Abstract
Background
Breast cancer and its treatment-related adverse effects are harmful to physical, psychological, and social functioning, leading to health-related quality of life (HRQoL) impairment in patients. Many programs have been used with this population for HRQoL improvement; however, few studies have considered the physical, psychological, and social health domains comprehensively, and few have constructed multimodal standard nursing interventions based on specific theories. The purpose of this trial is to examine the effect of a health belief model (HBM)-based multimodal standard nursing program (MSNP) on HRQoL in female patients with breast cancer.
Methods
This is a two-arm single-blind cluster randomized controlled trial (cRCT) in clinical settings. Twelve tertiary hospitals will be randomly selected from the 24 tertiary hospitals in Xi’an, China, and allocated to the intervention arm and control arm using a computer-generated random numbers table. Inpatient female patients with breast cancer from each hospital will receive either MSNP plus routine nursing care immediately after recruitment (intervention arm), or only routine nursing care (control arm). The intervention will be conducted by trained nurses for 12 months. All recruited female patients with breast cancer, participating clinical staff, and trained data collectors from the 12 hospitals will be blind with respect to group allocation. Patients of the control arm will not be offered any information about the MSNP during the study period to prevent bias. The primary outcome is HRQoL measured through the Functional Assessment of Cancer Therapy-Breast version 4.0 at 12 months. Secondary outcomes include pain, fatigue, sleep, breast cancer-related lymphedema, and upper limb function, which are evaluated by a visual analogue scale, the circumference method, and the Constant-Murley Score.
Discussion
This trial will provide important evidence on the effectiveness of multimodal nursing interventions delivered by nurses in clinical settings. Study findings will inform strategies for scaling up comprehensive standard intervention programs on health management in the population of female patients with breast cancer.
Trial registration
Chictr.org.cn ChiCTR-IOR-16008253 (April 9, 2016)
Similar content being viewed by others
Background
Breast cancer is the most common malignant tumor in the female population. Global statistics in 2012 indicated that about 1.7 million new cases were diagnosed and 522,000 died from the disease [1, 2]. In China, as in most other countries, breast cancer is prevalent in women. According to a report in 2014, Chinese cases accounted for 12.2 % of all newly diagnosed breast cancer cases and 9.6 % of all deaths from breast cancer worldwide [3]. Over the course of illness and treatment, breast cancer patients experience many acute and chronic adverse effects. They also face unique challenges to health and well-being as a result of their cancer, its treatment, and comorbidities [4–7].
Health-related quality of life (HRQoL) is broadly conceptualized as individuals’ perceptions of their physical health, psychological health, social relationships, relationship to their environment, independence level, and personal beliefs [8]. With changing medical models, HRQoL has been regarded as a key index for evaluating global therapeutic effects and survival status in populations of patients with cancer [9]. Given negative influences of the illness and treatment, breast cancer patients experience pain, fatigue, negative psychological states, self-image alteration, body function limitations, self-esteem reduction, and risk of recurrence, which severely impact physical, psychological, and social functioning [10–17]. Breast cancer patients also have been shown to have poorer HRQoL in comparison with the general population, especially among patients under 50 years of age [18, 19].
To improve HRQoL for breast cancer patients, many programs have been used with this population, such as art therapy (e.g., music therapy, dance/movement therapy) [20–22], exercise interventions (e.g., physical exercise/activity, resistance exercise, aerobic exercise, yoga) [23–28], psychoeducational support (e.g., health education, psychosocial support, spiritual group therapy) [29–33], and multimodal programs (e.g., rehabilitation programs, physiotherapy programs, exercise programs) [34–37], with different effects on HRQoL. However, existing or potential health problems in physical, psychological, and social domains have not been considered comprehensively. Additionally, the previously mentioned programs fail to describe intervention parameters such as time, frequency, or strength in an explicit manner, leading to their unsuitability as standard nursing interventions. Moreover, few studies have constructed a multimodal standard nursing program (MSNP) for breast cancer patient populations [38].
Theoretical framework
The MSNP is developed based on the Health Belief Model (HBM), which attempts to explain and predict health behaviors by focusing on the attitudes and beliefs of individuals. The HBM comprises four constructs: perceived susceptibility, perceived severity, perceived benefits, and perceived barriers. These concepts are proposed to account for people’s readiness to act [39]. Cues to action are thought to activate readiness and stimulate overt behavior. Self-efficacy or confidence promotes the performance of an action. Moreover, a person will take a health-related action if it is felt that a negative health condition can be avoided, there is a positive expectation about taking a recommended action, and a belief exists that a recommended health action can be taken successfully [40].
The HBM has been widely used in different populations [41–45]. However, it has rarely been employed as a theoretical guide in developing nursing intervention strategies in patients with breast cancer [46–48]. Based on the HBM, influencing factors can be explored from physical, psychological, and social viewpoints. Therefore, perceived susceptibility (e.g., risk of deterioration or recurrence), perceived severity (e.g., complications due to delayed treatment), perceived benefits (e.g., positive feedback following MSNP implementation), and perceived barriers (e.g., impediments to MSNP implementation) can be identified during the treatment and nursing process to aid in the development of the MSNP (Fig. 1).
Aims and objectives
The aim of this trial is to evaluate the effectiveness of a MSNP for female patients with breast cancer in Xi’an, China. The primary objective is to test for effects of the MSNP on HRQoL enhancement. The secondary objectives are to assess improvements in pain, fatigue, sleep, breast cancer-related lymphedema (BCRL), and upper limb function.
Based on these objectives, the primary hypothesis is that breast cancer patients receiving MSNP will achieve better HRQoL than a control arm at 12 months. The secondary hypotheses are that patients receiving the intervention will have (i) improved HRQoL at 1, 3, and 6 months; (ii) lower pain scores and less BCRL occurrence; and (iii) improved fatigue, sleep, and upper limb function at 1, 3, 6, and 12 months.
Methods
Design
This is a two-arm single-blind cluster randomized controlled trial (cRCT) in clinical settings with female breast cancer patients.
Participants
Participants are inpatients with breast cancer. Inclusion criteria are newly diagnosed with breast cancer, female, aged 18 years and older, preparing to receive radical mastectomy and other auxiliary treatments (e.g., chemotherapy, radiotherapy, endocrine therapy), and providing written informed consent. Patients with cognitive disorders, psychiatric disorders, other malignant tumors, active infection, or other severe potential infection will be excluded. Cognitive disorders and psychiatric disorders will be screened using DSM-V (Diagnostic and Statistical Manual of Mental Disorders, 5th ed.) criteria [49]. Reasons for refusal to participate will be documented.
Sample size and randomization
The sample size was calculated based on the FACT-Bv4.0 total score of similar intervention studies (two-arm trials with Chinese mainland female patients with breast cancer employing a 12-month follow-up period). According to an eligible study [50], 74 patients (37 in each arm) will be needed to detect a between-arm change of 6.74 in the FACT-Bv4.0 total score with a power of 80 % at a 5 % level of statistical significance. The sample size was increased to 90 patients (45 in each arm) to allow for a 20 % dropout rate. Assuming an intra-cluster correlation coefficient (ICC) of about 0.1 and 30 patients per cluster, the sample size adjusting for clustering is 12 clusters or 360 patients [51]. That is, a total of 12 tertiary hospitals will be required in the trial, with 6 hospitals in each arm and 30 patients per hospital on average.
The 12 tertiary hospitals will be randomly selected from the 24 tertiary hospitals in Xi’an district and allocated to the intervention and control arms using a computer-generated random numbers table. Selection and allocation of the 12 hospitals will be carried out independently by a member of the research team.
Procedure
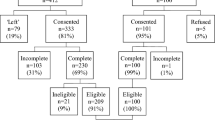
Following random selection and allocation of the 12 tertiary hospitals, eligible female inpatients with breast cancer from each hospital will receive either MSNP plus routine nursing care immediately after recruitment (intervention arm), or only routine nursing care (control arm). The patients in both arms will provide demographic data and complete the pre-test of the FACT-Bv4.0, VAS, arm circumference, and CMS prior to the intervention. After the baseline measurement, four post-tests (i.e., FACT-Bv4.0, VAS, arm circumference, and CMS) at 1, 3, 6, and 12 months will be conducted. Items on the questionnaire will be asked of all patients and their answers will be recorded by trained data collectors. The flow chart of the cRCT procedure is depicted in Fig. 2.
Outcomes and measurements
The primary outcome is HRQoL measured by the Functional Assessment of Cancer Therapy-Breast version 4.0 (FACT-Bv4.0) at 12 months.
FACT-Bv4.0
The 36-item Chinese (simple) FACT-Bv4.0 consists of a general cancer subscale (FACT-G) and a breast-cancer-specific subscale for additional concerns (BCS). The FACT-G comprises physical well-being (PWB, seven items), social/family well-being (SFWB, seven items), emotional well-being (EWB, six items), and functional well-being (FWB, seven items). The BCS comprises nine items, each rated on a 5-point Likert scale (from 0 to 4). The FACT-Bv4.0 total score, which is the sum of scores of the FACT-G and BCS ranges from 0 to 144. A higher score indicates better HRQoL of the patient [52]. The reliability, validity, and responsiveness of the Chinese (simple) FACT-Bv4.0 has been confirmed in Chinese patients with breast cancer [53].
The secondary outcomes include pain, fatigue, sleep, BCRL, and upper limb function evaluated by a visual analogue scale (VAS), the circumference method, and the Constant-Murley Score (CMS) at 1, 3, 6, and 12 months.
VAS
The VAS is a psychometric response scale used to measure subjective characteristics or attitudes that cannot be directly assessed. When answering a VAS item, respondents specify their level of agreement with a statement by indicating a position along a continuous line between two end-points [54]. In this study, a 0–10 cm VAS will be used in the subjective evaluation of pain (no pain to severe pain), fatigue (no fatigue to severe fatigue) and sleep (good sleep to poor sleep) in the patients.
Circumference method
Arm circumference will be measured at 10 cm above the wrist and 10 cm above the elbow using a leather measuring tape. Lymphedema is defined as a difference in arm circumference of more than 2 cm between the treated and untreated side at either of the two measured locations on the limb [55]. The measurement of arm circumference of all recruited patients will be consistently performed at 5 pm at the baseline survey and at each post-test.
CMS
The Chinese CMS has four subscales, including pain (15 points maximum), activities of daily living (20 points maximum), range of motion (ROM, 40 points maximum), and strength (25 points maximum). The total score ranges from 0 to 100, with a higher score indicating a higher quality of functioning [56]. A systematic review showed that the original CMS satisfied psychometric properties of functional assessment [57].
Interventions
The intervention arm
Patients in the intervention arm will receive the MSNP based on routine nursing care delivered by trained clinical nurses immediately after recruitment. The MSNP includes physical care, help with psychological adjustment, and social support, aiming to improve the physical, psychological, and social functioning of the patients, respectively. Detailed information on the MSNP content and implementation are shown in Table 1.
The control arm
Patients in the control arm will only receive routine nursing care, including vital signs observation, nursing specific to surgery, drainage tube nursing, fundamental exercises after surgery, and post-operative complications monitoring.
Masking
All recruited female patients with breast cancer, participating clinical staff, and trained data collectors of the 12 hospitals will be blinded to the allocation information. Patients in the control arm will not be offered any information on the MSNP during the study period in case of bias.
The trial statistician will also be blinded to the treatment code during development of the statistical analysis plan and writing of the statistical programs, which will be validated and completed using dummy randomization codes. The actual allocation will only be provided to the study team after locking of the database.
Data management and analyses
SAS 9.4 and SPSS 22.0 will be employed to perform all statistical analyses. All quantitative data will be collected using paper questionnaires with unique ID numbers for each recruited patient. Data will be stored at the research team office at the end of each day. Daily checking of data will be carried out by the research coordinator, with queries identified and resolved promptly. A database will be built using Epidata3.1; double entry and checking will be performed by an assigned data entry team. Discrepancies will be resolved by a third data manager. Once in an electronic file, all data will be password protected, with data managers controlling access to passwords and ensuring the database is backed up daily.
Findings of the trial will be reported according to the CONSORT guidelines for cRCT. Primary analyses will be based on an intention-to-treat (ITT) population and secondary analyses on a per-protocol (PP) population. The primary endpoint will be analyzed using a linear mixed model with intervention, time, and interaction between intervention and time as fixed effects, baseline measurement as covariate, and cluster and patient as random effects. The intervention difference at each time, together with its 95 % confidence interval will be derived from the mixed model. Missing data will be treated as missing at random in the above mixed model analysis and no imputation will be made. To assess the sensitivity of the result of this assumption, the last observation carried forward (LOCF) strategy will be used to compute missing HRQoL scores during follow-up. A covariate-adjusted mixed model of the primary endpoint will be tested by adding pre-specified covariates at baseline into the linear mixed model. Subgroup analysis will be performed on the pre-specified covariates.
Continuous secondary outcomes will be analyzed in a similar way to the primary endpoint analysis. For the analysis of binary secondary outcomes, a generalized mixed model will be employed with intervention, time, and the interaction between intervention and time as fixed effects, baseline measurement as covariate, and cluster and patient as random effects. The odds ratio (OR) between the two arms at each time, together with its 95 % CI will be derived from the generalized mixed model.
All analyses will be described in detail in the finalized and signed statistical analysis plan before unmasking the study.
Ethical approval
The trial protocol received ethical approval from the Biomedical Ethics Committee of Xi’an Jiaotong University Health Science Center. Written informed consent will be obtained from each recruited patient before the intervention and questionnaire survey.
Discussion
To improve HRQoL in female patients with breast cancer, novel intervention strategies that can improve physical, psychological, and social functions as well as sustain those improvements over time are required. This trial will examine the effectiveness of an MSNP in improving HRQoL compared to routine nursing care. The efficacy of the intervention on pain, fatigue, sleep, BCRL, and upper limb function will also be tested. The MSNP has several advantages over routine nursing care: (i) it is constructed based on validated evidence and within the theoretical framework of the HBM; (ii) taking into consideration physical, psychological, and social health domains, it outlines comprehensive nursing strategies for HRQoL improvement mainly in clinical contexts; and (iii) it may improve self-management of health in female patients with breast cancer, because patients will receive follow-up health instructions from nurses at home or in community settings.
Even in the case of null results, this trial will produce a large amount of illuminating data. Investigators will be able to closely monitor HRQoL in both arms during the 12-month follow-up period. If the MSNP is effective, it will provide an additional nursing care option for comprehensive health management of the population of female patients with breast cancer.
Abbreviations
- BCRL:
-
Breast cancer-related lymphedema
- CMS:
-
Constant-Murley Score
- cRCT:
-
Cluster randomized controlled trial
- FACT-Bv4.0:
-
Functional Assessment of Cancer Therapy-Breast version 4.0
- HBM:
-
Health Belief Model
- HRQoL:
-
Health-related quality of life
- MSNP:
-
Multimodal standard nursing program
- VAS:
-
Visual analogue scale
References
Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65(2):87–108.
Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136(5):e359–86.
Fan L, Strasser-Weippl K, Li JJ, St Louis J, Finkelstein DM, Yu KD, Chen WQ, Shao ZM, Goss PE. Breast cancer in China. Lancet Oncol. 2014;15(7):e279–89.
Chow R, Pulenzas N, Zhang L, Ecclestone C, Leahey A, Hamer J, DeAngelis C, Bedard G, McDonald R, Bhatia A, Ellis J, Rakovitch E, Vuong S, Chow E, Verma S. Quality of life and symptom burden in patients with breast cancer treated with mastectomy and lumpectomy. Support Care Cancer. 2016;24(5):2191–9.
Leggett S, Koczwara B, Miller M. The impact of complementary and alternative medicines on cancer symptoms, treatment side effects, quality of life, and survival in women with breast cancer—a systematic review. Nutr Cancer. 2015;67(3):373–91.
Yagata H, Ohtsu H, Komoike Y, Saji S, Takei H, Nakamura T, Ohashi Y, Iwase T, Shimozuma K. Joint symptoms and health-related quality of life in postmenopausal women with breast cancer who completed 5 years of anastrozole. Support Care Cancer. 2016;24(2):683–9.
Mayer M, Lang K, Hurvitz S, Lalla D, Federico V, Brammer M, Menzin J, Tripathy D. Symptom burden and quality of life among women with HER2(+) metastatic breast cancer. Breast J. 2015;21(2):208–10.
World Health Organization. Measuring quality of life. 1997; http://www.who.int/mental_health/media/68.pdf. Accessed 1 Sept 2015.
van Nieuwenhuizen AJ, Buffart LM, Brug J, Rene Leemans C, Verdonck-de Leeuw IM. The association between health related quality of life and survival in patients head and neck cancer: a systematic review. Oral Oncol. 2015;51(1):1–11.
Smyth EN, Shen W, Bowman L, Peterson P, John W, Melemed A, Liepa AM. Patient-reported pain and other quality of life domains as prognostic factors for survival in a phase III clinical trial of patients with advanced breast cancer. Health Qual Life Outcomes. 2016;14(1):52.
Karlsen RV, Frederiksen K, Larsen MB, von Heymann-Horan AB, Appel CW, Christensen J, Tjonneland A, Ross L, Johansen C, Bidstrup PE. The impact of a breast cancer diagnosis on health-related quality of life. A prospective comparison among middle-aged to elderly women with and without breast cancer. Acta Oncol. 2016;55(6):720–7.
Penha TR, Botter B, Heuts EM, Voogd AC, von Meyenfeldt MF, van der Hulst RR. Quality of life in patients with breast cancer-related lymphedema and reconstructive breast surgery. J Reconstr Microsurg. 2016;32(6):484–90.
Edib Z, Kumarasamy V, Binti Abdullah N, Rizal AM, Al-Dubai SA. Most prevalent unmet supportive care needs and quality of life of breast cancer patients in a tertiary hospital in Malaysia. Health Qual Life Outcomes. 2016;14(1):26.
Gold M, Dunn LB, Phoenix B, Paul SM, Hamolsky D, Levine JD, Miaskowski C. Co-occurrence of anxiety and depressive symptoms following breast cancer surgery and its impact on quality of life. Eur J Oncol Nurs. 2016;20:97–105.
Jeffe DB, Perez M, Cole EF, Liu Y, Schootman M. The effects of surgery type and chemotherapy on early-stage breast cancer patients’ quality of life over 2-year follow-up. Ann Surg Oncol. 2016;23(3):735–43.
Kibar S, Dalyan Aras M, Unsal DS. The risk factors and prevalence of upper extremity impairments and an analysis of effects of lymphoedema and other impairments on the quality of life of breast cancer patients. Eur J Cancer Care (Engl). 2016. doi:10.1111/ecc.12433.
Paterson CL, Lengacher CA, Donovan KA, Kip KE, Tofthagen CS. Body image in younger breast cancer survivors: a systematic review. Cancer Nurs. 2016;39(1):e39–58.
Champion VL, Wagner LI, Monahan PO, Daggy J, Smith L, Cohee A, Ziner KW, Haase JE, Miller KD, Pradhan K, Unverzagt FW, Cella D, Ansari B, Sledge Jr GW. Comparison of younger and older breast cancer survivors and age-matched controls on specific and overall quality of life domains. Cancer. 2014;120(15):2237–46.
Stover AM, Mayer DK, Muss H, Wheeler SB, Lyons JC, Reeve BB. Quality of life changes during the pre- and postdiagnosis period and treatment-related recovery time in older women with breast cancer. Cancer. 2014;120(12):1881–9.
Boehm K, Cramer H, Staroszynski T, Ostermann T. Arts therapies for anxiety, depression, and quality of life in breast cancer patients: a systematic review and meta-analysis. Evid Based Complement Alternat Med. 2014;2014:103297.
Sandel SL, Judge JO, Landry N, Faria L, Ouellette R, Majczak M. Dance and movement program improves quality-of-life measures in breast cancer survivors. Cancer Nurs. 2005;28(4):301–9.
Hanser SB, Bauer-Wu S, Kubicek L, Healey M, Manola J, Hernandez M, Bunnell C. Effects of a music therapy intervention on quality of life and distress in women with metastatic breast cancer. J Soc Integr Oncol. 2006;4(3):116–24.
Zeng Y, Huang M, Cheng AS, Zhou Y, So WK. Meta-analysis of the effects of exercise intervention on quality of life in breast cancer survivors. Breast Cancer. 2014;21(3):262–74.
Diggins AD, Hearn LE, Lechner SC, Annane D, Antoni MH, Whitehead NE. Physical activity in black breast cancer survivors: implications for quality of life and mood at baseline and 6-month follow-up. Psychooncology. 2016. doi:10.1002/pon.4095.
van Vulpen JK, Peeters PH, Velthuis MJ, van der Wall E, May AM. Effects of physical exercise during adjuvant breast cancer treatment on physical and psychosocial dimensions of cancer-related fatigue: a meta-analysis. Maturitas. 2016;85:104–11.
Pan Y, Yang K, Wang Y, Zhang L, Liang H. Could yoga practice improve treatment-related side effects and quality of life for women with breast cancer? A systematic review and meta-analysis. Asia Pac J Clin Oncol. 2015. doi:10.1111/ajco.12329.
Vardar Yagli N, Sener G, Arikan H, Saglam M, Inal Ince D, Savci S, Calik Kutukcu E, Altundag K, Kaya EB, Kutluk T, Ozisik Y. Do yoga and aerobic exercise training have impact on functional capacity, fatigue, peripheral muscle strength, and quality of life in breast cancer survivors? Integr Cancer Ther. 2015;14(2):125–32.
Hagstrom AD, Marshall PW, Lonsdale C, Cheema BS, Fiatarone Singh MA, Green S. Resistance training improves fatigue and quality of life in previously sedentary breast cancer survivors: a randomised controlled trial. Eur J Cancer Care (Engl). 2015. doi:10.1111/ecc.12422.
Ashing KT, Miller AM. Assessing the utility of a telephonically delivered psychoeducational intervention to improve health-related quality of life in African American breast cancer survivors: a pilot trial. Psychooncology. 2016;25(2):236–8.
Yuste Sanchez MJ, Lacomba MT, Sanchez BS, Merino DP, da Costa SP, Tellez EC, Zapico GA. Health related quality of life improvement in breast cancer patients: secondary outcome from a simple blinded, randomised clinical trial. Breast. 2015;24(1):75–81.
Sajjad S, Ali A, Gul RB, Mateen A, Rozi S. The effect of individualized patient education, along with emotional support, on the quality of life of breast cancer patients-A pilot study. Eur J Oncol Nurs. 2016;21:75–82.
Zamaniyan S, Bolhari J, Naziri G, Akrami M, Hosseini S. Effectiveness of spiritual group therapy on quality of life and spiritual well-being among patients with breast cancer. Iran J Med Sci. 2016;41(2):140–4.
Napoles AM, Ortiz C, Santoyo-Olsson J, Stewart AL, Gregorich S, Lee HE, Duron Y, McGuire P, Luce J. Nuevo Amanecer: results of a randomized controlled trial of a community-based, peer-delivered stress management intervention to improve quality of life in Latinas with breast cancer. Am J Public Health. 2015;105 Suppl 3:e55–63.
Do J, Cho Y, Jeon J. Effects of a 4-week multimodal rehabilitation program on quality of life, cardiopulmonary function, and fatigue in breast cancer patients. J Breast Cancer. 2015;18(1):87–96.
Cuesta-Vargas AI, Buchan J, Arroyo-Morales M. A multimodal physiotherapy programme plus deep water running for improving cancer-related fatigue and quality of life in breast cancer survivors. Eur J Cancer Care (Engl). 2014;23(1):15–21.
Cantarero-Villanueva I, Fernández-Lao C, Díaz-Rodriguez L, Fernández-de-las-Penas C, del Moral-Avila R, Arroyo-Morales M. A multimodal exercise program and multimedia support reduce cancer-related fatigue in breast cancer survivors: a randomised controlled clinical trial. Eur J Integr Med. 2011;3:e189–200.
Haines TP, Sinnamon P, Wetzig NG, Lehman M, Walpole E, Pratt T, Smith A. Multimodal exercise improves quality of life of women being treated for breast cancer, but at what cost? Randomized trial with economic evaluation. Breast Cancer Res Treat. 2010;124(1):163–75.
Jones CJ, Smith H, Llewellyn C. Evaluation the effectiveness of health belief model interventions in improving adherence: a systematic review. Health Psychol Rev. 2014;8(3):253–69.
U.S. Department of Health and Human Services, National Institutes of Health, National Cancer Institute. Theory at a glance - A guide for health promotion practice. 2nd ed. US: NIH Publication No. 05-3896. 2005. http://www.sbccimplementationkits.org/demandrmnch/wpcontent/uploads/2014/02/Theory-at-a-Glance-A-Guide-For-Health-Promotion-Practice.pdf.
Glanz K, Rimer BK, Lewis FM. Health Behavior and Health Education. Theory, Research and Practice. San Fransisco: Wiley; 2002.
Zare M, Ghodsbin F, Jahanbin I, Ariafar A, Keshavarzi S, Izadi T. The effect of health belief model-based education on knowledge and prostate cancer screening behaviors: a randomized controlled trial. Int J Community Based Nurs Midwifery. 2016;4(1):57–68.
Sharafkhani N, Khorsandi M, Shamsi M, Ranjbaran M. The effect of an educational intervention program on the adoption of low back pain preventive behaviors in nurses: an application of the health belief model. Global Spine J. 2016;6(1):29–34.
Li X, Lei Y, Wang H, He G, Williams AB. The health belief model: a qualitative study to understand high-risk sexual behavior in Chinese men who have sex with men. J Assoc Nurses AIDS Care. 2016;27(1):66–76.
Khoramabadi M, Dolatian M, Hajian S, Zamanian M, Taheripanah R, Sheikhan Z, Mahmoodi Z, Seyedi-Moghadam A. Effects of education based on health belief model on dietary behaviors of Iranian pregnant women. Glob J Health Sci. 2016;8(2):230–9.
Bayu H, Berhe Y, Mulat A, Alemu A. Cervical cancer screening service uptake and associated factors among age eligible women in Mekelle Zone, Northern Ethiopia, 2015: a community based study using health belief model. PLoS One. 2016;11(3):e0149908.
AI-Sakkaf KA, Basaleem HO. Breast cancer knowledge, perception and breast self-examination practices among Yemeni Women: an application of the health belief model. Asian Pac J Cancer Prev. 2016;17(3):1463–7.
Lee HY, Stange MJ, Ahluwalia JS. Breast cancer screening behaviors among Korean American immigrant women: findings from the health belief model. J Transcult Nurs. 2015;26(5):450–7.
Hajian-Tilaki K, Auladi S. Health belief model and practice of breast self-examination and breast cancer screening in Iranian women. Breast Cancer. 2014;21(4):429–34.
American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th edition. US: Washington DC. 2013.
Wang L, Li HP, Wang DB, Duan YQ. The effects of health belief model system education on lymphedema incidence and quality of life in post-operation breast cancer patients. Chin J Behav Med & Brain Sci. 2012;21(9):803–6.
Wang DL, Bakhai A. Clinical trials: a practical guide to design, analysis, and reporting. London: Remedica; 2006. p. 141–51.
Brady MJ, Cella DF, Mo F, Bonomi AE, Tulsky DS, Lloyd SR, Deasy S, Cobleigh M, Shiomoto G. Reliability and validity of the functional assessment of cancer therapy-breast quality-of-life instrument. J Clin Oncol. 1997;15(3):974–86.
Wan C, Zhang D, Yang Z, Tu X, Tang W, Feng C, Wang H, Tang X. Validation of the simplified Chinese version of the FACT-B for measuring quality of life for patients with breast cancer. Breast Cancer Res Treat. 2007;106(3):413–8.
Bailly F, Fautrel B, Gossec L. Pain assessment in rheumatology - How can we do better? A literature review. Joint Bone Spine. 2016;83(4):384–8.
Harris SR, Hugi MR, Olivotto IA, Levine M. Clinical practice guide-lines for the care and treatment of breast cancer: 11. Lymphedema CMAJ. 2001;164(2):191–9.
Guo HL, Shi CQ, Li LL. Effects of a comprehensive rehabilitation intervention on shoulder function and quality of life in breast cancer patients following radical mastectomy. Chin J Phys Med Rehabil. 2014;36(7):559–61.
Roy JS, MacDermid JC, Woodhouse LJ. A systematic review of the psychometric properties of the Constant-Murley score. J Shoulder Elbow Surg. 2010;19(1):157–64.
Acknowledgments
Funding from the National Natural Science Foundation of China (grant no. 81502700) is gratefully acknowledged. We also would like to thank Editage (http://www.editage.cn/) for English language editing.
Funding
The trial is supported by the National Natural Science Foundation of China (grant no. 81502700). The funding body will not play any role in the study.
Availability of data and material
None declared.
Authors’ contributions
KZ, DW, and XL contributed to the protocol and grant proposal. KZ, XL, XH, LH, JA, ML, and WW contributed to the intervention program design. KZ and DW were primarily involved in developing the statistical analysis plan. KZ prepared the manuscript and DW and XL assisted with the manuscript writing. The manuscript was amended based on comments from all authors. All authors read and approved the final manuscript.
Competing interests
The authors declare that they have no competing interests.
Consent for publication
Not applicable.
Ethics approval and consent to participate
The trial protocol has received ethical approval from the Biomedical Ethics Committee of Xi’an Jiaotong University Health Science Centre (No. 2015-170), which covers all participating centers of this study. Written informed consent will be obtained from each recruited patient before the intervention and questionnaire survey.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Zhou, K., Wang, D., He, X. et al. Effectiveness of a multimodal standard nursing program on health-related quality of life in Chinese mainland female patients with breast cancer: protocol for a single-blind cluster randomized controlled trial. BMC Cancer 16, 698 (2016). https://doi.org/10.1186/s12885-016-2726-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12885-016-2726-y