Abstract
Background
Pulmonary emphysema combined with systemic sclerosis (SSc)-associated interstitial lung disease (ILD) occurs more often in smokers but also in never-smokers. This study aimed to describe a new finding characterized by peculiar emphysematous change with SSc-associated ILD (SSc-ILD).
Methods
We conducted a retrospective review of 21 consecutive patients with SSc-ILD diagnosed by surgical lung biopsy and focused on the radio-pathological correlation of the emphysematous change.
Results
Pathological pulmonary emphysema (p-PE) with SSc-ILD was the predominant complication in 16 patients (76.2%) with/without a smoking history, of whom 62.5% were never-smokers. A low attenuation area (LAA) within interstitial abnormality on high-resolution computed tomography (HRCT) was present in 31.3%. Diffusing capacity of the lung for carbon monoxide (DLCO) was lower, disease extent on HRCT higher, and intimal/medial thickening in muscular pulmonary arteries more common in the patients with p-PE with SSc-ILD. However, forced vital capacity (FVC) was well preserved regardless of whether p-PE was observed. Most SSc-ILD patients had pulmonary microvasculature changes in arterioles (90.5%), venules (85.7%), and interlobular veins (81.0%).
Conclusions
Pulmonary emphysematous changes (LAA within interstitial abnormalities on HRCT and destruction of fibrously thickened alveolar walls) are specific and novel radio-pathological features of SSc-ILD. Peripheral vasculopathy may help to destroy the fibrously thickened alveolar walls, resulting in emphysematous change in SSc-ILD.
Similar content being viewed by others
Background
Interstitial lung disease (ILD) is common in patients with systemic sclerosis (SSc), and a minority of patients evolve to end-stage respiratory insufficiency [1]. Ground-glass opacity, reticular intralobular interstitial thickening, and traction bronchiectasis are the most common findings on high-resolution computed tomography (HRCT) [2, 3]. The HRCT features of ILD in patients with SSc closely resemble those in patients with idiopathic nonspecific interstitial pneumonia (NSIP) [3]. In terms of pathological findings, NSIP is increasingly regarded as the more prevalent histologic pattern in patients with SSc-associated ILD (SSc-ILD) and in those with other connective tissue diseases (CTD) [4,5,6,7,8].
We previously reported that SSc-related autoantibody-positive ILD, but not CTD, is a different disease entity from that of SSc or mixed CTD associated with ILD [9]. In that report, pathological pulmonary emphysema (p-PE) with ILD was observed more frequently (56%) in the patients with SSc or mixed CTD associated with ILD. Interestingly, a high proportion (64.3%) of these patients were never-smokers [9], and another recent report found that pulmonary emphysema combined with SSc-ILD was also present in some never-smokers [10]. In contrast, Overbeek et al. attached importance to the correlation between vasculopathy and ILD in the patients with SSc [11]. Detailed radiological and pathological features of pulmonary emphysema combined with SSc-ILD have not yet been described. Therefore, this study aimed to clarify the clinico-radio-pathological characteristics of SSc-ILD, focusing on pulmonary emphysema and vasculopathy.
Methods
This retrospective cohort study was approved by the institutional review board of Kanagawa Cardiovascular and Respiratory Center (no. 28–11). Because of the retrospective nature of the study, the review board waived the need for written informed consent from the patients.
The study population consisted of patients who had been diagnosed as having SSc-ILD, which was proven by surgical lung biopsy, at Kanagawa Cardiovascular and Respiratory Center between March 1997 and April 2016. The patients fulfilled the criteria for SSc of the American College of Rheumatology/European League Against Rheumatism classification [12]. One patient with SSc-rheumatoid arthritis overlap was excluded from this study, whereas 3 patients who developed manifestations of SSc during their follow-up period (0.42–2.17 years) were included. Baseline clinical measurements, radiological finding of HRCT, and right ventricular systolic pressure of echocardiography as indicator of pulmonary hypertension were obtained within 3 months of the histological diagnosis of ILD by surgical lung biopsy. The definition of chronic kidney disease were not a GFR below 60 mL/min/1.73m2 for three months or more or a GFR 60 mL/min/1.73m2 with kidney damage (i.e. marked of high levels of albumin in urine) [13]. Two radiologists (T. I. and S. I.) reviewed the HRCT scans for consensus of the diagnosis of ILD without knowledge of the patients’ clinical data. Patients were classified as presenting a HRCT pattern either “suggestive or consistent with NSIP” or “suggestive of usual interstitial pneumonia (UIP)” [14, 15]. The HRCT scans were analyzed for the following characteristics: honeycombing, ground-glass opacity, consolidation, reticulation, emphysema, cyst, traction bronchiectasis, bronchial wall thickening, mosaic attenuation, pulmonary artery dilation, enlarged mediastinal lymph nodes, micro-nodules, pleural thickening or effusion, and volume loss. These features were selected on the basis of previous studies [9, 16]. Moreover, low attenuation areas (LAA) within the interstitial abnormalities were also evaluated. This term was defined as low attenuation within an area such as a reticular shadow or traction bronchiectasis/bronchiolectasis, and the LAA itself had an apparent wall thickness of not more than 1 mm. Any disagreements between the two radiologists after the first assessment were resolved by discussion. The extent (%) of pulmonary abnormalities on HRCT was calculated with computer-aided 3D quantitative analysis [9, 17]. We also categorized the study patients by the easily applied disease staging of Goh’s criteria: l) limited disease or 2) extensive disease [18]. The surgical lung biopsy slides were reviewed by two pulmonary pathologists (K. O. and T. T.) who were blinded to the patients’ clinical and radiologic information. Histologic patterns were classified according to the current classification of idiopathic interstitial pneumonia [15]. The pathological features of lung parenchyma, airway, and pleural lesions were semi-quantitatively graded as 0 (absent), 1 (mild), 2 (moderate), or 3 (severe) [9, 19,20,21]. Pathological emphysema was defined as grade based on the number of alveolar pores due to alveolar wall destruction and the degree of enlarged alveolar lumina [22]. “Cicatricial emphysema,” defined as permanent enlargement of air spaces distal to terminal bronchioles caused by severe fibrosis (e.g. idiopathic pulmonary fibrosis), was excluded from the definition of emphysema [23]. Pulmonary vasculopathies, which include 1) muscular pulmonary arteries (from 200 to 300 μm in diameter) by the Heath-Edwards scoring system [24], 2) cellular and/or fibrotic intimal thickening and muscularisation in the interlobular veins and venules, and 3) changes such as medial hypertrophy and/or intimal thickening in the arterioles along the alveolar ducts (< 100 μm in diameter), were also evaluated based on previous reports [11, 25]. Any disagreements between the two pathologists were discussed until consensus was reached.
Data analysis
Categorical baseline characteristics are summarized by frequency and percentage, and continuous characteristic are reported as mean ± SD. To detect differences between groups, the Wilcoxon test, Fisher’s exact test, or Mann-Whitney U test was used as appropriate. We considered P < 0.05 to represent statistical significance. All data were analyzed with SPSS version 22.0 (IBM Japan, Tokyo, Japan).
Results
Patient characteristics
Clinical characteristics of the 21 patients with SSc-ILD are summarized in Table 1. The proportion of patients who had smoke was 38.1%, and 3 patients (14.3%) had Sjögren syndrome. The median follow-up period was 2.44 years, and only 2 patients (9.5%) died during this period.
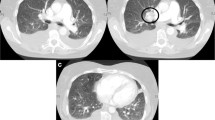
p-PE was observed in 16 patients (76.2%) of the patients with SSc-ILD. We classified the patients into two groups according to the existence or non-existence of p-PE with SSc-ILD. The proportion of never-smokers in the patients with p-PE with SSc-ILD was 62.5%. Smoking history for patients with each group was not significantly different. The diffusing capacity of the lung for carbon monoxide (DLCO) was significantly lower (P = 0.0163) and the disease extent on HRCT was significantly greater (P = 0.013) in the patients with p-PE with SSc-ILD. However, forced vital capacity (FVC) was well preserved in the patients regardless of whether p-PE was observed. The radiological and pathological characteristics of the patients are summarized in Tables 2, 3. The major HRCT pattern was NSIP in 19 patients (90.5%). The frequency of diffuse distribution of reticulation was more common in the patients with p-PE with SSc-ILD than in those without p-PE (P = 0.009). The association of vasculopathy with SSc-ILD patients are summarized in Table 4. LAA within the interstitial abnormalities was seen only in patients with p-PE with SSc-ILD (31.3%) (Figs. 1a, 2a, 3a, 4a). Fibrotic NSIP was the major histologic pattern seen in the SSc-ILD patients (85.7%). Of the SSc-ILD patients with p-PE, 12 (75.0%) had grade 1 emphysema, and 4 (25.0%) had grade 2 emphysema (Figs. 1b, 2b, 3b, 4b). Notably, emphysematous change in the SSc-ILD patients seemed to occur in the fibrotic alveolar walls and mainly presented as the destruction of fibrous thickened alveolar walls (Fig. 1c).
HRCT and surgical lung biopsy of a 45-year-old never-smoking woman. a Computed tomography scan shows traction bronchiectasis, reticulation predominantly in the peribronchovascular zone, and a low attenuation area (circle) in the subpleural area of both lower lungs. b Pathologic specimen (H & E staining, × 40) shows fibrotic nonspecific interstitial pneumonia with emphysematous change (grade 2). c At high-power magnification, emphysematous change occurs at the fibrously thickened alveolar walls showing an increase in pores (arrows) (Elastica van Gieson staining, × 100); AD: alveolar duct. d Muscular pulmonary artery shows intimal thickening (grade 1) (Elastica van Gieson staining, × 80). e Pulmonary arteriole lying along the alveolar ducts shows intimal thickening and muscularisation (Elastica van Gieson staining, × 200). f A venule shows intimal thickening and muscularisation (Elastica van Gieson staining, × 200)
HRCT and surgical lung biopsy of a 70-year-old never-smoking woman. a Computed tomography shows reticulation and a low attenuation area within a ground glass opacity and reticulation (circle). b Pathologic specimen (H & E staining, × 40) shows fibrotic nonspecific interstitial pneumonia with emphysematous change (grade 1). c Vascular intimal and medial thickening of a muscular pulmonary artery (grade 2) (Elastica van Gieson staining, × 100). d Intimal fibrosis of alveolar interstitial vessels (arteriole) and e) (venule) (Elastica van Gieson staining, × 200)
HRCT and surgical lung biopsy of 66-year-old ex-smoking man. a Computed tomography shows reticulation and a low attenuation area within the interstitial abnormalities (circle). b Pathologic specimen (H & E staining, × 40) shows fibrotic nonspecific interstitial pneumonia with emphysematous change (grade 2). c Intimal fibrosis of alveolar interstitial vessels (arteriole) and d (venule) (Elastica van Gieson staining × 200)
HRCT and surgical lung biopsy of 58-year-old never-smoking woman. a Computed tomography scan shows traction bronchiectasis, reticulation, and ground-glass opacity predominantly in the peribronchovascular zone of both lower lungs. b Pathologic specimen (H & E staining, × 40) shows fibrotic nonspecific interstitial pneumonia with emphysematous change (grade 2). c Muscular pulmonary artery shows medial hypertrophy (grade 1) (Elastica van Gieson staining, × 100). d Pulmonary venule lying along the alveolar ducts shows no apparent intimal thickening (Elastica van Gieson staining, × 200). e Venule and interlobular vein show intimal thickening (Elastica van Gieson staining, × 200)
Although the severity of changes in muscular pulmonary arteries in the patients with p-PE with SSc-ILD was not significantly different from that in the patients without p-PE (P = 0.105), its incidence was significantly higher (P = 0.047) in the patients with p-PE. Histological analysis found that 61.9% of the muscular pulmonary arteries were of Heath-Edwards grade 1–2 (Figs. 1d, 2c, 4c). Heath-Edwards grading of muscular pulmonary arteries of more than grade 2 (e.g. plexiform lesions) was not detected in our subjects. The incidences of changes in other pulmonary microvasculature were as follows: 90.5% showing arteriolar changes of intimal fibrosis and musculisation (Figs. 1e, 2d, 3c, 4d), 85.7% showing venular intimal thickening (Fig. 1f, 2e, 3d, 4e), and 81.0% showing intimal thickening of the interlobular veins (Fig. 4e). Most of the patients with SSc-ILD had arteriolar and venous-venular changes in their pulmonary microvasculature whether pathological emphysema coexist or not.
Discussion
We investigated characteristic radiological and pathological features of 21 patients with SSc-ILD. This study presented that pulmonary emphysematous changes (LAA within interstitial abnormalities on HRCT and destruction of fibrously thickened alveolar walls) are specific and novel radio-pathological features of SSc-ILD. We provided two important findings. First, among patients with SSc-ILD, the complication of p-PE was frequently present in patients regardless of whether they had ever smoked. Surprisingly, 62.5% of the patients with p-PE with SSc-ILD were never-smokers. Historically, the syndrome of combined pulmonary fibrosis and emphysema (CPFE) has been individualized within the spectrum of smoking-induced chronic lung disease [26]. Later, Cottin et al. reported that the syndrome of CPFE warrants recognition as a novel distinct pulmonary manifestation within the spectrum of lung diseases associated with CTD, especially in patients with rheumatoid arthritis and SSc, whether current- or ex-smokers; however, 11.8% of their patients with CTD complicated by CPFE never smoked. [27] Antoniou et al. recently reported a prevalence of emphysema in patients with SSc-ILD of 12%; emphysema was present more often in current or former smokers, but it was also present in 7.5% of the never-smokers [10]. In both reports, although emphysema was analyzed as a radiological finding, the patients with CPFE had a lower DLCO, preserved FVC, which were also found in our study, and wider disease extent. However, their definition of CPFE was based on the presence of both emphysema predominant in the upper lobes and pulmonary fibrosis predominant in the lower lung zones [10, 27]. Because the emphysema in our study was analyzed pathologically, it is unclear whether these disease entities were evaluated from the same viewpoint in the previous studies. Our analysis emphasizes that the emphysematous change occurring with SSc-ILD is different from the usual form of smoking-related emphysema, which presents as destructive holes in the center of the secondary lobules and is generally well demarcated from the adjacent parenchyma and which also centers around the respiratory bronchioles and alveolar ducts [28]. In contrast, the emphysematous change in SSc-ILD mainly presents as destruction of fibrously thickened alveolar walls, resulting in abnormal dilatation of the alveolar lumina and alveolar ducts. Obviously, as mentioned above [23], “cicatricial emphysema” together with bronchiolectasis presents a characteristic gross appearance known as microscopic honeycombing that reflects end-stage fibrosis that is different from the emphysematous change in SSc-ILD. We believe that the presence of p-PE with SSc-ILD is more commonly seen in SSc patients than previously realized, even if they have never smoked, and that this is a new finding in SSc-ILD.
The second important finding was that LAA within interstitial abnormalities on HRCT might be a specific radiological finding in patients with SSc-ILD. Although LAAs are also shown on HRCT in a variety of fibrotic ILDs (in the extreme, honeycombing is composed of cystic spaces) [29], this finding refers to the fusing of discrete cysts with walls, whereas LAAs in our analysis were closely adjacent to interstitial abnormalities, and the LAAs themselves did not have an apparent wall thickness of more than 1 mm. This radiological finding was only seen in some of the patients with p-PE with SSc-ILD, and therefore, it might correlate with the pathological emphysema of SSc-ILD, though all our hypotheses were not corroborated.
In the vast majority of people, smoking is the cause of emphysema [30]. A previous radiological analysis showed that pulmonary microvascular blood flow is reduced in mild chronic obstructive pulmonary disease (COPD) [31]. In the aspects of the pathological analysis, pulmonary vascular changes in COPD or CPFE are characterized by a thickening of the vessel wall that begins early in the process of emphysematous change [25]. Thickening of the intima is the first structural change and is followed by an increase in smooth muscle cells and infiltration of the vessel wall by inflammatory cells [32]. In contrast, pulmonary vascular abnormalities are common in SSc patients, and the pulmonary hypertensive vascular changes manifest as concentric intimal thickening by fibromyxoid tissue and medial hypertrophy leading to thickened and stenotic pulmonary arterioles [33]. Most SSc patients with or without pulmonary artery hypertension (PAH) have intimal fibrosis and medial hypertrophy of the pulmonary arteries with an external diameter between 101 and 200 μm, which lie beside bronchioles [34]. Overbeek et al. reported that PAH in SSc patients is characterized by intimal fibrosis in the small vessels (arterioles, venules, and interlobular veins) [11]. Because changes were seen in 61.9% of the muscular pulmonary arteries in our patients with SSc-ILD, and in 90.5% of the arterioles and 85.7% of the venules in the microvasculature, these findings could be caused by reduced pulmonary microvascular blood flow, which might be one factor contributing to the emphysematous changes occurring in ILD.
Our study has several limitations. First, it was a single-institution, retrospective study with a small sample size. Second, there was selection bias because only the patients who could undergo surgical lung biopsy were included. Additionally, only two patients received PAH-specific drug therapy during the follow-up period, and most patients could not be evaluated for PAH by right heart catheterization. Therefore, more severe cases might present different radiological and pathological findings. Third, although this study reveals characteristic radio-pathological features in SSc-ILD, a comparable analysis was not performed for other etiologies of ILD (e.g. CTD-ILD). Fourth, although previous research showed that increased matrix metalloproteinases expression may play a role in accelerating the process of destruction as emphysema [35], immunohistochemical analysis could not be performed in our analysis and would be an issue in the future. Fifth, we used several types of HRCT scanning with the times. Therefore, imaging protocol may lead to a little different interpretation of radiological finding.
Conclusions
Emphysematous changes, i.e. LAA within the interstitial abnormalities on HRCT and destruction of the fibrously thickened alveolar walls, are specific and novel radio-pathological features of SSc-ILD. Histopathological analysis suggested that peripheral vasculopathy may participate in the destruction of the fibrously thickened alveolar walls, resulting in the emphysematous changes seen in patients with SSc-ILD. Our results might help to provide more information for patients who do not fulfil the diagnosis of SSc (such as patients classified as having interstitial pneumonia with autoimmune features [36]) and suffer from a specific CTD diagnosis from the pulmonology viewpoint. Further studies are needed to clarify the quantitative loss of alveolar septa in the peripheral vascular bed that occurs with the emphysematous changes of SSc-ILD.
Abbreviations
- COPD:
-
Chronic obstructive pulmonary disease
- CPFE:
-
Combined pulmonary emphysema with fibrosis
- CTD:
-
Connective tissue disease
- DLCO :
-
Diffusing capacity of the lung for carbon monoxide
- FVC:
-
Forced vital capacity
- HRCT:
-
High-resolution computed tomography
- ILD:
-
Interstitial lung disease
- LAA:
-
Low attenuation area
- MCTD:
-
Mixed connective tissue disease
- NSIP:
-
Nonspecific interstitial pneumonia
- PAH:
-
Pulmonary artery hypertension
- PMBF:
-
Pulmonary microvascular blood flow
- p-PE:
-
Pathological pulmonary emphysema
- SSc:
-
Systemic sclerosis
References
Bussone G, Mouthon L. Interstitial lung disease in systemic sclerosis. Autoimmun Rev. 2011;10:248–55.
Goldin JG, Lynch DA, Strollo DC, Suh RD, Schraufnagel DE, Clements PJ, et al. High-resolution CT scan findings in patients with symptomatic scleroderma-related interstitial lung disease. Chest. 2008;134:358–67.
Desai SR, Veeraraghavan S, Hansell DM, Nikolakopolou A, Goh NS, Nicholson AG, et al. CT features of lung disease in patients with systemic sclerosis: comparison with idiopathic pulmonary fibrosis and nonspecific interstitial pneumonia. Radiology. 2004;232:560–7.
Fujita J, Yoshinouchi T, Ohtsuki Y, Tokuda M, Yang Y, Yamadori I, et al. Non-specific interstitial pneumonia as pulmonary involvement of systemic sclerosis. Ann Rheum Dis. 2001;60:281–3.
Kim DS, Yoo B, Lee JS, Kim EK, Lim CM, lee SD, et al. the major histopathologic pattern of pulmonary fibrosis in scleroderma is nonspecific interstitial pneumonia. Sarcoidosis Vasc Diffuse Lung Dis. 2002;19:121–7.
Bouros D, Wells AU, Nicholson AG, Colby TV, Polychronopoulos V, Pantelidis P, et al. Histopathologic subsets of fibrosing alveolitis in patients with systemic sclerosis and their relationship to outcome. Am J Respir Crit Care Med. 2002;165:1581–6.
Katzenstein AL, Fiorelli RF. Nonspecific interstitial pneumonia/fibrosis. Histologic features and clinical significance Am J Surg Pathol. 1994;18:136–47.
Nicholson AG, Colby TV, wells AU. Histopathological approach to patterns of interstitial pneumonia in patient with connective tissue disorders. Sarcoidosis Vasc Diffuse Lung Dis. 2002;19:10–7.
Yamakawa H, Hagiwara E, Kitamura H, Yamanaka Y, Ikeda S, Sekine A, et al. Clinical features of idiopathic interstitial pneumonia with systemic sclerosis-related autoantibody in comparison with interstitial pneumonia with systemic sclerosis. PLoS One. 2016;11:e0161908.
Antoniou KM, Margaritopoulos GA, Goh NS, Karagiannis K, Desai SR, Nicholson AG, et al. Combined pulmonary fibrosis and emphysema in scleroderma-related lung disease has a major confounding effect on lung physiology and screening for pulmonary hypertension. Arthritis Rheumatol. 2016;68:1004–12.
Overbeek MJ, Vonk MC, Boonstra a, Voskuyl AE, Vonk-Noordegraaf a, smit EF, et al. pulmonary arterial hypertension in limited cutaneous systemic sclerosis: a distinctive vasculopathy. Eur Respir J. 2009;34:371–9.
van den Hoogen F, Khanna D, Fransen J, Johnson SR, Baron M, Tyndall A, et al. 2013 classification criteria for systemic sclerosis: an American College of Rheumatology/European league against rheumatism collaborative initiative. Arthritis Rheum. 2013;65:2737–47.
Lopez-Giacoman S, Madero M. Biomarkers in chronic kidney disease, from kidney function to kidney damage. World J Nephrol. 2015;4:57–73.
American Thoracic Society. European Respiratory Society. American Thoracic Society/European Respiratory Society international multidisciplinary consensus classification of the idiopathic interstitial pneumonias. This joint statement of the American Thoracic Society (ATS), and the European Respiratory Society (ERS) was adopted by the ATS board of directors, June 2001 and by the ERS executive committee, June 2001. Am J Respir Crit Care Med. 2002;166:426.
Travis WD, Costabel U, Hansell DM, King TE Jr, Lynch DA, Nicholson AG, et al. ATS/ERS Committee on idiopathic interstitial pneumonias. An official American Thoracic Society/European Respiratory Society statement: update of the international multidisciplinary classification of the idiopathic interstitial pneumonias. Am J Respir Crit Care Med. 2013;188:733–48.
Hansell DM, Bankier AA, MacMahon H, McLoud TC, Müller NL, Remy J. Fleischner society: glossary of terms for thoracic imaging. Radiology. 2008;246:697–722.
Iwasawa T, Kato S, Ogura T, Kusakawa Y, Iso S, Baba T, et al. Low-normal lung volume correlates with pulmonary hypertension in fibrotic idiopathic interstitial pneumonia: computer-aided 3D quantitative analysis of chest CT. AJR Am J Roentgenol. 2014;203:W166–73.
Goh NS, Desai SR, Veeraraghavan S, Hansell DM, Copley SJ, Maher TM, et al. Interstitial lung disease in systemic sclerosis: a simple staging system. Am J Respir Crit Care Med. 2008;177:1248–54.
Song JW, Do KH, Kim MY, Jang SJ, Colby TV, Kim DS. Pathologic and radiologic differences between idiopathic and collagen vascular disease-related usual interstitial pneumonia. Chest. 2009;136:23–30.
Enomoto Y, Takemura T, Hagiwara E, Iwasawa T, Okudela K, Yanagawa N, et al. Features of usual interstitial pneumonia in patients with primary Sjögren’s syndrome compared with idiopathic pulmonary fibrosis. Respir Investig. 2014;52:227–35.
Flaherty KR, Colby TV, Travis WD, Toews GB, Mumford J, Murray S, et al. Fibroblastic foci in usual interstitial pneumonia: idiopathic versus collagen vascular disease. Am J Respir Crit Care Med. 2003;167:1410–5.
Wright JL. The importance of ultramicroscopic emphysema in cigarette smoke-induced lung disease. Lung. 2001;179:71–81.
Corrin, B, Nicholson AG: Pathology of the lungs. 3rd ed. Philadelphia, Elsevier, 2011,pp 91–134.
Heath D, Edwards JE. The pathology of hypertensive pulmonary vascular disease; a description of six grades of structural changes in the pulmonary arteries with special reference to congenital cardiac septal defects. Circulation. 1958;18:533–47.
Awano N, Inomata M, Ikushima S, Yamada D, Hotta M, Tsukuda S, et al. Histological analysis of vasculopathy associated with pulmonary hypertension in combined pulmonary fibrosis and emphysema: comparison with idiopathic pulmonary fibrosis and emphysema alone. Histopathology. 2017;70:896–905.
Cottin V, Nunes H, Brillet PY, Delaval P, Devouassoux G, Tillie-Leblond I, et al. Groupe d'Etude et de Recherche sur les maladies Orphelines Pulmonaires (GERM O P): combined pulmonary fibrosis and emphysema: a distinct underrecognised entity. Eur Respir J. 2005;26:586–93.
Cottin V, Nunes H, Mouthon L, Gamondes D, Lazor R, Hachulla E, et al. Groupe d'Etudes et de Recherche sur les maladies "Orphelines" Pulmonaires: combined pulmonary fibrosis and emphysema syndrome in connective tissue disease. Arthritis Rheum. 2011;63:295–304.
Wright JL, Tazelaar HD, Churg A. Fibrosis with emphysema. Histopathology. 2011;58:517–24.
Raoof S, Bondalapati P, Vydyula R, Ryu JH, Gupta N, Raoof S, et al. cystic lung diseases: algorithmic approach. Chest. 2016;150:945–65.
Wilson JS, Galvin JR. Normal diffusing capacity in patients with PiZ alpha(1)-antitrypsin deficiency, severe airflow obstruction, and significant radiographic emphysema. Chest. 2000;118:867–71.
Hueper K, Vogel-Claussen J, Parikh MA, Austin JH, Bluemke DA, Carr J, et al. pulmonary microvascular blood flow in mild chronic obstructive pulmonary disease and emphysema. The MESA COPD study. Am J Respir Crit Care Med. 2015;192:570–80.
Seimetz M, Parajuli N, Pichl A, Veit F, Kwapiszewska G, Weisel FC, et al. Inducible NOS inhibition reverses tobacco-smoke-induced emphysema and pulmonary hypertension in mice. Cell. 2011;147:293–305.
Vivero M, Padera RF. Histopathology of lung disease in the connective tissue diseases. Rheum Dis Clin N Am. 2015;41:197–211.
Yousem SA. The pulmonary pathologic manifestations of the CREST syndrome. Hum Pathol. 1990;21:467–74.
Cardoso WV, Sekhon HS, Hyde DM, Thurlbeck WM. Collagen and elastin in human pulmonary emphysema. Am Rev Respir Dis. 1993;147:975–81.
Fischer A, Antoniou KM, Brown KK, Cadranel J, Corte TJ, du bois RM, et al. “ERS/ATS task force on undifferentiated forms of CTD-ILD”. An official European Respiratory Society/American Thoracic Society research statement: interstitial pneumonia with autoimmune features. Eur Respir J. 2015;46:976–87.
Acknowledgments
We offer our sincerest thanks to Yosuke Sasaki of Satista Co., Ltd. for his advice on statistical analysis. The authors would like to thank Rise Japan LLC for the professional English language review.
Funding
Not applicable.
Availability of data and materials
The data will not be shared with participant confidentiality.
Author information
Authors and Affiliations
Contributions
HY was the primary author of the manuscript, had full access to all of the data in the study, and takes responsibility for the integrity of the data and the accuracy of the data analysis. TT, AS, HK, TB, and TO contributed to the study design; YY, SaI, AS, HK, TB, and TO contributed to collecting samples and carrying out experiments; AS, TI, and KK contributed to data analysis; TT, SaI, ShI, KO, and TO contributed to data interpretation; TT, TI, KO, and TO contributed to writing the manuscript. All authors read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This retrospective cohort study was approved by the institutional review board of Kanagawa Cardiovascular and Respiratory Center (no. 28–11). The patients’ approval or informed consent was not required for a retrospective review of their records, pursuant to the ethical guidelines of the Japanese Ministry of Health, Labor, and Welfare.; however, the present retrospective study was carried out by the opt-out method of our hospital website.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Yamakawa, H., Takemura, T., Iwasawa, T. et al. Emphysematous change with scleroderma-associated interstitial lung disease: the potential contribution of vasculopathy?. BMC Pulm Med 18, 25 (2018). https://doi.org/10.1186/s12890-018-0591-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12890-018-0591-y