Abstract
Background
A patent ductus arteriosus (PDA) is frequently found in very preterm neonates and is associated with increased risk of morbidity and mortality. A shunt across a PDA can result in an unfavorable distribution of the cardiac output and may in turn result in poor renal perfusion. Urinary Neutrophil Gelatinase-associated Lipocalin (U-NGAL) is a marker of renal ischemia and may add to the evaluation of PDA. Our primary aim was to investigate if U-NGAL is associated with PDA in very preterm neonates. Secondary, to investigate whether U-NGAL and PDA are associated with AKI and renal dysfunction evaluated by fractional excretion of sodium (FENa) and urine albumin in a cohort of very preterm neonates.
Methods
A cohort of 146 neonates born at a gestational age less than 32 weeks were consecutively examined with echocardiography for PDA and serum sodium, and urine albumin and sodium were measured on postnatal day 3 and U-NGAL and serum creatinine day 3 and 6. AKI was defined according to modified neonatal Acute Kidney Injury Network (AKIN) criteria. The association between U-NGAL and PDA was investigated. And secondly we investigated if PDA and U-NGAL was associated with AKI and renal dysfunction.
Results
U-NGAL was not associated with a PDA day 3 when adjusted for gestational age and gender. A PDA day 3 was not associated with AKI when adjusted for gestational age and gender; however, it was associated with urine albumin. U-NGAL was not associated with AKI, but was found to be associated with urine albumin and FENa.
Conclusions
Based on our study U-NGAL is not considered useful as a diagnostic marker to identify very preterm neonates with a PDA causing hemodynamic changes resulting in early renal morbidity. The interpretation of NGAL in preterm neonates remains to be fully elucidated.
Similar content being viewed by others
Background
A patent ductus arteriosus (PDA) in preterm neonates is associated with an increased risk of morbidity and mortality [1, 2]. On day 3 of life, up to 50% of neonates born before 32 weeks of gestation have a PDA [1, 3]. However, not every PDA challenges the preterm neonate. We have previously demonstrated that a PDA diameter above 1.5 mm on postnatal day 3 can be used to identify a clinically significant PDA [1]. However, further diagnostic tools are needed in order to understand the potential impact of PDA presence and optimize handling of PDA in very preterm neonates.
Neutrophil gelatinase-associated lipocalin (NGAL) is expressed in low concentrations in many organs and the expression is up-regulated during infection, inflammation, and ischemia [4, 5]. Furthermore, NGAL is believed to be involved in renal development [6]. Urinary NGAL is produced in the renal tubule of the thick ascending limb of Henle and the collecting ducts [7]. In preterm neonates, NGAL has been suggested to be associated with Acute Kidney Injury (AKI) [8, 9].
U-NGAL has been hypothesized to be increased in the presence of a PDA [10]. A shunt across a PDA may result in an unfavorable distribution of the cardiac output between the pulmonary and the systemic circulation. This may in turn result in poor renal perfusion. This could cause AKI or renal dysfunction due to ischemia which could increase U-NGAL [11].
The aim of this study was to evaluate if U-NGAL can be used as a diagnostic marker to identity very preterm neonates with a PDA causing hemodynamic changes resulting in renal morbidity within the first week of life. To do this we investigated if U-NGAL is associated with PDA in very preterm neonates. Secondly we investigated whether U-NGAL and PDA were associated with AKI and renal dysfunction evaluated by fractional excretion of sodium and urine albumin in a cohort of very preterm neonates.
Methods
Study population
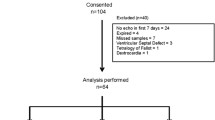
The study population has previously been described [1], in brief, newborns born at gestational age below 32 weeks admitted to Aarhus University Hospital were eligible. We excluded neonates with chromosomal abnormalities or congenital heart malformations other than atrial septum defects from the study (Fig. 1).
Data collection and definitions
Irrespective of clinical symptoms, all very preterm neonates had an echocardiography on day 3 (equal to 72 h). Urine samples and blood samples were collected day 3 and 6. Urine was collected with a cotton ball placed at the perineum [8, 9, 12]. The diaper was checked at least every 4 h and the cotton transferred to a refrigerator. Cotton was centrifuged at 1000 rpm for 1 min and the urine was stored at −80 °C within 24 h after collection.
From the patient medical records we collected the following clinical details: gestational age at birth (determined by ultrasound), birth weight, multiple births, Apgar score, early onset sepsis (defined as seven days of antibiotics initiated within the first 3 days after birth) [13, 14], surfactant administration, packed red blood cell transfusion (within the first 3 days), use of inotropes (within the first 3 days), maternal preeclampsia, antenatal steroid administration, and mode of delivery.
Clinical guidelines
Management of the PDA was in accordance with institutional clinical guideline. Treatment with Ibuprofen (Pedea, Orphan Europe SARL Puteaux, France) was initiated based on detection by echocardiography alone if the gestational age at birth was less than 28 weeks; otherwise treatment was initiated based on a combination of specific echocardiographic findings and clinical symptoms. Ibuprofen was never administered before day 3.
Echocardiographic measurements
A complete diagnostic echocardiography was performed by two senior pediatric echocardiographers (J Bjerre and MR Schmidt) by use of standard neonatal windows. A Philips IE33 Ultrasound machine with a 12-MHz cardiology probe (Philips Healthcare, Andover, Massachusetts, USA) was used. A PDA was defined as present if flow could be visualized on colour Doppler. The PDA diameter was measured in two-dimensional mode at the most narrow point. A mean value of three cardiac cycles was calculated. A large PDA was defined as a PDA with a diameter of 1.5 mm or more and a small PDA as a PDA with a diameter below 1.5 mm.
U-NGAL measurement
The U-NGAL test Reagent Kit from BioPorto was used (BioPorto, Copenhagen, Denmark) as routine analysis on the Cobas 6000 analyzer system (Roche, Basel, Switzerland) at the Department of Biochemistry, Aarhus University Hospital. Also, serum creatinine (SCr), serum sodium, urinary albumin and sodium were measured as routine analyses at the Department. AKI was defined according to the definition proposed by Jetton et Askenazi based on the neonatal acute kidney injury (AKIN) classification [15–17]. AKI Stage 0: no change or rise SCr < 0.3 mg/dl; Stage 1: increase SCr 0.3 mg/dl or increase SCr 150–200% from previous trough value; Stage2: increase SCr 200–300% from previous trough value; Stage 3: increase SCr 200% from previous trough value or 2.5 mg/dl or recipient of dialysis [15]. Six stable neonates only had one SCr measured, this was within normal range and they were categorized as not having AKI. To evaluate renal dysfunction we measured urine albumin and calculated fractional excretion of sodium (FENa). Fractional excretion of sodium was calculated using the following formula: FENa (%) = (UNa/ PNa) * (SCr/ UCr) * 100, where UNa = urine sodium (mmol/l); PNa = plasma sodium (mmol/l); SCr serum creatine (μmol/l); and UCr = urine creatinine (μmol/l).
Statistical analysis
U-NGAL, urine albumin, SCr, and FENa were transformed by the natural logarithm (ln) to normalize the distribution of residuals. Geometric means were compared using the Student’s t-test and proportions using Fisher’s exact test. Univariate and multivariate log-linear regression was performed in order to examine the association between U-NGAL and PDA day 3, PDA size, perinatal characteristics, and sepsis. We consequently exponentiated the differences estimated on the natural log scale to obtain ratios of the medians. The formula (expbeta -1) * 100% was used to express the percent change. These ratios are presented as crude and adjusted percent change per unit of explanatory variable or difference from reference group with 95% confidence interval (CI). Univariate and multivariate log-log regression was performed to examine the association between U-NGAL and parameters of renal dysfunction. The formula (1.01beta-1)* 100% was used to express the percent change in U-NGAL per 1% change in urine albumin, SCr or FENa with 95% CI. Univariate and multivariate logistic regression was performed to examine the association between AKI and PDA. Odds ratio (OR) was presented with 95% CI. Estimates were adjusted for gestational age as a continuous variable (weeks) and gender and sepsis as dichotomized variables. Robust cluster standard errors were used in order to take into account the correlation between twins. Data were analyzed with STATA special edition version 11 (College Station, Texas, USA). All tests were two-sided. P-values of less than 0.05 were considered statistically significant.
Results
Patient characteristics
A total of 146 neonates were included in the study (Fig. 1). A PDA day 3 was found in 67 (46%) neonates on day 3 and 41 (61%) of neonates with PDA had a large PDA. Characteristics of the 146 very preterm neonates in the study are found in Table 1. Neonates with large and small PDA had comparable characteristics (data not shown).
U-NGAL in neonates with PDA
U-NGAL levels day 3 in neonates with PDA and no PDA on day 3 in four gestational age groups are found in Fig. 2. The highest level of U-NGAL day 3 was found in neonates with a small PDA compared to neonates with no PDA or a large PDA day 3 (Table 2). Median U-NGAL day 3 was 53% higher in neonates with a PDA compared to neonates without a PDA (Table 3). However, after adjusting for gender, gestational age, and sepsis by multivariate linear regression the association was no longer present. And no association was found between PDA or PDA size day 3 and U-NGAL.
AKI in neonates with PDA
A total of seven neonates were found to have AKI. AKI was found more frequently in neonates with PDA compared to neonates with no PDA and especially in neonates with a large PDA compared to no PDA day 3 (Table 2). However, when adjusted for gestational age no association between PDA day 3 and AKI was found. The odds of AKI decreased some 40% for every 1 week increase in gestational age at birth (OR = 0.64; 95% CI 0.4–0.96).
Mean SCr, urinary albumin, and FENa are described in Table 2. No difference in SCr was found between PDA groups. We found that urine albumin and FENa was higher in neonates with PDA day 3 compared to neonates with no PDA. On regression we found PDA and PDA size to be associated with urine albumin also when adjusted for adjusting for gender, gestational age, and sepsis. No association between PDA or PDA size and FENa was found when adjusted for gestational age.
U-NGAL in neonates with AKI
We found no association between U-NGAL measured day 3 and AKI (Table 3). In 126 neonates we measured U-NGAL day 6. However, also on day 6 we found no association between U-NGAL and AKI. No association between U-NAGL day 3 and SCr was found. We did find an association between U-NGAL day 3 and both urine albumin and FENa also when adjusting for gender, gestational age, and sepsis.
Discussion
In 146 consecutively examined very preterm neonates, we found that the mere presence of a PDA and the PDA size day 3 was not associated with U-NGAL after adjusting for gestational age and gender. AKI was found more often in neonates with PDA, but after adjusting for gestational age there was no association between PDA day 3 and AKI. We found that neonates with PDA day 3 had higher urine albumin compared to neonates with no PDA. PDA and PDA size was associated with urine albumin also when adjusted for gestational age, gender, and sepsis. U-NGAL was not associated with AKI, it was however found to be associated with both urine albumin and FENa.
Tosse et al. found that U-NGAL in neonates with a birth weight below 1,500 g was higher if a PDA that needed either medical or surgical intervention was present than in neonates with no PDA or a PDA with no need for intervention [10]. They diagnosed the PDA by echocardiography on day 1–3 but the need for medical or surgical intervention was based on respiratory setback, oliguria or failure to thrive. Recently PDA diameter has been recognized as an important marker to evaluate the magnitude and clinical significance of the PDA [1, 18–20]. We used a PDA diameter above 1.5 mm on day 3 to identify the clinically significant PDA as we have previously shown that it is associated with severe morbidity and mortality [1]. Markers of organ dysfunction prior to clinical morbidity are needed in order to evaluate the PDA. The hemodynamic effects of a PDA may cause renal hypoperfusion and renal ischemia leading to AKI [21, 22]. Also management of the PDA including Indomethacin, Ibuprofen or fluid restrictions may alter renal function or cause AKI [23, 24]. Neonates in this study were not subjected to any of these treatment strategies prior to the first echocardiography and collection of urine day 3.
In concordance with previous studies we found that U-NGAL decrease with higher gestational age at birth or birth weight and also that U-NAGL is higher in girls than in boys in preterm neonates [8, 9, 25]. Gestational age could therefore partly explain why Tosse et al. report higher U-NGAL levels in neonates with PDA needing intervention compared to neonates with no PDA or a PDA with no need for intervention. Neonates who required intervention in the study by Tosse et al. had a lower gestational age at birth than the neonates with PDA in our study, whereas the non-intervention group and the neonates with no PDA had similar gestational ages as ours. Tosse et al. did not adjust for gestational age in their analysis. When adjusted for gestational age and gender we found no association between PDA and U-NGAL which is in also in agreement with Laundry et al. [9].
AKI is defined as a sudden decline in kidney function resulting in derangements in fluid balance, electrolytes, and waste products. Currently a modified neonatal Kidney Injury: Improving Global Outcomes (KIDGO) classification of AKI accepted at the National Institute of Diabetes and Kidney Disease (NIDDK) workshop 2013 is used [26]. The applicability of these criteria for AKI in neonates is problematic because they include SCr and urinary output. Neonates often have non-oliguric renal failure [27]. SCr is difficult to interpret in preterm neonates due to incomplete nephrogenesis and the potential impact of bilirubin on the measure of SCr. In the first days of life maternal renal function also plays a significant role in the magnitude of newborn SCr due to placental transfer [27]. Previous studies using a plethora of AKI definitions have found an association between AKI or altered renal function and PDA [28, 29]. At the time our study was designed and performed the modified neonatal AKIN classification as proposed by Jetton et Askenazi was used [15]. This definition does not include urinary output. In a cohort of very preterm neonates that all had an echocardiography day 3 to diagnose PDA we found that seven neonates (5%) had AKI within the first week of life. We found that AKI was associated with gestational age, but not PDA.
The renal function in premature neonates is affected by both immaturity with a decreased ability to reabsorb electrolytes and protein and to concentrate urine and by an increased risk of injury during the early postnatal period due to multifactorial morbidity, medication, umbilical artery catheter, and hemodynamic instability [26, 30, 31]. Urinary albumin is inversely related to gestational age, which may reflect tubular immaturity, tubular damage and glomerular dysfunction [12, 30, 32]. We found urine albumin to be associated with PDA and PDA size also when adjusted for gestational age, gender, and sepsis. A recent study found urinary albumin to be increased in preterm neonates treated with indomethacin compared to neonates that did not receive indomethacin. No information on the PDA was given in that study [33]. Urinary albumin has been found to be increased in preterm neonates with asphyxia and respiratory distress and has been suggested as a possible biomarker of AKI in newborns [32, 34] and preterm neonates [35]. In concordance with our findings FENa is known to be very high in preterm neonates and inversely related to gestational age [36]. Several mechanisms may cause this including immature tubular function, low density of sodium transporters in the tubular epithelium, and a physiological response to redistribution of extracellular fluids. After adjusting for gestational age, gender and sepsis we found no association between FENa and PDA.
Due to the difficulties in diagnosing AKI in preterm neonates, studies have investigated different biomarkers in order to allow also for earlier identification of neonates with AKI [26, 37]. An association between AKI and U-NGAL has been described in preterm neonates [8, 9, 38–40]. The definition of AKI differed in these studies, but they all included SCr. We found that U-NGAL was not an early marker of AKI in our population as we found no association between U-NGAL day 3 and AKI. Furthermore U-NGAL day 6 was not associated with AKI. In concordance with our findings Gubhaju et al. found no association between AKI and U-NGAL [30]. Gubhaju et al. recently described an association between U-NGAL and urine total protein, but contrary to us they did not find a positive association between U-NGAL and urine albumin [30]. Increased U-NGAL in extremely preterm neonates may be associated with infection, inflammation or indicate differentiation and growth of renal epithelium caused by immature nephrons stimulating glomerulogenesis [38, 41, 42]. We found U-NGAL to be associated with sepsis in very preterm neonates.
In our analyses we adjusted for gestational age, gender, and sepsis as previous studies found that they may influence U-NGAL levels. We included a total of 146 very preterm neonates in this study. Despite this being the largest cohort evaluating U-NGAL in preterm neonates, a larger cohort would be needed to allow control for more covariates and to facilitate subanalyses by gestational-age. Day-to-day variation has been described in both U-NGAL and SCr. Unfortunately; we could not collect all samples at the exact same time after birth. However, the variation in time of collection was similar in neonates with and without PDA. Renal complications in neonates with PDA may be due to hemodynamic effects of the PDA or to treatment strategies used. We were able to measure U-NGAL (day 3) before Ibuprofen was administered. However, all but one of the six neonates with PDA that were diagnosed with AKI received Ibuprofen in the interval between the two measurements of SCr used to define AKI.
Conclusion
In this cohort of 146 very preterm neonates we found no association between U-NGAL and PDA day 3 after adjusting for gestational age and gender. We found that PDA day 3 was associated with urine albumin; however PDA was not associated with AKI and FENa when adjusted for gestational age and gender. No association between U-NGAL and AKI was found. U-NGAL day 3 was associated with both urine albumin and FENa. Non-renal disease may constitute important confounders in the interpretation of NGAL and highlight the need for an enhanced understanding of the interactions between cardiovascular function, inflammation, AKI, and the risk of adverse outcome. At the present time U-NGAL is not useful as a diagnostic marker to identity very preterm neonates with a PDA causing hemodynamic changes resulting in renal morbidity within the first week of life.
Abbreviations
- AKI:
-
Acute kidney injury
- FENa:
-
Fractional excretion of sodium
- GA:
-
Gestational age
- PDA:
-
Patent ductus arteriosus
- SCr:
-
Serum creatinine
- U-NGAL:
-
Urinary neutrophil gelatinase-associated lipocalin
References
Sellmer A, Bjerre JV, Schmidt MR, McNamara PJ, Hjortdal VE, Host B, et al. Morbidity and mortality in preterm neonates with patent ductus arteriosus on day 3. Arch Dis Child Fetal Neonatal Ed. 2013;98(6):F505–10.
Noori S, McCoy M, Friedlich P, Bright B, Gottipati V, Seri I, et al. Failure of ductus arteriosus closure is associated with increased mortality in preterm infants. Pediatrics. 2009;123(1):e138–44.
Schneider DJ. The patent ductus arteriosus in term infants, children, and adults. Semin Perinatol. 2012;36(2):146–53.
Borregaard N, Cowland JB. Neutrophil gelatinase-associated lipocalin, a siderophore-binding eukaryotic protein. Biometals. 2006;19(2):211–5.
Mussap M, Degrandi R, Fravega M, Fanos V. Acute kidney injury in critically ill infants: the role of urine Neutrophil Gelatinase-Associated Lipocalin (NGAL). J Matern Fetal Neonatal Med. 2010;23 Suppl 3:70–2.
Mori K, Nakao K. Neutrophil gelatinase-associated lipocalin as the real-time indicator of active kidney damage. Kidney Int. 2007;71(10):967–70.
Mori K, Lee HT, Rapoport D, Drexler IR, Foster K, Yang J, et al. Endocytic delivery of lipocalin-siderophore-iron complex rescues the kidney from ischemia-reperfusion injury. J Clin Invest. 2005;115(3):610–21.
Askenazi DJ, Montesanti A, Hunley H, Koralkar R, Pawar P, Shuaib F, et al. Urine biomarkers predict acute kidney injury and mortality in very low birth weight infants. J Pediatr. 2011;159(6):907–12.
Lavery AP, Meinzen-Derr JK, Anderson E, Ma Q, Bennett MR, Devarajan P, et al. Urinary NGAL in premature infants. Pediatr Res. 2008;64(4):423–8.
Tosse V, Pillekamp F, Verde P, Hadzik B, Sabir H, Mayatepek E, et al. Urinary NT-proBNP, NGAL, and H-FABP May predict hemodynamic relevance of patent ductus arteriosus in very low birth weight infants. Neonatology. 2012;101(4):260–6.
Mishra J, Ma Q, Prada A, Mitsnefes M, Zahedi K, Yang J, et al. Identification of neutrophil gelatinase-associated lipocalin as a novel early urinary biomarker for ischemic renal injury. J Am Soc Nephrol. 2003;14(10):2534–43.
Fell JM, Thakkar H, Newman DJ, Price CP. Measurement of albumin and low molecular weight proteins in the urine of newborn infants using a cotton wool ball collection method. Acta Paediatr. 1997;86(5):518–22.
Stoll BJ, Gordon T, Korones SB, Shankaran S, Tyson JE, Bauer CR, et al. Early-onset sepsis in very low birth weight neonates: a report from the National Institute of Child Health and Human Development Neonatal Research Network. J Pediatr. 1996;129(1):72–80.
Simonsen KA, Anderson-Berry AL, Delair SF, Davies HD. Early-onset neonatal sepsis. Clin Microbiol Rev. 2014;27(1):21–47.
Jetton JG, Askenazi DJ. Update on acute kidney injury in the neonate. Curr Opin Pediatr. 2012;24(2):191–6.
Askenazi DJ, Griffin R, McGwin G, Carlo W, Ambalavanan N. Acute kidney injury is independently associated with mortality in very low birthweight infants: a matched case-control analysis. Pediatr Nephrol. 2009;24(5):991–7.
Mehta RL, Kellum JA, Shah SV, Molitoris BA, Ronco C, Warnock DG, et al. Acute kidney injury network: report of an initiative to improve outcomes in acute kidney injury. Crit Care. 2007;11(2):R31.
Kluckow M, Evans N. Early echocardiographic prediction of symptomatic patent ductus arteriosus in preterm infants undergoing mechanical ventilation. J Pediatr. 1995;127(5):774–9.
Evans N, Iyer P. Assessment of ductus arteriosus shunt in preterm infants supported by mechanical ventilation: effect of interatrial shunting. J Pediatr. 1994;125(5 Pt 1):778–85.
Kluckow M, Jeffery M, Gill A, Evans N. A randomised placebo-controlled trial of early treatment of the patent ductus arteriosus. Arch Dis Child Fetal Neonatal Ed. 2014;99(2):F99–104.
Shimada S, Kasai T, Hoshi A, Murata A, Chida S. Cardiocirculatory effects of patent ductus arteriosus in extremely low-birth-weight infants with respiratory distress syndrome. Pediatr Int. 2003;45(3):255–62.
Bomelburg T, Jorch G. Abnormal blood flow patterns in renal arteries of small preterm infants with patent ductus arteriosus detected by Doppler ultrasonography. Eur J Pediatr. 1989;148(7):660–4.
Gersony WM, Peckham GJ, Ellison RC, Miettinen OS, Nadas AS. Effects of indomethacin in premature infants with patent ductus arteriosus: results of a national collaborative study. J Pediatr. 1983;102(6):895–906.
Ohlsson A, Shah SS. Ibuprofen for the prevention of patent ductus arteriosus in preterm and/or low birth weight infants. Cochrane Database Syst Rev. 2011;7:CD004213.
Askenazi DJ, Koralkar R, Levitan EB, Goldstein SL, Devarajan P, Khandrika S, et al. Baseline values of candidate urine acute kidney injury biomarkers vary by gestational age in premature infants. Pediatr Res. 2011;70(3):302–6.
Selewski DT, Charlton JR, Jetton JG, Guillet R, Mhanna MJ, Askenazi DJ, et al. Neonatal acute kidney injury. Pediatrics. 2015;136(2):e463–73.
Askenazi DJ, Ambalavanan N, Goldstein SL. Acute kidney injury in critically ill newborns: what do we know? What do we need to learn? Pediatr Nephrol. 2009;24(2):265–74.
Walker MW, Clark RH, Spitzer AR. Elevation in plasma creatinine and renal failure in premature neonates without major anomalies: terminology, occurrence and factors associated with increased risk. J Perinatol. 2011;31(3):199–205.
Iacobelli S, Bonsante F, Ferdinus C, Labenne M, Gouyon JB. Factors affecting postnatal changes in serum creatinine in preterm infants with gestational age <32 weeks. J Perinatol. 2009;29(3):232–6.
Gubhaju L, Sutherland MR, Horne RS, Medhurst A, Kent AL, Ramsden A, et al. Assessment of renal functional maturation and injury in preterm neonates during the first month of life. Am J Physiol Renal Physiol. 2014;307(2):F149–58.
Koralkar R, Ambalavanan N, Levitan EB, McGwin G, Goldstein S, Askenazi D. Acute kidney injury reduces survival in very low birth weight infants. Pediatr Res. 2011;69(4):354–8.
Tsukahara H, Fujii Y, Tsuchida S, Hiraoka M, Morikawa K, Haruki S, et al. Renal handling of albumin and beta-2-microglobulin in neonates. Nephron. 1994;68(2):212–6.
Kent AL, Brown L, Broom M, Broomfield A, Dahlstrom JE. Increased urinary podocytes following indomethacin suggests drug-induced glomerular injury. Pediatr Nephrol. 2012;27(7):1111–7.
Askenazi DJ, Koralkar R, Hundley HE, Montesanti A, Parwar P, Sonjara S, et al. Urine biomarkers predict acute kidney injury in newborns. J Pediatr. 2012;161(2):270–5.
DeFreitas MJ, Seeherunvong W, Katsoufis CP, RamachandraRao S, Duara S, Yasin S, et al. Longitudinal patterns of urine biomarkers in infants across gestational ages. Pediatr Nephrol. 2016;31(7):1179–88.
Gallini F, Maggio L, Romagnoli C, Marrocco G, Tortorolo G. Progression of renal function in preterm neonates with gestational age < or = 32 weeks. Pediatr Nephrol. 2000;15(1-2):119–24.
Zaffanello M, Antonucci R, Cuzzolin L, Cataldi L, Fanos V. Early diagnosis of acute kidney injury with urinary biomarkers in the newborn. J Matern Fetal Neonatal Med. 2009;22 Suppl 3:62–6.
Genc G, Avci B, Aygun C, Ozkaya O, Kucukoduk S. Urinary neutrophil gelatinase-associated lipocalin in septic preterm babies: a preliminary study. Am J Perinatol. 2013;30(8):655–60.
Sarafidis K, Tsepkentzi E, Diamanti E, Agakidou E, Taparkou A, Soubasi V, et al. Urine neutrophil gelatinase-associated lipocalin to predict acute kidney injury in preterm neonates. A pilot study. Pediatr Nephrol. 2014;29(2):305–10.
Tabel Y, Elmas A, Ipek S, Karadag A, Elmas O, Ozyalin F. Urinary neutrophil gelatinase-associated lipocalin as an early biomarker for prediction of acute kidney injury in preterm infants. Am J Perinatol. 2014;31(2):167–74.
La MG, Galletti S, Capelli I, Vandini S, Nisi K, Aquilano G, et al. Urinary neutrophil gelatinase-associated lipocalin at birth predicts early renal function in very low birth weight infants. Pediatr Res. 2011;70(4):379–83.
Parravicini E, Nemerofsky SL, Michelson KA, Huynh TK, Sise ME, Bateman DA, et al. Urinary neutrophil gelatinase-associated lipocalin is a promising biomarker for late onset culture-positive sepsis in very low birth weight infants. Pediatr Res. 2010;67(6):636–40.
Acknowledgements
The authors would like to thank the parents of the infants enrolled, the staff at the neonatal intensive care unit at Aarhus University Hospital, Aarhus, and a special thanks to laboratory technician Jane Knudsen for handling plasma and urine samples.
Funding
Funding for this study was obtained from Aarhus University Faculty of Health and The Institute of Clinical Medicine, Aase and Ejnar Danielsen Foundation, Sophus Jacobsen and Astrid Jacobsen Foundation, Kurt Bønnelycke and Grethe Bønnelycke Foundation, Helga and Peter Korning Foundation, and Karl G. Andersons Foundation. The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Availability of data and material
According to The Act on Processing of Personal Data (Act No. 429 of 31 May 2000), the data are protected by the Danish Data Protection Agency. Data are available upon request, and researchers may contact head of the Perinatal Epidemiological Research Unit, Tine B Henriksen Professor, PhD (tbh@dadlnet.dk), or the corresponding author, Anna Sellmer, PhD (anna.sellmer@clin.au.dk).
Authors’ contributions
AS designed the study, collected data, performed data analysis and wrote the first draft of the paper and edited the following versions. BHB designed the study, assisted data analysis and edited the manuscript. JVB designed the study, performed echocardiography and contributed to the final manuscript. MRS designed the study, performed echocardiography and contributed to the final manuscript. VEH designed the study, assisted data analysis and edited the manuscript. GE took part in collection of data and contributed to the final manuscript. SR assisted data analysis and edited the manuscript. TBH designed the study, took part in collection of data, assisted data analysis and edited the manuscript. All authors read and approved the final manuscript.
Competing interests
The authors declare that they have no competing interests.
Consent for publication
Not applicable.
Ethics approval and consent to participate
All parents gave informed, written consent for their child to participate in the study. The study was approved by the Central Denmark Region Committee on Health Research Ethics (journal number M-20090243), the Danish Data Protection Agency, and the National Board of Health.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Sellmer, A., Bech, B.H., Bjerre, J.V. et al. Urinary Neutrophil Gelatinase-associated Lipocalin in the evaluation of Patent Ductus Arteriosus and AKI in Very Preterm Neonates: a cohort study. BMC Pediatr 17, 7 (2017). https://doi.org/10.1186/s12887-016-0761-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12887-016-0761-0