Abstract
Thoracic diseases in patients with systemic lupus erythematosus (SLE), especially interstitial pneumonia (SLE-IP), are rare and have been poorly studied. The aims of this multicentre study were to evaluate SLE-IP and elucidate its clinical characteristics and prognosis. Fifty-five patients with SLE-IP who had attended the respiratory departments of participating hospitals were retrospectively evaluated in this multicentre study. Clinical information, high-resolution computed tomography (HRCT), and surgical lung biopsy/autopsy specimens were analysed by respiratory physicians, pulmonary radiologists, and pulmonary pathologists. IP patterns on HRCT and lung specimens were classified based on the international classification statement/guideline for idiopathic interstitial pneumonias. The most frequent form of SLE-IP at diagnosis was chronic IP (63.6%), followed by subacute (20.0%), and acute IP (12.7%). Radiologically, the most common HRCT pattern was “Unclassifiable” (54%). Histologically, “Unclassifiable” was the most frequently found (41.7%) among 12 patients with histologically proven IP. Interestingly, accompanying airway diseases were present in nine of these patients (75%). In multivariate analysis, current smoking (hazard ratio [HR] 6.105, p = 0.027), thrombocytopenia (HR 7.676, p = 0.010), anti-double-strand DNA titre (HR 0.956, p = 0.027), and nonspecific interstitial pneumonia (NSIP) + organizing pneumonia (OP) pattern on HRCT (vs. NSIP, HR 0.089, p = 0.023) were significant prognostic factors. In conclusion, chronic IP was the most frequent form of IP in patients with SLE-IP, and “Unclassifiable” was the commonest pattern radiologically and histologically.
Similar content being viewed by others
Introduction
Systemic lupus erythematosus (SLE) is one of the most significant connective tissue diseases (CTD) that affect multiple organs, primarily in young women. Lung involvement especially interstitial pneumonia (IP), occurs much less frequently in SLE than in other CTDs1. Pleuritis, the most frequently reported thoracic disorder, is found in 16–60% of patients with SLE2,3, whereas IP occurs in only 4–10% of such patients4,5. As for IP in patients with SLE, the late-onset group (≧50 years old) had 2.56 times higher incidence of IP than early-onset group (<18 years old)6.
However, many of the above-cited studies on lung involvement in SLE drew their patients from rheumatology departments; thus, details of severe lung diseases in patients who have been managed in respiratory departments have not been well-established. Further, although patients with SLE with neuropsychiatric7 or renal involvement8 are known to have poor prognoses, the prognostic significance of lung involvement has not been fully elucidated.
In this multicentre retrospective study, we investigated patients with SLE and thoracic diseases who had been treated in respiratory departments and thoroughly evaluated those with SLE-related interstitial pneumonia (SLE-IP). Furthermore, we used data from high-resolution computed tomography (HRCT) and surgical lung biopsy (SLB)/autopsy specimens to evaluate details of IP patterns and classify them based on the international classification statement/guideline for idiopathic interstitial pneumonias (IIPs)9 and idiopathic pulmonary fibrosis (IPF)10. To the best of our knowledge, this is the first study to comprehensively and precisely evaluate SLE-IP in a multicentre study by respiratory physicians, pulmonary radiologists, and pulmonary pathologists.
Results
Clinical characteristics, laboratory and physiological findings, and SLE activity scores in patients with SLE-related IP
Clinical characteristics of all patients, including serum markers, results of physiological tests, and SLE activity scores are shown in Table 1. These data were collected at the time of diagnosis of SLE-related IP. The median observation period was 85 months. The participants had a median age of 54 years, 42 of them (76.4%) were women and 39 (70.9%) were never-smokers. Twenty-five (45.5%) presented because of symptoms, mainly respiratory symptoms, and 25 (45.5%) were referred from other departments such as rheumatology departments. Most participants had been diagnosed with SLE prior to development of IP (21 patients, 38.2%) or were diagnosed with IP and SLE concomitantly (27 patients, 49.1%). Nineteen patients (34.5%) had comorbid other CTDs, the commonest being Sjogren syndrome (nine patients). Forced vital capacity (FVC) was preserved, whereas diffusion lung capacity for carbon monoxide (DLCO) was impaired (median 57.4%). At the time of diagnosis of IP, activity scores for SLE (SLEDAI-2K) were high (median score 12; <4 denoting mild activity, and ≧6 moderate to severe activity11,12).
Onset forms of IP and frequency of SLE-related thoracic diseases other than IP
The forms of IP at onset in the 55 patients with SLE-IP are shown in Fig. 1A. The chronic form was the commonest (35 patients, 63.6%), followed by subacute IP (11 patients, 20%) and acute IP (seven patients, 12.7%). The most frequent thoracic disease other than IP (Fig. 1B) was pleuritis (six patients, 10.9%), followed by pulmonary hypertension (five, 9.1%), pericarditis (three, 5.5%), and pulmonary thromboembolism (two, 3.6%). Serositis, including pleuritis and pericarditis, was diagnosed in 16.4% of the patients with SLE-IP.
Forms of systemic lupus erythematosus-related interstitial pneumonia (SLE-IP) at onset and frequency of other SLE-related thoracic diseases in 55 patients with SLE-IP. (A) Chronic IP accounted for 35 patients (63.6%) followed by subacute IP (11 patients, 20%) and acute IP (seven patients, 12.7%). (B) The most frequent thoracic disease other than IP was pleuritis (six patients, 10.9%) followed by pulmonary hypertension (five patients, 9.1%), pericarditis (three patients, 5.5%), and pulmonary thromboembolism (two patients, 3.6%). Serositis, including pleuritis and pericarditis, was present in 16.4% of patients with SLE-IP.
Relationships between form of IP at onset, serositis and activity of SLE
Activity of SLE was assessed and relationships between disease activity and form of IP at onset examined. SLEDAI-2K scores were significantly higher in patients with acute/subacute IP than in those with chronic IP (Supplementary Fig. S2A, p = 0.046). In addition, patients with SLE-IP and pleural/pericardial effusion had significantly higher SLEDAI-2K scores than those without such effusions (Supplementary Fig. S2B, p = 0.039).
Frequency of IP patterns on HRCT and prognosis
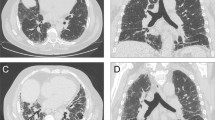
The most frequent IP pattern on HRCT according to the IIPs classification was “Unclassifiable” (30 patients, 54%). In “Unclassifiable”, nonspecific interstitial pneumonia (NSIP) + organizing pneumonia (OP) patterns accounted for 25% of all patients with SLE-IP (Fig. 2A). “Unclassifiable (others)” accounted for 29% of all patients; the breakdown of “Unclassifiable (others)” is shown in Supplementary Table S1. Twelve patients (22%) were classified as having OP pattern, followed by NSIP pattern (seven, 13%), usual interstitial pneumonia (UIP) pattern (definite UIP pattern two + possible UIP pattern; five, 9%), and diffuse alveolar damage (one, 2%). Frequency of IP patterns on HRCT in patients with SLE-IP without other CTDs was similar to that in all SLE-IP patients (Fig. 2B). Representative HRCT images of patients with UIP pattern (Fig. 2C), NSIP pattern (Fig. 2D), OP pattern (Fig. 2E), NSIP + OP pattern (Fig. 2F; i.e., within the “Unclassifiable”), diffuse alveolar damage (DAD) pattern (Fig. 2G) are shown. As for the prognosis, survival curves from the diagnosis of IP according to HRCT pattern are shown in Fig. 2H. Patients with NSIP + OP pattern had significantly better prognoses than those with NSIP (log-rank test, p = 0.042). UIP pattern was not associated with a worse prognosis than other IP patterns, unlike IPF/UIP10 or rheumatoid arthritis-related UIP13. Even in patients with SLE but without other CTDs, those with NSIP + OP pattern still had significantly better prognoses than those with NSIP (Fig. 2I, log-rank test, p = 0.021). The extent scores of lung fibrosis on HRCT were not significantly different between NSIP + OP and NSIP (p = 0.548).
Frequency of interstitial pneumonia (IP) patterns on high-resolution computed tomography (HRCT) and prognosis. IP patterns were re-evaluated in 55 patients with systemic lupus erythematosus (SLE)-IP. IP patterns on HRCT were classified according to the international classification statement/guideline for idiopathic interstitial pneumonia and idiopathic pulmonary fibrosis. (A) The most frequent IP pattern was “Unclassifiable” (30 patients, 54%). Of the patients with “Unclassifiable” SLE-IP, 25% had a nonspecific interstitial pneumonia (NSIP) + organizing pneumonia (OP) pattern, 12 (22%) an OP pattern, seven (13%) an NSIP pattern, and five an usual interstitial pneumonia (UIP) pattern (two definite and three possible UIP pattern; 9%). (B) Frequency of IP patterns on HRCT in patients with SLE-IP without other CTDs is similar to that in all SLE-IP patients. Representative HRCT images of patients with (C) UIP pattern, (D) NSIP pattern, (E) OP pattern, (F) NSIP + OP pattern (i.e., included in “Unclassifiable”), and (G) diffuse alveolar damage (DAD) pattern are shown. (C) UIP pattern showing bilateral and subpleural cystic changes with a basal honeycomb pattern. (D) NSIP pattern showing reticular and ground-glass opacities along bronchovascular bundles without consolidation. (E) OP pattern showing bilateral patchy areas of airspace consolidation with peri-bronchovascular predominance. (F) NSIP + OP pattern (i.e., included in “Unclassifiable”) showing both ground-glass and patchy air space consolidation. (G) DAD pattern showing extensive areas of ground-glass attenuation and mild reticulation with peribronchovascular predominance. Mild traction bronchiectasis is also suspected. Open arrowheads: reticular opacity, closed arrowheads: ground-glass opacity, and arrows: airspace consolidation. (H) Survival curves from the diagnosis of IP according to HRCT pattern are shown. Patients with NSIP + OP pattern had significantly better prognoses than those with NSIP (log-rank test, p = 0.042). UIP pattern did not have a worse prognosis than other IP patterns. (I) Even in patients with SLE but without other CTDs, those with NSIP + OP pattern still had significantly better prognosis than those with NSIP (log-rank test, p = 0.021).
Histopathological findings on surgical lung biopsy or autopsy specimens
Nine patients had undergone surgical lung biopsy and three had been autopsied. The results of histopathological examination are shown in Table 2. The most frequent IP pattern according to the IIPs classification was “Unclassifiable” (five patients, 41.7%), followed by NSIP pattern (three, 25%) and OP pattern (two, 16.7%). As to other pathological findings, mild airway diseases, mainly cellular bronchiolitis, were found in nine patients (75%), pleural diseases in eight (66.7%), and lymphoid follicles in seven (58.3%). Four patients having histopathological UIP (three were diagnosed as mixed pattern) showed no significant difference in their prognosis compared to those with other patterns (log-rank, p = 0.464).
Survival curves and prognostic factors in patients with SLE-IP
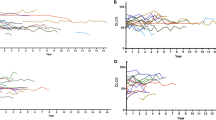
Overall survival from the diagnosis of IP is shown in Supplementary Fig. S3. The 5-year survival rate was 85.3% in 55 patients with SLE-IP. Fifty-one of them had been treated for IP, all of them had received corticosteroids, and 22 patients had also been given immunosuppressants. Kaplan-Meier survival curves from the diagnosis of IP according to the indicated clinical factors, are shown in Fig. 3 and Supplementary Fig. S4. Current smokers (log-rank, p = 0.001, Fig. 3A), patients with thrombocytopenia (log-rank, p = 0.036, Fig. 3C), those with high extent of lung fibrosis (extent scores of 2 or 3) on HRCT (log-rank, p = 0.002, Fig. 3F), and those with neuropsychiatric lesions (log-rank, p = 0.003, Supplementary Fig. S4D) had significantly worse prognoses than those without these characteristics. However, activity of SLE was not related to prognosis (≥12 based on median value, log-rank, p = 0.750, Fig. 3E). Age ≥54 years (based on median value, log-rank, p = 0.086, Supplementary Fig. S4A), male sex (log-rank, p = 0.116, Supplementary Fig. S4B), pleural/pericardial effusion (log-rank, p = 0.236, Supplementary Fig. S4C), histopathologic pattern having UIP (log-rank, p = 0.464, Supplementary Fig. S4E), absence of other CTDs (log-rank, p = 0.051, Fig. 3B), or low anti-double strand (ds) DNA antibody titres (<34.4 based on median value, log-rank, p = 0.093, Fig. 3D) were not related with prognosis either. Fifteen patients died during the observation period: causes of death are shown in Supplementary Table S2. Infection was the most frequent cause of death (six patients, 40%) followed by malignant tumours (four, 26.7%) and neuropsychiatric lesions (two, 13.3%). Respiratory failure due to IP occurred in only two patients (13.3%). An acute exacerbation occurred in one patient, who survived with steroid-pulse and immunosuppressant therapy. Finally, prognostic factors were assessed with Cox proportional hazards analyses. In univariate analysis (Table 3), age (hazard ratio [HR] 1.051, p = 0.033), current smoker (HR 6.689, p = 0.018), %FVC (HR 0.962, p = 0.011), serum Krebs von den Lungen-6 (KL-6; HR 1.001, p = 0.009), serum surfactant protein D (SP-D; HR 1.007, p = 0.048), NSIP + OP pattern on HRCT (vs. NSIP pattern; HR 0.111, p = 0.037), high extent of lung fibrosis on HRCT (HR 5.705, p = 0.015), comorbid other CTDs (HR 0.167, p = 0.029), and neuropsychiatric lesions (HR 5.762, p = 0.027) were significant prognostic factors. Next, multivariate Cox proportional hazards analyses adjusted for age are shown in Table 4. Current smoker (HR 6.105, p = 0.027), serum KL-6 (HR 1.001, p = 0.008), NSIP + OP pattern on HRCT (vs. NSIP pattern; HR 0.089, p = 0.023), high extent of lung fibrosis on HRCT (HR 5.332, p = 0.023), comorbid other CTDs (HR 0.138, p = 0.014), thrombocytopenia (HR 7.676, p = 0.010), and anti-dsDNA antibody titre (HR 0.956, p = 0.027), and neuropsychiatric lesions (HR 6.585, p = 0.020) were found to be significant prognostic factors.
Kaplan-Meier survival curves from the diagnosis of interstitial pneumonia (IP), according to indicated clinical factors in patients with systemic lupus erythematosus (SLE)-related IP. (A) Current smokers (log-rank, p = 0.0001), (C) patients with thrombocytopenia (log-rank, p = 0.036), and (F) patients with high extent of lung fibrosis (extent scores of 2 or 3) on HRCT (log-rank, p = 0.002) showed significantly worse prognoses than those without these characteristics. (B) Absence of comorbid other connective tissue diseases (CTDs) (log-rank, p = 0.051) and (D) low anti-dsDNA antibody titre (<34.4 based on median value, log-rank, p = 0.093) were not significantly related with prognosis. E, Activity of SLE (SLEDAI-2K score) was not associated with prognosis either (≥12 based on median value, log-rank, p = 0.750).
Discussion
In the present study, we retrospectively studied data of patients with SLE-IP who had attended respiratory departments with a particular focus on radiologic and histopathologic patterns in these patients. The most frequent form of SLE-IP at onset was chronic IP (63.6%). Further, according to IIPs/IPF classification statement/guidelines, “Unclassifiable” was the commonest pattern on both HRCT and SLB/autopsy specimens, and NSIP + OP pattern (i.e., included in “Unclassifiable”) on HRCT was associated with a better prognosis than NSIP pattern. To our knowledge, the present study includes the largest series of patients with SLE-IP thus far published and is the first in which pulmonary physicians, radiologists, and pathologists have precisely evaluated SLE-IP.
Previous studies have reported that IP is less common in patients with SLE than in those with other CTDs, comprising 4–10% of patients with SLE and being diagnosed mainly on chest radiographs4,5. In addition, chronic IP is reportedly found in 3–13% of patients with SLE3. The most frequently reported intrathoracic disorder in patients with SLE is pleuritis, which occurs in 16–60% of such patients2,3. However, many of the above-cited studies were conducted on patients who were attending rheumatology departments. In contrast, in the present study, we found that the commonest thoracic disease in 60 patients with SLE and thoracic diseases who visited or were referred to respiratory departments was IP (91.7%), pleuritis having been identified in only 18.3%. Furthermore, chronic IP accounted for 63.3% of patients with SLE-IP. Thus, it seems that the characteristics of patients with SLE who visit rheumatology departments are quite different from those of patients who visit respiratory departments.
In the current study, we found unexpectedly high frequency of comorbid other CTDs (19 patients [34.5%]) in patients with SLE-IP. Several studies have suggested that SLE-Sjogren overlap syndrome phenotype may have contributed to increased risk of IP, especially in older patients with SLE6. Further, in the current study, the presence of comorbid other CTDs was found to be an independent prognostic factor. These results seem to be important and useful real-world information in a clinical practice in this rare disease.
As for the relationship between disease activity and prognosis, SLEDAI-2K scores of ≤414 or <315 are reportedly associated with better prognoses irrespective of the presence of thoracic diseases. However, in the present study, SLEDAI-2K scores were not associated with prognosis in patients with SLE-IP. Additionally, SLEDAI-2K scores were significantly higher in patients with acute/subacute IP or pleural/pericardial effusion. These clinical features may therefore be useful in predicting SLE activity in patients with SLE-IP.
In patients with IIPs, IP patterns are important for predicting prognosis and selecting therapies9,10,16. In the present study, the most frequent IP pattern in both HRCT and SLB/autopsy specimens was “Unclassifiable”; in contrast, a previous study indicated that the NSIP pattern on HRCT was frequent in patients with SLE17. This “Unclassifiable” in patients with SLE-IP may represent heterogeneity of lung inflammation and/or fibrosis; this being unlike other CTDs, which mainly have NSIP-predominant patterns18. Further, in the present study, patients with NSIP + OP on HRCT had better prognoses than those with NSIP alone regardless of lung fibrosis extent. This difference in prognosis may be associated with whether the predominant milieu in the lung is inflammatory or fibrotic. UIP pattern was not associated with a worse prognosis than other IP patterns, unlike rheumatoid arthritis-related UIP13 or IPF/UIP10. The better prognosis of UIP in SLE than of IPF/UIP is consistent with previous reports on CTD-IP19,20.
Interestingly, in our study, mild airway diseases such as cellular bronchiolitis were found in most SLB/autopsy specimens from patients with SLE-IP (75%). Among patients with CTD-IP, those with rheumatoid arthritis or Sjogren syndrome reportedly frequently have airway diseases1; however, few studies have documented histologically-proven airway diseases in patients with SLE-IP. Our observations suggest that involvement of small airways is a characteristic feature of SLE-IP.
Regarding prognostic factors, old age, male sex, renal damage, psychiatric involvement, and high disease activity are reportedly significant predictors of poor prognosis in patients with SLE7,8,11,14,15,21. Although many studies have not found lung involvement to be a significant prognostic factor, Haye Salinas et al. have reported that pleuropulmonary manifestations are predictors of significantly worse prognosis11. In our cohort, which comprised only patients with SLE and IP, current smoking, serum KL-6, and NSIP + OP pattern on HRCT (vs. NSIP pattern) were significant prognostic factors according to multivariate Cox proportional hazards analyses, as were comorbid other CTDs, thrombocytopenia, and anti-dsDNA antibody titre. However, the most frequent cause of death was infection (six patients) and respiratory failure caused by IP occurring in only two patients. The risk of death from infection is reportedly 4.98-fold higher in patients with SLE than in the general population22. In the present study, 51 patients (92.7%) received corticosteroids for their IP and 22 of them received additional immunosuppressants; thus, careful attention to immunosuppressive therapy should be paid in clinical practice.
The present study has several limitations. First, SLE-IP is rare; accordingly, our patient cohort is small. Second, the data were retrospectively collected. Third, the treatments for SLE-IP were not uniform; however, most patients had been treated with corticosteroids with or without immunosuppressants. Fourth, proportion of comorbid other CTDs, mainly Sjogren syndrome, was relatively high (34.5%) and this condition may have influenced our observations. Fifth, we were unable to directly compare the features of SLE patients who were attending respiratory departments with those of patients who were attending rheumatology departments. The proportions of IP or other lung involvements may differ between these two settings. A larger and prospective study, including both respiratory and rheumatology departments, would be ideal for further evaluating SLE-IP.
In conclusion, in this multicentre study of patients with SLE-IP, we found that the most frequent form of IP was chronic IP (63.6%). Further, the most common pattern on HRCT and SLB/autopsy was “Unclassifiable” (54.0% and 41.7%, respectively). Additionally, histological examination revealed a high prevalence of accompanying mild airway disease in patients with SLE-IP. To more comprehensively evaluate this rare lung disease, larger and prospective studies across rheumatology and respiratory medicine departments are needed.
Methods
Study design and participants
In this multicentre study, respiratory physicians, pulmonary radiologists, and pulmonary pathologists retrospectively reviewed the data of 62 patients with SLE and thoracic diseases who had visited respiratory departments in nine hospitals in Japan between 1987 and 2016 (Supplementary Fig. S1). Two patients were excluded from this study because their thoracic diseases were deemed to be attributable to infections. A further five without interstitial pneumonia were also excluded. Three of these patients had pleuritis, another pleuritis and pericarditis, and the fifth pulmonary hypertension. Therefore, 55 patients with SLE and IP were studied. All diagnoses of SLE had been made in accordance with the diagnostic criteria of the American College of Rheumatology (ACR) 1997 and/or Systemic Lupus International Collaborating Clinics (SLICC) 2012 in collaboration with specialists in other areas such as rheumatologists and dermatologists23. Nine of the participants had undergone SLB and three had been autopsied. Acute exacerbation of IP was diagnosed according to the diagnostic criteria of that in IPF24. The study protocol was approved by the Ethics Committees of the participating institutions (Hamamatsu University School of Medicine [Approval Number (No.) E16-132], National Centre for Global Health and Medicine [No. NCGM-G-002219-00], Ogaki Municipal Hospital [No. 20161124-4], Toranomon Hospital [No. 1328], Okinawa Chubu Hospital [No. 42], Tosei General Hospital [No. 600], Kanazawa University Graduate School of Medical Sciences [No. 2447-1], University of Fukui [No. 20160158], Kinki-Chuo Chest Medical Centre [No. 694], Saga University [No. 2016-08-10], Nagasaki University Hospital [No. 16103114]), and this study was carried out in accordance with the approved protocol. The need for patient approval and informed consent was waived due to the retrospective nature of the study.
Data collection and evaluation for disease activity of SLE
Clinical data, including symptoms, laboratory and pulmonary function tests, treatments, and period from the diagnosis of IP were obtained from the participant’s medical records. These data were evaluated by 10 respiratory physicians. Chronic, subacute, and acute IP was defined as duration of ≧3 months, 3-1 months, and <1 month, respectively. These were periods from the onset of respiratory symptoms to the diagnosis of IP. Activity of SLE at the time of diagnosis of IP was evaluated using SLE-disease activity index 2000 (SLEDAI-2K) scores, which are derived from 23 items25,26.
Review of chest HRCT and lung pathological specimens
Three pulmonary radiologists independently evaluated the HRCT features in 55 patients with SLE-IP and subsequently reached a consensus on diagnosis and IP pattern. The extent of lung fibrosis on HRCT was semi-quantitatively evaluated based on honeycombing and reticulation. The extent scores of lung fibrosis were as follows: score 0, none; 1, <25%; 2, 25–50%; 3, ≥50%. Scores of 0 and 1 were defined as low extent scores, and those of 2 and 3 as high extent scores. Where specimens obtained by SLB or autopsy were available, they were evaluated histologically. Four pulmonary pathologists evaluated histological features independently, and subsequently reached consensus diagnoses. IP patterns, lymphoid follicle with germinal centres, small airway disease, vasculopathy, and pleural lesions were identified and assessed. IP patterns on HRCT and lung specimens were classified based on the international classification statement/guideline for IIPs9 and IPF10. The definition of “Unclassifiable” on HRCT and/or histological examination is as follows: (1) multiple HRCT and/or pathologic patterns; (2) new entity or unusual variant of recognized entity, not adequately characterized by the international IIPs classification statements9,27; and (3) inadequate radiologic or pathologic data.
Statistical analysis
Statistical analyses were performed using JMP-13.1.0 (SAS Institute Inc., Cary, NC, USA). Categorical data were compared using the χ2 test or Fisher’s exact probability test for independence, and continuous data using the Wilcoxon rank sum test. Overall survival of patient groups was estimated using Kaplan-Meier curves, and was compared between groups using the log-rank test. The relationships between variables and mortality were evaluated by Cox proportional hazards regression analysis. All tests were two-sided and statistical significance was set at p < 0.05.
References
Fischer, A. & du Bois, R. Interstitial lung disease in connective tissue disorders. Lancet 380, 689–698, https://doi.org/10.1016/S0140-6736(12)61079-4 (2012).
Torre, O. & Harari, S. Pleural and pulmonary involvement in systemic lupus erythematosus. Presse Med 40, e19–29, https://doi.org/10.1016/j.lpm.2010.11.004 (2011).
Keane, M. P. & Lynch, J. P. 3rd Pleuropulmonary manifestations of systemic lupus erythematosus. Thorax 55, 159–166 (2000).
Haupt, H. M., Moore, G. W. & Hutchins, G. M. The lung in systemic lupus erythematosus. Analysis of the pathologic changes in 120 patients. Am J Med 71, 791–798 (1981).
Ghosh, A., Das, T., Ghosh, A., Karmakar, P. & Pal, J. Evaluation of respiratory manifestations in systemic lupus erythematosus with special reference to pulmonary interstitial involvement. J Indian Med Assoc 110, 109–111 (2012).
Medlin, J. L., Hansen, K. E., McCoy, S. S. & Bartels, C. M. Pulmonary manifestations in late versus early systemic lupus erythematosus: A systematic review and meta-analysis. Semin Arthritis Rheum 48, 198–204, https://doi.org/10.1016/j.semarthrit.2018.01.010 (2018).
Riveros Frutos, A. et al. Systemic lupus erythematosus in Spanish males: a study of the Spanish Rheumatology Society Lupus Registry (RELESSER) cohort. Lupus 26, 698–706, https://doi.org/10.1177/0961203316673728 (2017).
Rahman, P., Gladman, D. D., Urowitz, M. B., Hallett, D. & Tam, L. S. Early damage as measured by the SLICC/ACR damage index is a predictor of mortality in systemic lupus erythematosus. Lupus 10, 93–96, https://doi.org/10.1191/096120301670679959 (2001).
Travis, W. D. et al. An official American Thoracic Society/European Respiratory Society statement: Update of the international multidisciplinary classification of the idiopathic interstitial pneumonias. Am J Respir Crit Care Med 188, 733–748, https://doi.org/10.1164/rccm.201308-1483ST (2013).
Raghu, G. et al. An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med 183, 788–824, https://doi.org/10.1164/rccm.2009-040GL (2011).
Haye Salinas, M. J. et al. Pleuropulmonary involvement in patients with systemic lupus erythematosus from a Latin American inception cohort (GLADEL). Lupus 26, 1368–1377, https://doi.org/10.1177/0961203317699284 (2017).
Wallace, D. J. et al. Efficacy and safety of epratuzumab in patients with moderate/severe active systemic lupus erythematosus: results from EMBLEM, a phase IIb, randomised, double-blind, placebo-controlled, multicentre study. Ann Rheum Dis 73, 183–190, https://doi.org/10.1136/annrheumdis-2012-202760 (2014).
Tsuchiya, Y. et al. Lung diseases directly associated with rheumatoid arthritis and their relationship to outcome. Eur Respir J 37, 1411–1417, https://doi.org/10.1183/09031936.00019210 (2011).
Franklyn, K. et al. Definition and initial validation of a Lupus Low Disease Activity State (LLDAS). Ann Rheum Dis 75, 1615–1621, https://doi.org/10.1136/annrheumdis-2015-207726 (2016).
Polachek, A., Gladman, D. D., Su, J. & Urowitz, M. B. Defining Low Disease Activity in Systemic Lupus Erythematosus. Arthritis Care Res (Hoboken) 69, 997–1003, https://doi.org/10.1002/acr.23109 (2017).
Raghu, G. et al. An Official ATS/ERS/JRS/ALAT Clinical Practice Guideline: Treatment of Idiopathic Pulmonary Fibrosis. An Update of the 2011 Clinical Practice Guideline. Am J Respir Crit Care Med 192, e3–19, https://doi.org/10.1164/rccm.201506-1063ST (2015).
Devaraj, A., Wells, A. U. & Hansell, D. M. Computed tomographic imaging in connective tissue diseases. Semin Respir Crit Care Med 28, 389–397, https://doi.org/10.1055/s-2007-985611 (2007).
Kim, E. J., Collard, H. R. & King, T. E. Jr. Rheumatoid arthritis-associated interstitial lung disease: the relevance of histopathologic and radiographic pattern. Chest 136, 1397–1405, https://doi.org/10.1378/chest.09-0444 (2009).
Nakamura, Y. et al. Nonspecific interstitial pneumonia in collagen vascular diseases: comparison of the clinical characteristics and prognostic significance with usual interstitial pneumonia. Sarcoidosis Vasc Diffuse Lung Dis 20, 235–241 (2003).
Enomoto, N. et al. Quantitative analysis of fibroblastic foci in usual interstitial pneumonia. Chest 130, 22–29, https://doi.org/10.1378/chest.130.1.22 (2006).
Lopez, R., Davidson, J. E., Beeby, M. D., Egger, P. J. & Isenberg, D. A. Lupus disease activity and the risk of subsequent organ damage and mortality in a large lupus cohort. Rheumatology (Oxford) 51, 491–498, https://doi.org/10.1093/rheumatology/ker368 (2012).
Yurkovich, M., Vostretsova, K., Chen, W. & Avina-Zubieta, J. A. Overall and cause-specific mortality in patients with systemic lupus erythematosus: a meta-analysis of observational studies. Arthritis Care Res (Hoboken) 66, 608–616, https://doi.org/10.1002/acr.22173 (2014).
Aberle, T. et al. Use of SLICC criteria in a large, diverse lupus registry enables SLE classification of a subset of ACR-designated subjects with incomplete lupus. Lupus Sci Med 4, e000176, https://doi.org/10.1136/lupus-2016-000176 (2017).
Collard, H. R. et al. Acute Exacerbation of Idiopathic Pulmonary Fibrosis. An International Working Group Report. Am J Respir Crit Care Med 194, 265–275, https://doi.org/10.1164/rccm.201604-0801CI (2016).
Bombardier, C., Gladman, D. D., Urowitz, M. B., Caron, D. & Chang, C. H. Derivation of the SLEDAI. A disease activity index for lupus patients. The Committee on Prognosis Studies in SLE. Arthritis Rheum 35, 630–640 (1992).
Gladman, D. D., Ibanez, D. & Urowitz, M. B. Systemic lupus erythematosus disease activity index 2000. J Rheumatol 29, 288–291 (2002).
American Thoracic, S. & European Respiratory, S. American Thoracic Society/European Respiratory Society International Multidisciplinary Consensus Classification of the Idiopathic Interstitial Pneumonias. This joint statement of the American Thoracic Society (ATS), and the European Respiratory Society (ERS) was adopted by the ATS board of directors, June 2001 and by the ERS Executive Committee, June 2001. Am J Respir Crit Care Med 165, 277–304, https://doi.org/10.1164/ajrccm.165.2.ats01 (2002).
Acknowledgements
We thank Dr. Trish Reynolds, MBBS, FRACP, from Edanz Group (www.edanzediting.com/ac) for editing a draft of this manuscript. Results of this study were previously presented at the ATS International Conference 2018.
Author information
Authors and Affiliations
Contributions
N.E. and T.S. contributed to the study conception and design. N.E., R.E., M.H., Y.W., T.I., S.W., K.K., S.I., A.S., A.M., K.K., T.K., C.S. and K.K. contributed acquisition of data. R.E., K.T., M.H., M.K., Y.T., T.B., H.S., T.T., H.S. and N.E. analyzed and interpreted the data. N.E. drafted the manuscript. Y.I., Y.K. and T.S. contributed to critical revision.
Corresponding author
Ethics declarations
Competing Interests
N.E., R.E., K.T., M.H., M.K., Y.W., T.I., S.W., K.K., S.I., A.S., A.M., K.K., T.K., C.S., Y.I., K.K., Y.T., T.B., H.S., T.T., H.S. and T.S. declare no competing interests. Dr. Kondoh has received advisory board fees and personal fees from Asahi Kasei Pharma Co., Ltd., Boehringer Ingelheim Co., Ltd., Janssen Pharmaceutical K.K. Co., Ltd., Chugai Pharmaceutical Co., Ltd., Eisai inc., KYORIN Pharmaceutical Co., Ltd., Mitsubishi Tanabe Pharma Co., Ltd., Novartis Pharma K.K. Co., Ltd., and Shionogi Co., Ltd. outside the submitted work.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Enomoto, N., Egashira, R., Tabata, K. et al. Analysis of systemic lupus erythematosus-related interstitial pneumonia: a retrospective multicentre study. Sci Rep 9, 7355 (2019). https://doi.org/10.1038/s41598-019-43782-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-43782-7
- Springer Nature Limited