve repair in rabbits [24]. In the present study, early return of continence after application of HA/CMC to the nerve-sparing side may have been related to enhanced regeneration of axons in the cavernous nerves. Figure 3 outlines how application of HA/CMC could prevent three potential causes of postoperative loss of tissue elasticity due to surgical invasion.
A retrospective study showed that wrapping HA/CMC around the suture line led to a higher frequency of sequelae associated with intestinal anastomotic leakage after abdominopelvic surgery [25]. However, covering the prostate bed with HA/CMC did not increase including postoperative urine leakage or other complications in the present study, even though RP involves vesicourethral anastomosis. Joung et al. reported earlier recovery of erectile function when the neurovascular bundle was coated with HA/CMC gel during RARP than in the control group [26]. They also reported that complication rates did not differ between the two groups, a similar finding to the results of the present study. It is possible that precise suturing with robotic assistance [27] could contribute to reducing urine leakage and prevent migration of dissolved HA/CMC after radical prostatectomy. Excellent hemostasis of the prostatic bed achieved with RARP could also have enabled safe application of HA/CMC.
Several limitations of this study should be considered. First, the retrospective design and small number of patients limit the conclusions that can be drawn. Second, reasons of the rather low continence outcomes of the non-HA/CMC groups in the present study might be ascribed to the strict continence definition used and the fact that not all the four surgeons were experts. Third, the treatment protocol was not randomized, so results might have been influenced by the learning curve effect. Thompson et al. reported that RARP had a long learning curve with inferior early results that progressively transform to superior functional and oncological outcomes [28]. Although patients receiving HA/CMC were assigned according to institutional policy, the caseload was still an independent predictor of postoperative continence by Cox proportional hazards analysis in the present study. However, use of HA/CMC was also an independent predictor of the earlier achievement of pad-free status by Cox analysis. Thus, intraoperative application of HA/CMC to the prostate bed during nerve-sparing RARP improved postoperative continence.
Conclusions
This study demonstrated that application of HA/CMC to the prostate bed and neurovascular plate significantly shortened the duration of postoperative incontinence after both unilateral and bilateral nerve-sparing RARP. Further researches on this working mechanism and a prospective randomized controlled trial of HA/CMC in patients undergoing RARP seems to be warranted.
Abbreviations
- HA:
-
hyaluronic acid
- HA/CMC:
-
hyaluronic acid-carboxymethyl cellulose membrane
- RARP:
-
robot-assisted radical prostatectomy
References
Ficarra V, Novara G, Rosen RC, Artibani W, Carroll PR, Costello A, et al. Systematic review and meta-analysis of studies reporting urinary continence recovery after robot-assisted radical prostatectomy. Eur Urol. 2012;62(3):405–17.
Heesakkers J, Farag F, Bauer RM, Sandhu J, De Ridder D, Stenzl A. Pathophysiology and contributing factors in Postprostatectomy incontinence: a review. Eur Urol. 2017;71(6):936–44.
Tuygun C, Imamoglu A, Keyik B, Alisir I, Yorubulut M. Significance of fibrosis around and/or at external urinary sphincter on pelvic magnetic resonance imaging in patients with postprostatectomy incontinence. Urology. 2006;68(6):1308–12.
Paparel P, Akin O, Sandhu JS, Otero JR, Serio AM, Scardino PT, et al. Recovery of urinary continence after radical prostatectomy: association with urethral length and urethral fibrosis measured by preoperative and postoperative endorectal magnetic resonance imaging. Eur Urol. 2009;55(3):629–37.
Kielty CM, Sherratt MJ, Shuttleworth CA. Elastic fibres. J Cell Sci. 2002;115(Pt 14):2817–28.
Watson RE, Griffiths CE, Craven NM, Shuttleworth CA, Kielty CM. Fibrillin-rich microfibrils are reduced in photoaged skin. Distribution at the dermal-epidermal junction. J Invest Dermatol. 1999;112(5):782–7.
Korn GP, Martins JR, Park SW, Mendes A, Kobayashi EY, Nader HB, et al. Concentration of hyaluronic acid in human vocal folds in young and old subjects. Otolaryngol Head Neck Surg. 2011;145(6):981–6.
Kawase T, Shibata S, Katori Y, Ohtsuka A, Murakami G, Fujimiya M. Elastic fiber-mediated enthesis in the human middle ear. J Anat. 2012;221(4):331–40.
Scott JE, Cummings C, Brass A, Chen Y. Secondary and tertiary structures of hyaluronan in aqueous solution, investigated by rotary shadowing-electron microscopy and computer simulation. Hyaluronan is a very efficient network-forming polymer. Biochem J. 1991;274(Pt 3):699–705.
Rosenbloom J, Abrams WR, Mecham R. Extracellular matrix 4: the elastic fiber. FASEB J. 1993;7(13):1208–18.
Kendall K. Molecular adhesion and its applications: the sticky universe: Springer; 2001.
Hinata N, Murakami G, Abe S, Shibata S, Morizane S, Honda M, et al. Coexistence of elastic fibers with hyaluronic acid in the human urethral sphincter complex: a histological study. J Urol. 2013;190(4):1313–9.
Di Saverio S, Coccolini F, Galati M, Smerieri N, Biffl WL, Ansaloni L, et al. Bologna guidelines for diagnosis and management of adhesive small bowel obstruction (ASBO): 2013 update of the evidence-based guidelines from the world society of emergency surgery ASBO working group. World J Emerg Surg. 2013;8(1):42.
Hinata N, Sejima T, Takenaka A. Progress in pelvic anatomy from the viewpoint of radical prostatectomy. Int J Urol. 2013;20(3):260–70.
Becker JM, Dayton MT, Fazio VW, Beck DE, Stryker SJ, Wexner SD, et al. Prevention of postoperative abdominal adhesions by a sodium hyaluronate-based bioresorbable membrane: a prospective, randomized, double-blind multicenter study. J Am Coll Surg. 1996;183(4):297–306.
Joshi N, de Blok W, van Muilekom E, van der Poel H. Impact of posterior musculofascial reconstruction on early continence after robot-assisted laparoscopic radical prostatectomy: results of a prospective parallel group trial. Eur Urol. 2010;58(1):84–9.
Weigel PH, Fuller GM, LeBoeuf RD. A model for the role of hyaluronic acid and fibrin in the early events during the inflammatory response and wound healing. J Theor Biol. 1986;119(2):219–34.
Hiro D, Ito A, Matsuta K, Mori Y. Hyaluronic acid is an endogenous inducer of interleukin-1 production by human monocytes and rabbit macrophages. Biochem Biophys Res Commun. 1986;140(2):715–22.
Goldberg RL, Toole BP. Hyaluronate inhibition of cell proliferation. Arthritis Rheum. 1987;30(7):769–78.
Kumar S, Wong PF, Leaper DJ. Intra-peritoneal prophylactic agents for preventing adhesions and adhesive intestinal obstruction after non-gynaecological abdominal surgery. Cochrane Database Syst Rev. 2009;1:CD005080.
Ozgenel GY. Effects of hyaluronic acid on peripheral nerve scarring and regeneration in rats. Microsurgery. 2003;23(6):575–81.
Wang KK, Nemeth IR, Seckel BR, Chakalis-Haley DP, Swann DA, Kuo JW, et al. Hyaluronic acid enhances peripheral nerve regeneration in vivo. Microsurgery. 1998;18(4):270–5.
Reeves F, Preece P, Kapoor J, Everaerts W, Murphy DG, Corcoran NM, et al. Preservation of the neurovascular bundles is associated with improved time to continence after radical prostatectomy but not long-term continence rates: results of a systematic review and meta-analysis. Eur Urol. 2015;68(4):692–704.
Adanali G, Verdi M, Tuncel A, Erdogan B, Kargi E. Effects of hyaluronic acid-carboxymethylcellulose membrane on extraneural adhesion formation and peripheral nerve regeneration. J Reconstr Microsurg. 2003;19(1):29–36.
Beck DE, Cohen Z, Fleshman JW, Kaufman HS, van Goor H, Wolff BG. A prospective, randomized, multicenter, controlled study of the safety of Seprafilm adhesion barrier in abdominopelvic surgery of the intestine. Dis Colon Rectum. 2003;46(10):1310–9.
Joung JY, Ha YS, Singer EA, Ercolani MC, Favaretto RL, Lee DH, et al. Use of a hyaluronic acid-carboxymethylcellulose adhesion barrier on the neurovascular bundle and prostatic bed to facilitate earlier recovery of erectile function after robot-assisted prostatectomy: an initial experience. J Endourol. 2013;27(10):1230–5.
Ploussard G, de la Taille A, Moulin M, Vordos D, Hoznek A, Abbou CC, et al. Comparisons of the perioperative, functional, and oncologic outcomes after robot-assisted versus pure extraperitoneal laparoscopic radical prostatectomy. Eur Urol. 2014;65(3):610–9.
Thompson JE, Egger S, Bohm M, Haynes AM, Matthews J, Rasiah K, et al. Superior quality of life and improved surgical margins are achievable with robotic radical prostatectomy after a long learning curve: a prospective single-surgeon study of 1552 consecutive cases. Eur Urol. 2014;65(3):521–31.
Acknowledgements
Not applicable.
Funding
Not applicable.
Availability of data and materials
The datasets used and/or analyzed in this study are available from the corresponding author by request.
Author information
Authors and Affiliations
Contributions
Conception and design: NH, MF, Data acquisition: NH, YB, KC, JF, KH, TI, Data analysis and interpretation: KC, Drafting the manuscript: YB, NH, MF, Critical revision of the manuscript for scientific and factual content: NH, Statistical Analysis: YB, NH Supervision: MF. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
All procedures performed in this study were in accordance with the Declaration of the Helsinki and approved by the institutional review board of Kobe University Hospital (KUH-170108).
Informed consent from the participants was waived by the institutional review board as the current study satisfied all of the following requirements for the waiver of informed consent. The research involved no more than minimal risk to the participants (retrospective data analysis of previously collected medical record).
Consent for publication
Not applicable.
Competing interests
The author declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Hinata, N., Bando, Y., Chiba, K. et al. Application of hyaluronic acid/carboxymethyl cellulose membrane for early continence after nerve-sparing robot-assisted radical prostatectomy. BMC Urol 19, 25 (2019). https://doi.org/10.1186/s12894-019-0458-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12894-019-0458-4