Abstract
Background
Childhood overweight and obesity is a serious public health issue with an increase being observed in preschool-aged children. Treating childhood obesity is difficult and few countries use standardized treatments. Therefore, there is a need to find effective approaches that are feasible for both health care providers and families. Thus, the overall aim of this study is to assess the acceptance and effectiveness of a parent support program (the More and Less, ML) for the management of overweight and obesity followed by a mobile health (mHealth) program (the MINISTOP application) in a socially diverse population of families.
Methods/design
A two-arm, parallel design randomized controlled trial in 300 2-to 6-year-old children with overweight and obesity from Romania, Spain and Sweden (n = 100 from each). Following baseline assessments children are randomized into the intervention or control group in a 1:1 ratio. The intervention, the ML program, consists of 10-weekly group sessions which focus on evidence-based parenting practices, followed by the previously validated MINISTOP application for 6-months to support healthy eating and physical activity behaviors. The primary outcome is change in body mass index (BMI) z-score after 9-months and secondary outcomes include: waist circumference, eating behavior (Child Eating Behavior Questionnaire), parenting behavior (Comprehensive Feeding Practices Questionnaire), physical activity (ActiGraph wGT3x-BT), dietary patterns (based on metabolic markers from urine and 24 h dietary recalls), epigenetic and gut hormones (fasting blood samples), and the overall acceptance of the overweight and obesity management in young children (semi-structured interviews). Outcomes are measured at baseline and after: 10-weeks (only BMI z-score, waist circumference), 9-months (all outcomes), 15- and 21-months (all outcomes except physical activity, dietary patterns, epigenetics and gut hormones) post-baseline.
Discussion
This study will evaluate a parent support program for weight management in young children in three European countries. To boost the effect of the ML program the families will be supported by an app for 6-months. If the program is found to be effective, it has the potential to be implemented into routine care to reduce overweight and obesity in young children and the app could prove to be a viable option for sustained effects of the care provided.
Trial registration
ClinicalTrials.gov NCT03800823; 11 Jan 2019.
Similar content being viewed by others
Background
According to the World Health Organization childhood obesity is one of the gravest public health challenges of today’s society [1], with approximately 108 million 2- to 19-year-old children being classified as having obesity [2]. More specifically, in children less than 5 years, there has been a swift increase in childhood overweight and obesity and if these trends continue it is predicted that 70 million children will be overweight or obese by 2025 [3]. These statistics are concerning as Geserick et al. [4] found that 90% of 3 year olds with obesity still had overweight or obesity in adolescence. Furthermore, for those adolescents with overweight or obesity, the majority of weight gain happened between two and 6 years of age [4]. Thus, this demonstrates the need for evidence-based treatment programs in the pre-school years in order to attempt to rectify the increased prevalence of childhood overweight and obesity.
According to Colquitt et al. [5] for children under 6 years of age multicomponent interventions (i.e., diet, physical activity, and behavioral interventions) seem to be effective at treating overweight and obesity. However, the authors did state that evidence is limited [5]. To date, the majority of the treatment interventions for overweight and obesity use face-to-face delivery methods [6]. A recent meta-analysis by Ling et al. [6] found small effect sizes on treatment interventions for preschool-aged children for body mass index (BMI) (− 0.28 kg/m2, p < 0.001) using various in person delivery methods. Furthermore, the More and Less (ML) study found that at the 12-month follow-up, a 10-week group treatment program focusing on parenting practices had a greater reduction in BMI z-scores than standard treatment in health care (− 0.30 vs. -0.07, p < 0.05). An even greater reduction was observed in the intervention group who received booster sessions (a 30-min phone call every 4 to 6 weeks over a 9-month period) [7]. These results are promising; however, sustained contacts with families after treatment programs are burdensome on both health care providers and participants, which makes it difficult to scale-up. Therefore, different types of boosters need to be used in order to reduce the burden on both health care and participants.
The universal use of smartphones makes the use of mobile health (mHealth) an option for boosting the effects of treatment programs. mHealth is increasingly being used for promoting healthy habits and as treatment of many types of health conditions and diseases. In adults, two meta-analyses have found that mHealth interventions focusing on weight loss significantly decreased participants’ weight in the intervention groups compared to the control groups [8, 9]. In children and adolescents few studies have utilized mHealth in the prevention or treatment of obesity [10,11,12,13,14] and hardly any have been conducted in the preschool-age group [15, 16]. The Mobile-based Intervention Intended to Stop Obesity in Preschoolers (MINISTOP) trial was a mHealth obesity prevention intervention that was developed and led by Marie Löf and her team to improve 4-year-old children’s body composition, dietary, physical activity, and sedentary behaviors [17, 18]. The MINISTOP intervention had a significant effect on a composite score composed of body composition, diet, and physical activity variables, with this effect being more evident among children with a higher fat mass index [18]. There are numerous advantages of mHealth over conventional intervention approaches such as: the programs can be delivered any time and place; are interactive; can be tailored to different groups (e.g., translated into multiple languages); and reduces burden on health care professionals and participants. These advantages further motivates the use of mHealth in families with young children with overweight and obesity.
The mechanisms that drive weight gain such as epigenetics and gut hormones are still unclear [19, 20]. Epigenetics has received attention during the recent years for the putative involvement in transmitting obesity risk to offspring and in the heritable regulation of gene expression without altering their coding sequence [21]. The most relevant epigenetic mechanisms involved in gene activity control are histone modifications, non-coding RNAs (ncRNA) and DNA methylation [20]. Further, obesity has been associated with the epigenetic modulation of several genes. For example, a relationship has been reported between increased BMI and adiposity as well as higher DNA methylation levels at the hypoxia-inducible transcription factor 3A (HIF3A) gene [22]. Moreover, an increased methylation in the gene RXRA measured at birth has been associated with greater adiposity in later childhood [23]. Two other investigations identified a strong correlation between obesity and serum levels of micro RNA (miR)-122 and miR-519d [24] and found DNA methylation to be related to insulin resistance [25]. However, these findings need to be confirmed and further explored in young children.
Another field of interest for obesity is the gastrointestinal tract (GIT) [26]. The GIT plays an important role in acute appetite regulation through a number of mechanisms: (1) the release of hormones that play a role in appetite regulation such as anorectic hormones (Peptide YY, PYY, and glucagon-like peptide, GLP-1) and orexogenic gut hormones (e.g., ghrelin), (2) the enteric nervous system and signals through the vagus to the brain to influence appetite and (3) secondary to stimulating signals from other organs such as liver adipose. Previous research in adults has demonstrated that the infusion of the GIT anorectic hormones PYY and GLP-1 at physiological doses has profound effects to suppress appetite [26]. Also weight loss appears to lead to a suppression of PYY and GLP-1 suggesting a role in the feelings of hunger during weight reduction. However, evidence of the role of GIT hormones in overweight and obesity among young children is sparse.
A major challenge in the management of obesity in both adults and children is understanding what people eat. Most dietary assessment methodologies use methods of self-reported food intake which is a subject to large misreporting error [27, 28]. It is therefore impossible to understand what children eat. Garcia et al. has developed a new metabolomic methodology of dietary assessment using urine, which is not subject to the same misreporting errors [29]. This method has been validated in adults. Our aim is to do this is children.
To the best of our knowledge there is no study to date that has the ambition to assess a broad array of key biological and social determinants of obesity in young children. This study protocol outlines the design of a multi-country study that incorporates both a parent support program and mHealth in an overweight and obesity intervention in 2- to 6-year-old children with overweight and obesity.
Aim
The overall aim of this study is to assess the feasibility, acceptance and effectiveness of an overweight and obesity intervention in a socially diverse population of families. The specific aims are:
-
1.
To determine the effectiveness on child weight status (BMI z-score) of a 10-week parent support program delivered in groups focusing on evidence-based parenting practices (the ML program) followed by a mHealth component for 6-months (the MINISTOP application, app) for overweight and obesity in preschool-aged children.
-
2.
To assess change in secondary outcomes, which are: waist circumference, child eating behavior, parental feeding practices, and physical activity.
-
3.
To assess epigenetic mechanisms and physio pathological processes underlying childhood obesity including the role of gut hormones.
-
4.
To assess and validate child food intake with metabolic markers in urine metabolomics.
-
5.
To evaluate the feasibility of recruitment (facilitators and barriers), attrition and acceptability of the ML program, the standard treatment and the overall acceptance of overweight and obesity management according to patients and care providers.
Hypotheses
Our central hypothesis is that the intervention (the ML program followed by the MINISTOP app for boosting) will be more effective in decreasing children’s BMI z-score (primary outcome), improving eating and feeding behaviors, and physical activity (secondary outcomes) compared to standard care. Another study hypothesis is that the intervention will produce changes in urinary metabolites, which will serve as biomarkers of the nutritional outcomes or as targets for application. We also hypothesize that the parent program and the mHealth intervention will be well accepted by families and caregivers.
Methods
Study design
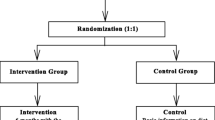
ML Europe is a two-arm parallel design randomized controlled trial (RCT) comparing overweight and obesity treatments in 2-to 6-year-old children in three countries (Romania, Spain, and Sweden). Following baseline assessments, participants will be randomized into the intervention and control group in a 1:1 ratio. The intervention group receives a 10-week parent support program (the ML program) which focuses on evidence-based parenting practices [7, 30] followed by a previously validated 6-month mHealth program (the MINISTOP app, PI: M Löf) to support healthy lifestyle changes [17, 18]. The control group receives standard treatment as offered in the country of participation. The different interventions are described in greater detail below. Assessments will be conducted at 10 weeks, 9 months, 15 months, and 21 months post-baseline (see Fig. 1 for study outline). This study protocol follows the SPIRIT 2013 statement [31, 32].
Sample size and power calculation
Based on power calculations, 75 children are needed in each group (adjusted for drop-out) to detect a difference of 0.3 BMI z-score with 85% power at the 9-month follow-up between the intervention and control group. These calculations are based on a previous study in this age group [33]. Thus, each site aims to recruit 100 participants to ensure adequate power.
Participants, eligibility, and recruitment
In total, we aim to include 300 families (n = 100 in Romania, Spain, and Sweden, respectively). To be included in this study: children must be between 2 and 6 years old and have overweight or obesity as classified by international cut-offs [34]; have no other underlying medical condition(s); the child has not started any treatment for overweight or obesity; and at least one parent has to have the ability to communicate in Romanian, Spanish, or Swedish depending on the country of participation. Parents who do not own a smartphone compatible with the MINISTOP app will be excluded from this study (i.e., version 10.0 or higher for iOS or version 5.0 or higher for Android).
Recruitment will follow a standardized protocol for all countries. In Romania, family physicians and pediatricians will be involved to hand out information regarding the study to families with 2- to 6-year-olds with overweight or obesity. Parents who want to learn more about the study are provided with a phone number, email address, web page and Facebook page with information of how to contact the research group. Participants will also be recruited, as self-referrals, using an official page for the study on Facebook to be shared with specialized groups.
In Spain, families with children who attend weight and height assessments at their pediatricians at primary care health centers and hospitals will be asked to participate in the study. If the parents are interested in participating, the pediatrician will schedule a visit within a maximum of 7 days to provide them with more detailed information regarding the study and for them to sign the informed consent.
Finally, in Sweden, the recruitment methods have been previously described in detail [7, 30]. Briefly, recruitment is done primarily at primary child health care centers, where all parents of children from birth to 5 years of age are offered free, yearly check-ups. If overweight or obesity is detected the nurse provides a verbal and a short one-page explanation of the study. If the parent(s) are interested in participating the nurse sends a referral to the research group that will send out more detailed information regarding the study together with a consent letter. After 1 week, a member from the research team will contact the families to answer any questions that they have. Recruitment is also conducted at secondary health care (i.e., out-patient pediatric clinics). Additionally, self-recruitment is being done through newspaper ads as well as by placing posters on primary health care bulletin boards.
For all countries, after fully informing the families, if they still want to participate they send back the signed consent letter, which is subsequently signed by a member of the research team and a copy is sent back to the family. A time for baseline assessments is then scheduled with the research group.
Randomization and blinding
After the consent form has been signed, the participants are randomly allocated to either the intervention group (parent support program and mHealth booster) or the control group (standard care as per country) at a 1:1 ratio via a random allocation sequence list (in blocks of three). The sequence list was generated using free software environment for statistical computing and graphics R (version 3.5.1) [35]. The random allocation sequence is managed by a person who has no relationship with recruitment or treatment and opaque envelopes are used to ensure concealment. Those assessing the outcomes are blinded to the treatment allocation; however, owing to the nature of the intervention participants are not blind to their allocation.
Intervention
The More and Less program
The ML program is based on the Keeping Foster and Kin Parents Supported and Trained (KEEP) parenting program, which has been tested in multiple settings [36,37,38,39]. KEEP is based on Bandura’s Social Learning Theory [40] and Patterson’s Social Interaction Learning Theory [41, 42]. The key concept of the programs is to support parents in evidence-based parenting practices, especially regarding positive reinforcement and limit setting, in order to improve parent and child communication. In ML, the improved communication lays the foundation for parents to support a healthy lifestyle for the child.
The ML program is comprised of 10 weekly sessions (1.5 h/week) and is culturally adapted for Romanian, Spanish, and Swedish families with preschool aged children with overweight or obesity. Table 1 displays the content of the ML program [7, 30]. Beyond the evidence-based parenting practices, the program includes content regarding healthy food habits, physical activity habits, as well as techniques to help parents regulate emotional control. Each session begins with a theoretical introduction to a parenting skill, the focus of the session is then discussed and practice is done through role play and homework assignments. To facilitate the implementation of the ML program it follows a manual where the sessions are described with precise instructions to the group leaders (2 per group). The parents receive a manual which summarizes what has been discussed during each session. For parents who are unable to attend sessions, the parental manual is sent home to the family and the family is contacted by phone for a brief review of the session. To facilitate session attendance the time and location for the groups are planned to suit the parents. Child care is also provided during the sessions.
The ML group leaders received an initial 4 day training in child overweight and obesity management and in the ML program content. The training was provided by the ML program developers PN and AE. During the training the sessions of the program were thoroughly discussed and the group leaders were trained in how to deliver the program by acting as group leaders while the other participants acted as parents. The training of group leaders will continue by external supervision after each weekly session for the first group in all countries. The group leaders will be asked to watch the filmed sessions and reflect on how they delivered the program. In Sweden and in Spain, groups will be held in health care facilities and in Romania in university facilities.
The MINISTOP app
The MINISTOP app was developed and evaluated in a population based study with preschool aged children (PI: Marie Löf) and has been previously described in detail [17, 18]. Briefly, MINISTOP comprises of an extensive program of information and push notifications built using current guidelines for a healthy diet and physical activity in pre-school aged children [43]. Over the 6-month period 12 themes will be covered (Table 1). A new theme is introduced bi-weekly, with parents being alerted by a push notification when this happens. Every theme is split into three parts (general information; advice; and strategies to change unwanted behavior). Through the app, parents have the ability to register their child’s consumption of sugar sweetened beverages, candy, fruits and vegetables, and physical activity and sedentary behavior. Parents then receive feedback on the registered parameters at the end of every week. Reminder messages are sent out to parents if they have not been in the app after a couple of days [17].
Two days before the tenth and final session of the ML program, parents receive an email with a username and password for the MINISTOP app as well as a text message with a link to download the app. At the final session, the ML program leaders will ensure that all parents were able to download the app and sign in. Thereafter, they will explain how the app works to the parents and answer any questions that they may have.
Control
The weight management offered to the control group follows the standard care procedure for each country of participation. In Romania and Spain, the control group receives an evaluation of a one-day food frequency questionnaire as well as a 30-min consultation with a doctor that is a specialist in childhood nutrition, where healthy lifestyle recommendations are made. The parents also receive a hand-out which provides general recommendations for healthy food and physical activity in 2 to 6 year olds. Furthermore, in Romania the children are re-evaluated after 3 months during a 15-min consultation. In Sweden, the control group receives standard care according to the Action plan for overweight and obesity for Stockholm County [44]. Children with overweight and children with obesity younger than 4 years receive support from their child health care nurse. Children older than for 4 years with obesity are followed in an outpatient pediatric clinic with yearly visits to a pediatrician and follow-up visits to a pediatric nurse, approximately 5 visits (30 min in duration) per year [7]. The treatment centers around supporting the family in creating healthy diet and physical activity habits for the child. Children may also be referred to dieticians, psychologists or physiotherapists.
Measures
Outcome measures are collected at baseline, 10 weeks, 9 months, 15 months, and 21 months post baseline. Table 2 presents when outcome measures are assessed and the instruments used to assess child and parental behaviors are displayed in Table 3.
Primary outcome
BMI z-score is the primary outcome measure which is the most commonly used indicator of weight change in pediatric obesity studies [47]. The children’s weight and height will be measured to the nearest 0.1 kg and 0.1 cm, respectively. A fixed stadiometer is used to assess height and weight will be measured with the children wearing only underwear. BMI is derived as weight (kg) divided by height (m) squared. BMI z-scores are then calculated using age and gender specific reference values [34].
Secondary outcomes
Waist circumference
Waist circumference is measured at the mid-point between the lower rib and iliac crest to the nearest 0.1 cm using a non-elastic tape measurer.
Weight, height and waist circumference are measured three times and mean values are then calculated. All children are measured in a standardized manner by trained health care professionals using calibrated instruments.
Eating behavior
The children’s eating behavior is assessed using the Child Eating Behavior Questionnaire (CEBQ) [45]. It includes 35 items on eating styles comprising eight factors related to the risk of obesity. Parents rate each behavior on a five-point Likert scale (`never´, `rarely´, `sometimes´, `mostly´, and `always´ for items 1 to 13 and `disagree´, `slightly disagree´, `neutral´, `slightly agree´, and `agree´ for items 14 to 49). Mean scores for each sub-scale are calculated. This questionnaire has been found to have high internal reliability and good validity [45, 48,49,50,51,52,53].
Parenting behavior
The Comprehensive Feeding Practices Questionnaire (CFPQ) is used to measure parenting behavior [46]. The CFPQ is a parent-report instrument, designed to measure feeding practices of parents of children aged 2–8 years. It contains 49 items comprising 12 factors, where parents rate each behavior on a five-point Likert scale (`never´, `rarely´, `sometimes´, `mostly´, and `always´). The CFPQ has previously been validated in Brazilian preschoolers [54].
Physical activity and sedentary behavior
The ActiGraph wGT3x-BT accelerometer (ActiGraph Corp, Pensacola, USA, www.actiGraphcorp.com) is used to assess physical activity and sedentary behavior over seven consecutive 24 h periods. The ActiGraph will be attached the child’s non-dominant wrist and be worn at all times, except for water-based activities (e.g., showering/bathing or swimming). The recorded movements will be used to estimate time in various activity levels based on appropriate cut-points.
Metabolites of food intake
First void urinary samples will be collected from the children and will be used to assess metabolites of food intake. Two urine samples from the child will be collected by the parents at home six and 3 days before the visit to the research group. The third urine sample is collected on the morning of the visit to the research group. The urine metabolite analysis will be carried out as previously described [29]. In brief, urine samples will be measured by proton nuclear magnetic resonance (1H-NMR) spectroscopy. Global urinary 1H-NMR profiles will be used to predict the quality of the diet using the World Health Organization guidelines as a reference. Individual urinary metabolites associated with the intake of foods will be used to assess the dietary profile of the child. The Dietary Metabotype Score that embodies concentrations of urinary metabolites related to food components and adherence to diet will be developed and validated against one 24-h dietary recall with a parent. The 24-h recall will cover the day before the visit to the research group. For children attending preschool a food diary for teachers to fill out will be collected to cover the food intake not provided by the parent.
Epigenetic markers and gut hormones
Fasting blood samples are collected to assess reversibility of metabolic markers through epigenetic markers and the role of gut hormones.
Epigenetic markers
The epigenetic analysis is carried out in white blood cells, which require DNA extraction, bisulphite transformation, and analysis with Polymerase chain reactions (PCRs) or other technologies involving hypothesis driven methylation (CpGs). The unit of measurement/criteria is changes in percentage CpGs. The methodology has been explained in detail elsewhere [55, 56]. Methylation levels will be analyzed following standardized epigenetic methods after bisulphite conversion as described previously [55, 56] in hypothesis- driven specific CpGs.
Gut hormones
PYY concentrations will be measured using an in-house radioimmunoassay (RIA). The assays are highly sensitive and do not cross-react with other gut hormones. Separation of the antibody-antigen complexes from the free antigen is achieved by secondary antibody. The reported intra- and inter-assay variation is 5.8 and 9.8% respectively.
GLP-1 concentrations will be measured using an in-house RIA. This assay is highly specific and sensitive with the antibody cross reacting with 100% of all amidated forms of GLP-1. The assay does not cross react with glycine extended forms (GLP1–37 and GLP9–37) or any other gut hormones. The lowest level of GLP-1 that can be detected by this assay is 7.5 pmol/l. Separation of the antibody-antigen complexes from the free antigen is achieved by charcoal adsorption. The reported in-house intra- and inter-assay variation is 5.4 and 11.5% respectively.
Feasibility, attrition, and acceptability
Using semi-structured interviews, the facilitators and barriers of recruitment as well as attrition to the intervention (first 10 weeks, i.e., the ML parent program) and feasibility and acceptability of the MINISTOP app and of the standard care offered are assessed. Both parents and healthcare professionals are interviewed by trained research staff. During the interviews a set of questions are asked to all participants follow-up questions are however based on individual responses. The questions have been tested in pilot interviews with both parents and health care professionals. The interviews are recorded and fully transcribed. Interviews will be conducted before and after the intervention.
Sociodemographic data
At baseline parents are asked to fill out a background questionnaire for the child and themselves. Questions for the parent include: health status, sociodemographic factors and social support. For the child, questions include: country of birth, health status, family structure and lifestyle related questions such as food and screen time behaviors.
Adverse events
Adverse events will be monitored, reported and handled appropriately. The risks imposed by this research project are deemed to be low, i.e., the burden of the experiments for the research subjects is limited. It is important to note that blood samples collected in the study are optional and not a criteria for participation. However, blood samples are taken by experienced nurses and a pain reducing cream is used to reduce any discomfort. Urinary samples are none invasive and thus cause no risk to the participants. In addition, the investigators have extensive experience conducting behavioral weight control studies, and active efforts will be taken by the research staff to ensure the participating families’ safety. Other adverse events may include psychosocial burden that parents may experience when made aware about their child’s weight status and the sense of guilt that may arise. To handle that, already in the first session of the ML program causes and consequences are reviewed in a non-judgmental way. Also, potential impact on the child’s self-esteem and the way to talk about body weight and obesity with children, if necessary, are addressed.
Data management
All collected data will be handled as approved by the ethical boards to protect confidentiality. Data is de-identified and entered manually into a database by research staff at the participating site where the data originated from. An identical database is used at each site. To ensure data quality and validity the researchers follow standard operation procedure protocols when entering data. The entered data will be double checked by the person entering the data and random checks will be performed regularly to ensure data validity. The database will be password protected and access is restricted to researchers with passwords. Original data forms will be stored in a secure place at each study site.
Statistical analysis
Intention-to-treat analysis using generalized linear mixed models with repeated measures will be used to examine the effects of the intervention on primary (aim 1) and secondary outcomes (aim 2) for the total study population (i.e., all three sites). The link function for the primary outcome (BMI z-score) will be the identity and the Gaussian family (equivalent to a linear regression). In secondary outcomes we will use a Gaussian identity and family link function for waist circumference, physical activity and sedentary behavior, and a logarithmic link and Poisson family function (equivalent to a Poisson regression) for child eating behavior and parental feeding practices. A random effect for country will be used to account for the clustered study design. In the models, we will control for relevant covariates such as sex, age, parental weight status, education level, income and foreign background. Random intercept and a random slope for time will be included in the model to control those non-observed confounders specific to each child that could be constant or vary in time, respectively. Furthermore, interactions between variables will be estimated. If missing values in the outcomes (primary and secondary) are more than 10%, these will be imputed through a two-part model (also known as a model for semi continuous data). In this model, we would simultaneously estimate the probability of not being missing (first part) and the outcome (second part), using a mixed generalized linear model, in which we would include, as explanatory variables: age, sex, parental weight status, foreign background, educational level, and the random effects which are aforementioned.
Statistical tests and analyses of the interaction of phenotypical outcomes with epigenetics will include Manhattan plots, volcano plots, principal component analysis (PCA)/cluster, heatmaps, partial least square-discriminant analysis (PLS-DA), correlations and association studies, linear regression models, receiver operating characteristic (ROC) curves and these will be implemented as appropriate.
The means and medians for the gut hormone values before and after the intervention will be compared, using Student’s t test and Mann-Whitney U test, respectively. The differences will be adjusted in a generalized linear mixed model, with an identity link and Gaussian family, including the confounders, both observed and unobserved, indicated above.
For the validation of child food intake with metabolic markers in urine, the urinary dietary model will be derived using previously described methodology [29]. Comparison between the study groups will be carried out using PCA and Monte Carlo cross-validated partial least square-discriminant analysis (MCCV-PLS-DA) methodology. The relationship between dietary biomarkers and the dietary metabolite profile will be carried out using a generalized linear mixed model, with an identity link and Gaussian family, including, again, the confounders.
The semi-structured interviews with parents and health care professional will be fully transcribed verbatim and analyzed using thematic analysis [57].
For our analyses we will use R [35], STATA version 12.1 (StataCorp 2011, College Station, TX, USA) and SPSS Statistics (IBM, Armonk, NY, USA).
Ethics approval
This trial was approved by: the Ethics Committee of Scientific Research in University of Medicine and Pharmacy “Victor Babes”, Timisoara, Romania, October 31st, 2018 (25/31.10.2018), the Balearic Islands Ethics Committee, Mallorca, Spain, February 13th, 2019 (IB 3814/18 PI), and the Research Ethics Committee, Stockholm, Sweden, December 11th, 2018 (2018/2082–31/1). Written informed consent is obtained from all parents/caregivers. The ethics committees approved the consent procedure.
Trial status
In Sweden and Romania recruitment began in January 2019 and Spain began to recruit in February 2019. Recruitment is expected to last until 2020.
Discussion
The ML Europe trial will assess the impact of parent support group sessions (the ML program) followed by a mHealth program (the MINISTOP app) to treat overweight and obesity in 2- to 6-year-old children from three European countries. Globally, there has been very few overweight and obesity treatment interventions targeted to pre-school aged children [6] and to date no intervention has coupled face-to-face delivery with mHealth to boost the effect of the intervention.
In this trial we aim to recruit a representative sample of the study population in each participating country. In Sweden this will be done by inviting all primary and secondary health care centers in Stockholm County to participate in recruitment, with a similar process being done in Spain (all primary health care centers and hospitals in Mallorca were invited to participate). However, the ability to get a representative sample of the study population in Romania might be more difficult as recruitment relies on families contacting the research team themselves through contacts with physicians and pediatricians and Facebook announcements. Therefore, certain parts of the population may be missed, e.g., those not likely to contact the research team and those who do not use Facebook.
Additionally, there are a few other factors that should be considered with regards to recruitment, which have the possibility to influence the representativeness of the overall sample. Firstly, the participating families need to be able to understand, speak, and read Romanian, Spanish, or Swedish sufficiently well (depending on the country of participation) in order to participate. Secondly, families with low socioeconomic status and parents with a lower educational background have been found to be less likely to participate in research [58, 59]. The inability to speak the language the intervention is being conducted in coupled with the possibility of low participation rates in families with low socioeconomic status is of concern. This is due to the fact that children of migrant parents and those of low socioeconomic status are more likely to have overweight or obesity [60, 61]. Furthermore, families will only be included if they own a smartphone compatible with the MINISTOP app, which could affect recruitment of low socioeconomic families; however, we believe this risk to be quite small as smartphones are so commonly used in most populations. Finally, we foresee that recruitment for this study will be a challenge as was found in the ML trial [7]. In the ML trial parents decided not to participate for various reasons, with the most common being parents’ work schedules or family situation [7]. When recruiting for ML Europe we used our experiences from previous clinical RCTs to ensure that recruitment and patient participation are organized in the most feasible way, e.g., time, date and place for the parent groups will be adjusted to suit as many families as possible. We anticipate the recruitment to be influenced by target population size which varies between the countries (330,000 in Timisoara, 860,000 in Mallorca and 2.3 million in Stockholm County). Also, we are aware that the prevalence of overweight and obesity among children differs in each site. While recent national data are yet to be published, in Romania, a study including 6-year-old children found the prevalence for overweight and obesity to be 19% [62]. In Spain, the prevalence of overweight and obesity was 21% in 3 to 5-year-old children [63]. In the Stockholm County, the prevalence of overweight and obesity among 4-year-old children is on average 11% ranging from 4% in the more affluent areas to over 15% in less affluent areas [64]. Thus, although the prevalence and obesity seems to be lowest in the Swedish site the larger population may compensate this challenge. It remains to be elucidated what the largest barrier in the recruitment process will be: not sufficiently large targeted population or low prevalence of overweight and obesity.
The randomized controlled design and multi-site recruitment (i.e., Timisoara, Romania; Mallorca, Spain; and Stockholm, Sweden) are strengths of this study. Furthermore, the fairly large sample size (n = 300) will allow us to assess the intervention’s effectiveness in samples within and across three very different European countries. With regards to the intervention, both components are based on behavior change theories (i.e., Bandura’s Social Learning Theory [40] and Patterson’s Social Interaction Learning Theory [41, 42] for the ML program and Social Cognitive Theory [65] for the MINISTOP program). Furthermore, the combination of group sessions followed by a previously evaluated mHealth app is a further strength, as it will allow for the reiteration of the material taught during the group sessions to be explained in different ways with different examples. This is important as the booster group in the ML study had a mean change in BMI z-score from baseline which was significantly larger in comparison to standard treatment and the group without boosters (− 0.54, p < 0.001; − 0.11, p = 0.551; and − 0.04 for the booster, without boosters, and standard treatment groups, respectively) [7]. In today’s society, telephone based booster sessions after an intervention such as ML is difficult to sustain due to parents’ busy schedules. Therefore, a mHealth solution such as MINISTOP to booster the effect of treatment may be a more feasible approach as it allows parents to work through the material at their own pace, when they have time. Finally, this study is limited by the fact that there is no standard overweight and obesity treatment across Europe. Therefore, the control group will receive different treatment depending on the country of participation, which could influence the results. However, standard treatment as per country is the best possible control as it would be considered unethical to withhold treatment for a condition if a treatment exists [66].
The use of objective assessments for anthropometrics and body composition, physical activity and sedentary behavior, food intake, as well as epigenetic and metabolic markers is a further strength of this study. Additionally, the use of qualitative methods, i.e., semi-structured interviews with health care professionals and parents from all sites will allow us to assess the feasibility of this new overweight and obesity management intervention in three European countries.
In conclusion, in the majority of countries, there is no standard management of overweight and obesity in the pre-school years. As overweight and obesity in this age may track into adolescence and adulthood, causing psychological and physical consequences, families should receive support as early as possible. Feasible and effective approaches for families with pre-school aged children are yet to be developed. If the ML Europe intervention is found to be effective, it has the potential to be implemented into routine care for overweight and obesity across Europe.
Availability of data and materials
Not applicable.
Abbreviations
- 1H-NMR:
-
proton nuclear magnetic resonance
- App:
-
Application
- BMI:
-
Body mass index
- CEBQ:
-
Child eating behaviour questionnaire
- CFPQ:
-
Comprehensive feeding practices questionnaire
- DNA:
-
Deoxyribonucleic acid
- GIT:
-
Gastrointestinal tract
- GLP:
-
Glucagon-like peptide
- HIF3A:
-
Hypoxia-inducible transcription factor 3A
- KEEP:
-
Keeping foster and kin parents supported and trained
- MCCV-PLS-DA:
-
Monte Carlo cross-validated partial least square-discriminant analysis
- mHealth:
-
Mobile health
- MINISTOP:
-
Mobile-based intervention intended to stop obesity in preschoolers
- miR:
-
Micro Ribonucleic acid
- ML:
-
More and Less
- ncRNA:
-
Non-coding Ribonucleic acid
- PCA:
-
Principal component analysis
- PCR:
-
Polymerase chain reaction
- PLS-DA:
-
Partial least square-discriminant analysis
- PYY:
-
Peptide YY
- RCT:
-
Randomized controlled trial
- RIA:
-
Radioimmunoassay
- ROC curves:
-
receiver operating characteristic curves
References
World Health Organization. The Commision on ending childhood obesity. https://www.who.int/end-childhood-obesity/publications/echo-plan-executive-summary/en/. Accessed 20 Feb 2019.
Collaborators GBDO, Afshin A, Forouzanfar MH, Reitsma MB, Sur P, Estep K, et al. Health effects of overweight and obesity in 195 countries over 25 years. N Engl J Med. 2017;377(1):13–27.
World Health Organization. Facts and figures on childhood obesity. http://www.who.int/end-childhood-obesity/facts/en/. Accessed 20 Feb 2019.
Geserick M, Vogel M, Gausche R, Lipek T, Spielau U, Keller E, et al. Acceleration of BMI in early childhood and risk of sustained obesity. N Engl J Med. 2018;379(14):1303–12.
Colquitt JL, Loveman E, O'Malley C, Azevedo LB, Mead E, Al-Khudairy L, et al. Diet, physical activity, and behavioural interventions for the treatment of overweight or obesity in preschool children up to the age of 6 years. Cochrane Database Syst Rev. 2016;(3):CD012105.
Ling J, Robbins LB, Wen F, Zhang N. Lifestyle interventions in preschool children: a meta-analysis of effectiveness. Am J Prev Med. 2017;53(1):102–12.
Ek A, Chamberlain KL, Sorjonen K, Hammar U, Malek ME, Sandvik P, et al. A Parent Treatment Program for Preschoolers with Obesity: A Randomized Controlled Trial. Pediatrics. 2019; In Press.
Schippers M, Adam PC, Smolenski DJ, Wong HT, de Wit JB. A meta-analysis of overall effects of weight loss interventions delivered via mobile phones and effect size differences according to delivery mode, personal contact, and intervention intensity and duration. Obes Rev. 2017;18(4):450–9.
Flores Mateo G, Granado-Font E, Ferre-Grau C, Montana-Carreras X. Mobile phone apps to promote weight loss and increase physical activity: a systematic review and meta-analysis. J Med Internet Res. 2015;17(11):e253.
Sharifi M, Dryden EM, Horan CM, Price S, Marshall R, Hacker K, et al. Leveraging text messaging and mobile technology to support pediatric obesity-related behavior change: a qualitative study using parent focus groups and interviews. J Med Internet Res. 2013;15(12):e272.
Schoeppe S, Alley S, Van Lippevelde W, Bray NA, Williams SL, Duncan MJ, et al. Efficacy of interventions that use apps to improve diet, physical activity and sedentary behaviour: a systematic review. Int J Behav Nutr Phys Act. 2016;13(1):127.
Darling KE, Sato AF. Systematic review and meta-analysis examining the effectiveness of Mobile health Technologies in Using Self-Monitoring for pediatric weight management. Child Obes. 2017;13(5):347–55.
Callender C, Thompson D. Family TXT: feasibility and acceptability of a mHealth obesity prevention program for parents of pre-adolescent African American girls. Children (Basel). 2018;5(6).
Grutzmacher SK, Munger AL, Speirs KE, Vafai Y, Hilberg E, Braunscheidel Duru E, et al. Predicting attrition in a text-based nutrition education program: survival analysis of Text2BHealthy. JMIR mHealth uHealth. 2019;7(1):e9967.
Militello L, Melnyk BM, Hekler EB, Small L, Jacobson D. Automated behavioral text messaging and face-to-face intervention for parents of overweight or obese preschool children: results from a pilot study. JMIR mHealth uHealth. 2016;4(1):e21.
Militello LK, Melnyk BM, Hekler E, Small L, Jacobson D. Correlates of healthy lifestyle beliefs and behaviors in parents of overweight or obese preschool children before and after a cognitive behavioral therapy intervention with text messaging. J Pediatr Health Care. 2016;30(3):252–60.
Delisle C, Sandin S, Forsum E, Henriksson H, Trolle-Lagerros Y, Larsson C, et al. A web- and mobile phone-based intervention to prevent obesity in 4-year-olds (MINISTOP): a population-based randomized controlled trial. BMC Public Health. 2015;15:95.
Nystrom CD, Sandin S, Henriksson P, Henriksson H, Trolle-Lagerros Y, Larsson C, et al. Mobile-based intervention intended to stop obesity in preschool-aged children: the MINISTOP randomized controlled trial. Am J Clin Nutr. 2017;105(6):1327–35.
Roth CL, Enriori PJ, Harz K, Woelfle J, Cowley MA, Reinehr T. Peptide YY is a regulator of energy homeostasis in obese children before and after weight loss. J Clin Endocrinol Metab. 2005;90(12):6386–91.
Egger G, Liang G, Aparicio A, Jones PA. Epigenetics in human disease and prospects for epigenetic therapy. Nature. 2004;429(6990):457–63.
van Dijk SJ, Molloy PL, Varinli H, Morrison JL, Muhlhausler BS. Epigenetics and human obesity. Int J Obes. 2015;39(1):85–97.
Dick KJ, Nelson CP, Tsaprouni L, Sandling JK, Aissi D, Wahl S, et al. DNA methylation and body-mass index: a genome-wide analysis. Lancet. 2014;383(9933):1990–8.
Godfrey KM, Sheppard A, Gluckman PD, Lillycrop KA, Burdge GC, McLean C, et al. Epigenetic gene promoter methylation at birth is associated with child's later adiposity. Diabetes. 2011;60(5):1528–34.
Adams BD, Arem H, Hubal MJ, Cartmel B, Li F, Harrigan M, et al. Exercise and weight loss interventions and miRNA expression in women with breast cancer. Breast Cancer Res Treat. 2018;170(1):55–67.
Arpon A, Milagro FI, Ramos-Lopez O, Mansego ML, Santos JL, Riezu-Boj JI, et al. Epigenome-wide association study in peripheral white blood cells involving insulin resistance. Sci Rep. 2019;9(1):2445.
Choudhury SM, Tan TM, Bloom SR. Gastrointestinal hormones and their role in obesity. Curr Opin Endocrinol Diabetes Obes. 2016;23(1):18–22.
Poslusna K, Ruprich J, de Vries JH, Jakubikova M, van't Veer P. Misreporting of energy and micronutrient intake estimated by food records and 24 hour recalls, control and adjustment methods in practice. Br J Nutr. 2009;101(Suppl 2):S73–85.
Lafay L, Mennen L, Basdevant A, Charles MA, Borys JM, Eschwege E, et al. Does energy intake underreporting involve all kinds of food or only specific food items? Results from the Fleurbaix Laventie Ville Sante (FLVS) study. Int J Obes Relat Metab Disord. 2000;24(11):1500–6.
Garcia-Perez I, Posma JM, Gibson R, Chambers ES, Hansen TH, Vestergaard H, et al. Objective assessment of dietary patterns by use of metabolic phenotyping: a randomised, controlled, crossover trial. Lancet Diabetes Endocrinol. 2017;5(3):184–95.
Ek A, Chamberlain KL, Ejderhamn J, Fisher PA, Marcus C, Chamberlain P, et al. The more and less study: a randomized controlled trial testing different approaches to treat obesity in preschoolers. BMC Public Health. 2015;15(1):735.
Chan AW, Tetzlaff JM, Gotzsche PC, Altman DG, Mann H, Berlin JA, et al. SPIRIT 2013 explanation and elaboration: guidance for protocols of clinical trials. BMJ. 2013;346:e7586.
Chan AW, Tetzlaff JM, Altman DG, Laupacis A, Gotzsche PC, Krleza-Jeric K, et al. SPIRIT 2013 statement: defining standard protocol items for clinical trials. Ann Intern Med. 2013;158(3):200–7.
Kleber M, Schaefer A, Winkel K, Hoffmann D, Wunsch R, Kersting M, et al. Lifestyle intervention "Obeldicks mini" for obese children aged 4 to 7 years. Klin Padiatr. 2009;221(5):290–4.
Cole TJ, Lobstein T. Extended international (IOTF) body mass index cut-offs for thinness, overweight and obesity. Pediatr Obes. 2012;7(4):284–94.
R Core Team. https://www.R-project.org/. Accessed 9 May 2019.
Price JM, Chamberlain P, Landsverk J, Reid JB, Leve LD, Laurent H. Effects of a foster parent training intervention on placement changes of children in foster care. Child Maltreat. 2008;13(1):64–75.
Chamberlain P, Price J, Leve LD, Laurent H, Landsverk JA, Reid JB. Prevention of behavior problems for children in foster care: outcomes and mediation effects. Prev Sci. 2008;9(1):17–27.
Forgatch MS, DeGarmo DS. Parenting through change: an effective prevention program for single mothers. J Consult Clin Psychol. 1999;67(5):711–24.
Horwitz SM, Chamberlain P, Landsverk J, Mullican C. Improving the mental health of children in child welfare through the implementation of evidence-based parenting interventions. Admin Pol Ment Health. 2010;37(1–2):27–39.
Bandura A. A social learning theory. New York: General Learning Press; 1971.
Patterson GR, Forgatch MS, Degarmo DS. Cascading effects following intervention. Dev Psychopathol. 2010;22(4):949–70.
Patterson GR: Performance models for parenting: a social interactional perspective. In: parenting and children's internalization of values: a handbook of contemporary theory. Grusec JE, Kuczynski L, ediors. New York: Wiley; 1997: 193–235.
National Food Agency, Sweden. Swedish Food Database. https://www.livsmedelsverket.se/livsmedel-och-innehall/naringsamne/livsmedelsdatabasen. Accessed 14 May 2019.
The healthcare administration in Stockholm County. Action plan overweight and obesity 2016–2020, Stockholm County. http://dok.slso.sll.se/CES/FHG/Folkhalsoarbete/Informationsmaterial/Handlingsprogram-overvikt-fetma-2016-2020.pdf. Accessed May 9 2019.
Wardle J, Guthrie CA, Sanderson S, Rapoport L. Development of the Children's eating behaviour questionnaire. J Child Psychol Psychiatry. 2001;42(7):963–70.
Musher-Eizenman D, Holub S. Comprehensive feeding practices questionnaire: validation of a new measure of parental feeding practices. J Pediatr Psychol. 2007;32(8):960–72.
Oude Luttikhuis H, Baur L, Jansen H, Shrewsbury VA, O'Malley C, Stolk RP, et al. Interventions for treating obesity in children. Cochrane Database Syst Rev. 2009;(1):CD001872.
Carnell S, Wardle J. Measuring behavioural susceptibility to obesity: validation of the child eating behaviour questionnaire. Appetite. 2007;48(1):104–13.
Sleddens EF, Kremers SP, Thijs C. The children's eating behaviour questionnaire: factorial validity and association with body mass index in Dutch children aged 6-7. Int J Behav Nutr Phys Act. 2008;5:49.
Svensson V, Lundborg L, Cao Y, Nowicka P, Marcus C, Sobko T. Obesity related eating behaviour patterns in Swedish preschool children and association with age, gender, relative weight and parental weight--factorial validation of the Children's eating behaviour questionnaire. Int J Behav Nutr Phys Act. 2011;8:134.
Spence JC, Carson V, Casey L, Boule N. Examining behavioural susceptibility to obesity among Canadian pre-school children: the role of eating behaviours. IJPO. 2011;6(2–2):e501–7.
Sparks MA, Radnitz CL. Confirmatory factor analysis of the Children's eating behaviour questionnaire in a low-income sample. Eat Behav. 2012;13(3):267–70.
Mallan KM, Liu WH, Mehta RJ, Daniels LA, Magarey A, Battistutta D. Maternal report of young children's eating styles. Validation of the Children's eating behaviour questionnaire in three ethnically diverse Australian samples. Appetite. 2013;64:48–55.
Warkentin S, Mais LA, Latorre Mdo R, Carnell S, Taddei JA. Validation of the comprehensive feeding practices questionnaire in parents of preschool children in Brazil. BMC Public Health. 2016;16:603.
Arpon A, Milagro FI, Razquin C, Corella D, Estruch R, Fito M, et al. Impact of consuming extra-virgin olive oil or nuts within a Mediterranean diet on DNA methylation in peripheral white blood cells within the PREDIMED-Navarra randomized controlled trial: a role for dietary lipids. Nutrients. 2017;10(1).
Mansego ML, Garcia-Lacarte M, Milagro FI, Marti A, Martinez JA. DNA methylation of miRNA coding sequences putatively associated with childhood obesity. Pediatr Obes. 2017;12(1):19–27.
Braun V, Clarke V. Using thematic analysis in psychology. Qual Res Psychol. 2006;3(2):77–101.
Ulijaszek SJ, Pentecost M, Marcus C, Karpe F, Fruhbeck G, Nowicka P. Inequality and childhood overweight and obesity: a commentary. Pediatr Obes. 2017;12(3):195–202.
Bambra CL, Hillier FC, Cairns JM, Kasim A, Moore HJ, In SCD. How effective are interventions at reducing socioeconomic inequalities in obesity among children and adults? Two systematic reviews. Southampton (UK): NIHR J Libr. 2015.
Iguacel I, Fernandez-Alvira JM, Ahrens W, Bammann K, Gwozdz W, Lissner L, et al. Prospective associations between social vulnerabilities and children's weight status. Results from the IDEFICS study. Int J Obes. 2018;42(10):1691–703.
Peeters A, Backholer K. Reducing socioeconomic inequalities in obesity: the role of population prevention. Lancet Diabetes Endocrinol. 2015;3(11):838–40.
Chirita-Emandi A, Barbu CG, Cinteza EE, Chesaru BI, Gafencu M, Mocanu V, et al. Overweight and underweight prevalence trends in children from Romania - pooled analysis of cross-sectional studies between 2006 and 2015. Obes Facts. 2016;9(3):206–20.
Cadenas-Sanchez C, Intemann T, Labayen I, Artero EG, Alvarez-Bueno C, Sanchis-Moysi J, et al. Prevalence of severe/morbid obesity and other weight status and anthropometric reference standards in Spanish preschool children: the PREFIT project. Pediatr Res. 2019.
The Child Health Care Unit Stockholm County. Yearly Report Child Health Care in Stockholm County 2017. https://www.vardgivarguiden.se/globalassets/behandlingsstod/barnhalsovard/bhv-rapporter/arsrapport-barnhalsovard-2017.pdf. Accessed 20 Feb 2019.
Bandura A. Human agency in social cognitive theory. Am Psychol. 1989;44(9):1175–84.
Holm JC, Nowicka P, Farpour-Lambert NJ, O'Malley G, Hassapidou M, Weiss R, et al. The ethics of childhood obesity treatment - from the childhood obesity task force (COTF) of European Association for the Study of obesity (EASO). Obesity facts. 2014;7(4):274–81.
European Commission. Cordis EU research results. https://cordis.europa.eu/project/rcn/214762/factsheet/en. Accessed 13 May 2019.
Acknowledgments
Many people have contributed with valuable ideas and practical support to this study. Among these are: (in Romania) Prof. Dr. Maria Puiu, Prof. Dr. Mihai Gafencu, Dr. Iulia Teodora Perva, Dr. Iulia Jurca Simina, Dr. Anamaria Dragomir, Casandra Chera (psychologist), Meda Bugi (dietician), (in Sweden) Fredika Gauffin (Head of the Astrid Lindgren’s Children’s Hospital’s out-patient pediatric clinics in Stockholm), Ola Eklund (Assisting Head of the Astrid Lindgren’s Children’s Hospital’s out-patient pediatric clinics in Stockholm), Christina Norling (Nursing Manager of the Astrid Lindgren’s Children’s Hospital’s out-patient pediatric clinics South Stockholm), Annelie Täppmark (Nursing Manager of the Astrid Lindgren’s Children’s Hospital’s out-patient pediatric clinics North Stockholm), Helena Martin (Head of the Stockholm County Child Health Care), Catharina Neovius (Child Health Care Developer in Stockholm County), Emmie Söderström (research assistant), My Sjunnestrand (research assistant) all child health care nurses, pediatricians and pediatric nurses that are involved in recruitment and providing standard care, (in Spain) David Mateos (nurse, Son Espases University Hospital), Diego de Sotto (Head of Pediatric Service, Rotger Clinics), Helena Corral (pediatrician, Hospital of Inca), Maria Àngels Martínez (pediatrician, Hospital of Inca), Maria Caimari (pediatric endocrinologist, Son Espases University Hospital), Marta Minguez (pediatrician, Hospital of Inca) for support and involvement in recruitment and providing standard care. The authors thank Nils Lidström and Jan Fjellström for help with the technical development of the MINISTOP app and Hanna Hennriksson for her work and support during the translation process of the app.
Funding
This study is funded through the STOP project, http://www.stopchildobesity.eu/. The STOP project received funding from the European Union’s Horizon 2020 research and innovation programme under Grant Agreement No. 774548 [67]. The content of this document reflects only the authors’ views and the European Commission is not liable for any use that may be made of the information it contains. JAT, CB, EA and JAM are also funded by CIBEROBN (CB12/03/30038), Instituto de Salud Carlos III and the European Regional Development Fund. CB is also funded by a Fernando Tarongí Bauzà Grant. MS was also funded by CIBERESP, Instituto de Salud Carlos III. The funders had no role in the design; collection, analysis, and interpretation of data; or in writing the manuscript.
Author information
Authors and Affiliations
Contributions
All authors were involved in the study design for More and Less Europe. AE is the project coordinator for the three sites and drafted the manuscript together with CDN who also aided in the development of the MINISTOP app’s content. ACE is the primary investigator for the Romanian site and JAT is the primary investigator for the Spanish site. Regarding recruitment and data collection KN is the coordinator in Sweden, CP in Romania and EA and CB in Spain. JAM is responsible for analyses of epigenetic and metabolic markers and their interpretations. GF and IGP are responsible for the analyses of gut hormones and validation of food intake through urine. MS is responsible for statistical analysis. ML created the original MINISTOP program and led the work when it was modified for ML Europe. PN is responsible for the Swedish site and the primary investigator of the ML Europe. All authors read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Ethics approval was obtained from: the Ethics Committee of Scientific Research in University of Medicine and Pharmacy “Victor Babes”, Timisoara, Romania, October 31st, 2018 (25/31.10.2018), the Balearic Islands Ethics Committee, Mallorca, Spain, February 13th, 2019 (IB 3814/18 PI), and the Research Ethics Committee, Stockholm, Sweden, December 11th, 2018 (2018/2082–31/1). Written informed consent is obtained from all parents or caregivers.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Ek, A., Delisle Nyström, C., Chirita-Emandi, A. et al. A randomized controlled trial for overweight and obesity in preschoolers: the More and Less Europe study - an intervention within the STOP project. BMC Public Health 19, 945 (2019). https://doi.org/10.1186/s12889-019-7161-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12889-019-7161-y