Abstract
Background
Diffusion kurtosis imaging (DKI) has the potential to provide microstructural insights into myelin and axonal pathology with additional kurtosis parameters. To our knowledge, few studies are available in the current literature using DKI by tract-based spatial statistics (TBSS) analysis in patients with multiple sclerosis (MS). The aim of this study is to assess the performance of commonly used parameters derived from DKI and diffusion tensor imaging (DTI) in detecting microstructural changes and associated pathology in relapsing remitting MS (RRMS).
Methods
Thirty-six patients with RRMS and 49 age and sex matched healthy controls underwent DKI. The brain tissue integrity was assessed by fractional anisotropy (FA), mean diffusivity (MD), axial diffusivity (Da), radial diffusivity (Dr), mean kurtosis (MK), axial kurtosis (Ka) and radial kurtosis (Kr) of DKI and FA, MD, Da and Dr of DTI. Group differences in these parameters were compared using TBSS (P < 0.01, corrected). To compare the sensitivity of these parameters in detecting white matter (WM) damage, the percentage of the abnormal voxels based on TBSS analysis, relative to the whole skeleton voxels for each parameter was calculated.
Results
The sensitivities in detecting WM abnormality in RRMS were MK (78.2%) > Kr (76.7%) > Ka (53.5%) and Dr (78.8%) > MD (76.7%) > FA (74.1%) > Da (28.3%) for DKI, and Dr (79.8%) > MD (79.5%) > FA (68.6%) > Da (40.1%) for DTI. DKI-derived diffusion parameters (FA, MD, and Dr) were sensitive for detecting abnormality in WM regions with coherent fiber arrangement; however, the kurtosis parameters (MK and Kr) were sensitive to discern abnormalities in WM regions with complex fiber arrangement.
Conclusions
The diffusion and kurtosis parameters could provide complementary information for revealing brain microstructural damage in RRMS. Dr and DKI_Kr may be regarded as useful surrogate markers for reflecting pathological changes in RRMS.
Similar content being viewed by others
Background
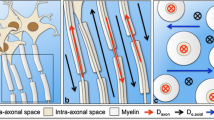
Multiple sclerosis (MS) is a chronic disorder of the CNS, characterized by focal white matter (WM) plaques along with diffuse normal appearing WM (NAWM) damage and cortical demyelination [1]. Diffusion tensor imaging (DTI) is one of the most widely used methods in detecting microstructural abnormalities based on water diffusion measures with the assumption that the diffusion displacement of water molecule in an unrestricted environment has a Gaussian approximation [2].In reality, water molecules often show non-Gaussian diffusion due to the presence of barriers of cell membranes, axon sheaths, and water compartments in biological tissues [3]. So it is thought that DTI may not be capable to provide accurate values at dense intersections of fiber tracts [4]. In contrast, as a clinically feasible extension of DTI, diffusion kurtosis imaging (DKI) has been proposed to characterize the deviation of water diffusion in neural tissues from Gaussian diffusion [5, 6]. Both diffusion parameters including fractional anisotropy (FA), mean diffusivity (MD), axial diffusivity (Da), radial diffusivity (Dr) and kurtosis parameters including mean kurtosis (MK), axial kurtosis (Ka), radial kurtosis (Kr) could be obtained from DKI data. DKI can be regarded as a more sensitive indicator of diffusional heterogeneity and can be used to investigate abnormalities in tissues with isotropic structure [6, 7].
The sensitivity of DKI has been evaluated in age-related diffusion patterns in the prefrontal brain [8], reactive astrogliosis in traumatic brain injury [9], and cuprizone-induced demyelination in mice [10], which showed better demonstration of microstructural changes than with DTI. However, there were few studies to validate the merits of DKI in evaluating patients with MS [11, 12]. Tract-based spatial statistics (TBSS) provides a powerful and objective method to perform multi-subject comparisons [13].
In this study, the microstructural alterations reflected by both DKI and DTI parameters in relapsing-remitting multiple sclerosis (RRMS) were investigated using TBSS. Our aim is to assess the performances of 11 commonly used parameters derived from DKI (MK, Ka, Kr, FA, MD, Da and Dr) and DTI (FA, MD, Da and Dr) in detecting microstructural abnormalities in RRMS.
Methods
Subjects
Thirty-six (13 male and 23 female) consecutive patients with RRMS (diagnosed by McDonald criteria [14]) were prospectively enrolled in this study. All patients underwent clinical assessments, including relapse history and disability assessment using EDSS before MRI examination. Patients were excluded if they had a history of other CNS disorders, corticosteroid use or relapses within three months prior to MRI. For comparison, 49 age- and gender-matched healthy controls (17 male and 32 female), with no previous history of neurological disorders were recruited (Table 1). Approval for this study was obtained from the Ethics Committee of Huashan Hospital, Fudan University and written informed consent was obtained from all the subjects.
MRI data acquisition
All scans were acquired using Discovery MR750 3.0 T scanner (GE Healthcare, Milwaukee, WI, USA) with an eight-channel phase array head coil.
An axial FLAIR sequence (SE: repetition time/ echo time = 8800/146 ms, slice thickness = 6.0 mm, field of view =512 × 512 mm, voxel size = 0.5 × 0.5 × 6.0 mm3) for white matter lesion volume calculation was performed. DKI was acquired with two values of b (b = 1250 and 2500 s/mm2) along 25 diffusion-encoding directions and b value of 0 s/mm2 along 25 non-diffusion-weighted images, with a spin-echo single-shot echo planar imaging (EPI) sequence (TR/TE = 4700/102 ms; matrix = 128 × 128; FOV = 240 × 240 mm; slice thickness = 4 mm without gap; 35 axial slices; acquisition time was 8 min and 42 s).
White matter lesion volume calculation
With MRIcron software (http://www.nitrc.org/projects/mricron/) we drew all the WM leisons manually on FLAIR images and calculated total WM lesion volume for each patient and summerized it in Table 1.
DKI data processing
We used the same methodology from a previously published work [15], and the differences were as following. In “calculation of diffusion and kurtosis parameters”, all the data (b = 0, 1250, 2500 s/mm2) were used for DKI fitting and only images with b = 0 and 1250 s/mm2 were employed for DTI fitting. In “tract-based spatial statistics”, group comparisons between RRMS patients and healthy controls were performed using a general linear model. The percentage of the abnormal voxels relative to the whole skeleton voxels for each parameter was calculated, so as to quantitatively compare the sensitivity of parameters from DKI and DTI in detecting brain tissue integrity impairments in RRMS.
Results
Kurtosis parameters from DKI
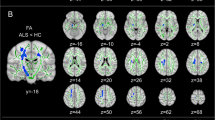
Compared with healthy controls, RRMS patients had significantly decreased DKI-derived kurtosis parameters in WM regions (P < 0.01, two-tailed, FWE corrected) with complex fiber arrangement, such as in the juxtacortical WM and corona radiata. DKI_MK, DKI_Ka and DKI_Kr could detect abnormal diffusion in 78.2%, 53.5% and 76.7% voxels of the whole WM skeleton respectively. Kurtosis parameters are shown in Fig. 1.
TBSS shows WM regions with significant differences in the DKI_MK, DKI_Ka and DKI_Kr between RRMS patients and healthy subjects (P < 0.01, FWE corrected). Green represents mean FA skeleton of all participants; blue represents reduction in RRMS patients. The percentage in the left column represents the percentage of the abnormal voxels relative to the whole skeleton voxels for each parameter
Diffusion parameters from DKI
Compared with healthy controls, RRMS patients demonstrated reduced DKI_FA in WM regions with coherent fiber arrangement, such as the corpus callosum and anterior limb of internal capsule, and increased DKI_MD, DKI_Da and DKI_Dr (P < 0.01, two-tailed, FWE corrected). DKI_FA, DKI_MD, DKI_Da and DKI_Dr could detect abnormal diffusion in 74.1%, 76.7%, 28.3% and 78.8% voxels of the whole WM skeleton respectively. DKI-derived diffusion parameters are shown in Fig. 2.
TBSS shows WM regions with significant differences in the DKI_FA, DKI_MD, DKI_Da and DKI_Dr between RRMS patients and healthy subjects (P < 0.01, FWE corrected). Green represents mean FA skeleton of all participants; red denotes increase and blue represents reduction in RRMS patients. The percentage in the left column represents the percentage of the abnormal voxels relative to the whole skeleton voxels for each parameter
Diffusion parameters from DTI
RRMS patients exhibited similar patterns with DKI-derived diffusion parameters. FA was reduced, MD, Da and Dr were increased (P < 0.01, two-tailed, FWE corrected). DTI_FA, DTI_MD, DTI_Da and DTI_Dr could detect abnormal diffusion in 68.6%, 79.5%, 40.1% and 79.8% voxels of the whole WM skeleton respectively. DTI-derived diffusion parameters are shown in Fig. 3.
TBSS shows WM regions with significant differences in the DTI_FA, DTI_MD, DTI_Da and DTI_Dr between RRMS patients and healthy subjects (P < 0.01, FWE corrected). Green represents mean FA skeleton of all participants; red denotes increase and blue represents reduction in RRMS patients. The percentage in the left column represents the percentage of the abnormal voxels relative to the whole skeleton voxels for each parameter
Discussion
Although DTI has been widely used in investigating structural changes in the NAWM in MS [16, 17], it may not provide accurate parameters at dense intersections of fiber tracts [4]. In contrast, DKI can be used to quantify non-Gaussian diffusion, thus providing accurate parameters at dense intersection of fiber tracts [6]. To our knowledge, there were only a limited number of studies using DKI in MS patients [11, 12, 18, 19], Raz E et al. measured FA, MD, and MK values of the entire cross-sectional cord area, normal-appearing gray matter (NAGM) and WM in MS patients by DKI using region-of-interest (ROI) analysis, they thought that DKI could provide additional and complementary information to DTI on spinal cord pathology [11]. In another research, DKI was used to evaluate diffusional changes in NAWM regions remote from MS plaques using ROI analysis, the results indicated that DKI might be an additional sensitive indicator for detecting tissue damage in MS patients [12]. They concluded that DKI was sensitive for detecting tissue damage in MS patients and could provide information that was complementary to that of conventional DTI-derived metrics. However, most of these above-mentioned studies adopted ROI-based analysis, which had poor reproducibility of ROI positioning and only a limited number of specific regions can be examined. In contrast, the TBSS method used in this study was relatively a novel hypothesis-free and user-independent voxel-wise analysis.
In this study, TBSS analysis of both DKI and DTI derived parameters showed widespread WM damage in RRMS patients compared with healthy controls, which was consistant with previous studies using DKI [19] or DTI [20, 21]. Similarly to a research study using DKI in schizophrenia patients, we also observed that DKI-derived kurtosis and diffusion parameters had differernt sensitivity to detect abnormality in WM areas with different fiber architecture [15]. Moreover, we found that the MK decrease in the WM of RRMS patients was predominantly caused by the Kr decrease, and the FA decrease was mainly driven by the increase of Dr.
FA measures anisotropic water diffusion and is proven to be most applicable for assessing WM regions with coherent fiber arrangement. However, it is not suitable for detecting diffusion changes of complex WM architecture, such as crossing fiber regions [22, 23]. As the most characteristic parameter of DKI, MK measures the deviation of the diffusion displacement profile from a Gaussian distribution and enables to probe WM regions with complex fiber arrangement [24]. Therefore, the combination of diffusion and kurtosis parameters may provide improved sensitivity and specificity in detecting alterations in various WM structures. This theoretical prediction has been validated by a previous study in schizophrenia patients [15], and confirmed by our findings that altered diffusion parameters (especially reduced DKI_FA) were observed mainly in WM regions with coherent fiber arrangement (such as the corpus callosum and anterior limb of internal capsule). The percentage of abnormal DKI_FA voxels (74.1%) relative to the whole skeleton voxels was higher than that of DTI_FA (68.6%) in this study, which suggest that DKI_FA might have higher sensitivity than DTI_FA in detecting abnormality in WM regions, while reduced kurtosis parameters were mainly located in WM regions with complex fiber arrangement (such as the juxtacortical WM and corona radiate). The percentage of abnormal MK voxels relative to the whole skeleton voxels was 78.2%, which suggest that MK might have higher sensitivity than DTI in detecting abnormality in WM regions. Therefore, appropriate DKI derived parameters should be selected to probe altered diffusion pattern in specific WM regions in RRMS patients.
As we know, there is strong directional dependence of water distribution within myelinated WM tracts. However, once inflammation and demyelination occur, diffusivity will increase and directionality will decrease. The increase of diffusivity manifested as increase of Dr (diffusion perpendicular to the long axis) and Da (diffusion along the long axis). However, decreased Da was reported in some animal experiments [25, 26]. In our opinion, these studies may not take into account the full complexity of pathological processes occurred in RRMS. The increase of Da found in our RRMS patients was consistent with a TBSS study using DTI in RRMS patients [20]. The cause may be explained by severe decreases in axonal packing density which would lead to a whole increase in extracellular water, resulting in larger Dr increases and subsequent Da increases. Other reported reasons include fiber re-organization, increased axonal diameter and membrane permeability [27, 28]. In our study, the percentage of abnormal DKI_Dr voxels (79.8%) relative to the whole skeleton voxels is significantly higher than that of DKI_Da (28.3%), which demonstrated that the increase of MD (mean diffusivity) and decreased FA mainly caused by the increased DKI_Dr. Similarly, this pattern of changes was also found in DTI_Da and DTI_Dr. Interestingly, when assessing the contribution of DKI_Ka and DKI_Kr in those regions showing significantly decreased DKI_MK, we found these were driven predominantly by decreases in DKI_Kr (76.5% vs DKI_Ka 53.5%). All these above-mentioned findings suggested demyelination might be regarded as a key factor among various pathological changes in RRMS. So Dr and DKI_Kr might be regarded as useful surrogate markers for reflecting pathological changes and improving clinical–radiological correlations in MS. Furthermore, Dr and DKI_Kr measured by TBSS might have great potential to be a MRI biomarker in monitoring remyelination in MS patients.
Conclusions
In conclusion, DKI-derived parameters were sensitive to detect abnormality in microstructural changes. The diffusion and kurtosis parameters could provide complementary information for revealing pathological changes in RRMS patients. Dr and DKI_Kr might be regarded as a useful surrogate marker for reflecting pathological changes and improving clinical–radiological correlations in MS patients.
Abbreviations
- Da:
-
Axial diffusivity
- Dr:
-
Radial diffusivity
- GM:
-
Gray matter
- Ka:
-
Axial kurtosis
- Kr:
-
Radial kurtosis
- MK:
-
Mean kurtosis
- NAGM:
-
Normal-appearing gray matter
- NAWM:
-
Normal-appearing white matter
- WM:
-
White matter
References
Frohman EM, Racke MK, Raine CS. Multiple sclerosis--the plaque and its pathogenesis. N Engl J Med. 2006;354(9):942–55.
Basser PJ, Jones DK. Diffusion-tensor MRI: theory, experimental design and data analysis - a technical review. NMR Biomed. 2002;15(7–8):456–67.
Tuch DS, Reese TG, Wiegell MR, Wedeen VJ. Diffusion MRI of complex neural architecture. Neuron. 2003;40(5):885–95.
Abdallah CG, Tang CY, Mathew SJ, Martinez J, Hof PR, Perera TD, Shungu DC, Gorman JM, Coplan JD. Diffusion tensor imaging in studying white matter complexity: a gap junction hypothesis. Neurosci Lett. 2010;475(3):161–4.
Hui ES, Cheung MM, Qi L, Wu EX. Towards better MR characterization of neural tissues using directional diffusion kurtosis analysis. NeuroImage. 2008;42(1):122–34.
Jensen JH, Helpern JA. MRI quantification of non-Gaussian water diffusion by kurtosis analysis. NMR Biomed. 2010;23(7):698–710.
Jensen JH, Helpern JA, Ramani A, Lu H, Kaczynski K. Diffusional kurtosis imaging: the quantification of non-gaussian water diffusion by means of magnetic resonance imaging. Magn Reson Med. 2005;53(6):1432–40.
Falangola MF, Jensen JH, Babb JS, Hu C, Castellanos FX, Di Martino A, Ferris SH, Helpern JA. Age-related non-Gaussian diffusion patterns in the prefrontal brain. Journal of magnetic resonance imaging: JMRI. 2008;28(6):1345–50.
Zhuo J, Xu S, Proctor JL, Mullins RJ, Simon JZ, Fiskum G, Gullapalli RP. Diffusion kurtosis as an in vivo imaging marker for reactive astrogliosis in traumatic brain injury. NeuroImage. 2012;59(1):467–77.
Guglielmetti C, Veraart J, Roelant E, Mai Z, Daans J, Van Audekerke J, Naeyaert M, Vanhoutte G, Delgado YPR, Praet J, Fieremans E, Ponsaerts P, Sijbers J, Van der Linden A, Verhoye M. Diffusion kurtosis imaging probes cortical alterations and white matter pathology following cuprizone induced demyelination and spontaneous remyelination. NeuroImage. 2016;125:363–77.
Raz E, Bester M, Sigmund EE, Tabesh A, Babb JS, Jaggi H, Helpern J, Mitnick RJ, Inglese M. A better characterization of spinal cord damage in multiple sclerosis: a diffusional kurtosis imaging study. AJNR Am J Neuroradiol. 2013;34(9):1846–52.
Yoshida M, Hori M, Yokoyama K, Fukunaga I, Suzuki M, Kamagata K, Shimoji K, Nakanishi A, Hattori N, Masutani Y, et al. Diffusional kurtosis imaging of normal-appearing white matter in multiple sclerosis: preliminary clinical experience. Jpn J Radiol. 2013;31(1):50–5.
Smith SM, Jenkinson M, Johansen-Berg H, Rueckert D, Nichols TE, Mackay CE, Watkins KE, Ciccarelli O, Cader MZ, Matthews PM, et al. Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. NeuroImage. 2006;31(4):1487–505.
Polman CH, Reingold SC, Banwell B, Clanet M, Cohen JA, Filippi M, Fujihara K, Havrdova E, Hutchinson M, Kappos L, et al. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol. 2011;69(2):292–302.
Zhu J, Zhuo C, Qin W, Wang D, Ma X, Zhou Y, Yu C. Performances of diffusion kurtosis imaging and diffusion tensor imaging in detecting white matter abnormality in schizophrenia. NeuroImage Clinical. 2015;7:170–6.
Vrenken H, Geurts JJ, Knol DL, van Dijk LN, Dattola V, Jasperse B, van Schijndel RA, Polman CH, Castelijns JA, Barkhof F, et al. Whole-brain T1 mapping in multiple sclerosis: global changes of normal-appearing gray and white matter. Radiology. 2006;240(3):811–20.
Roosendaal SD, Geurts JJ, Vrenken H, Hulst HE, Cover KS, Castelijns JA, Pouwels PJ, Barkhof F. Regional DTI differences in multiple sclerosis patients. NeuroImage. 2009;44(4):1397–403.
Bester M, Jensen JH, Babb JS, Tabesh A, Miles L, Herbert J, Grossman RI, Inglese M. Non-Gaussian diffusion MRI of gray matter is associated with cognitive impairment in multiple sclerosis. Mult Scler. 2015;21(7):935–44.
de Kouchkovsky I, Fieremans E, Fleysher L, Herbert J, Grossman RI, Inglese M. Quantification of normal-appearing white matter tract integrity in multiple sclerosis: a diffusion kurtosis imaging study. J Neurol. 2016;263(6):1146–55.
Liu Y, Duan Y, He Y, Yu C, Wang J, Huang J, Ye J, Parizel PM, Li K, Shu N. Whole brain white matter changes revealed by multiple diffusion metrics in multiple sclerosis: a TBSS study. Eur J Radiol. 2012;81(10):2826–32.
Yu HJ, Christodoulou C, Bhise V, Greenblatt D, Patel Y, Serafin D, Maletic-Savatic M, Krupp LB, Wagshul ME. Multiple white matter tract abnormalities underlie cognitive impairment in RRMS. NeuroImage. 2012;59(4):3713–22.
Douaud G, Jbabdi S, Behrens TE, Menke RA, Gass A, Monsch AU, Rao A, Whitcher B, Kindlmann G, Matthews PM, et al. DTI measures in crossing-fibre areas: increased diffusion anisotropy reveals early white matter alteration in MCI and mild Alzheimer's disease. NeuroImage. 2011;55(3):880–90.
Vos SB, Jones DK, Jeurissen B, Viergever MA, Leemans A. The influence of complex white matter architecture on the mean diffusivity in diffusion tensor MRI of the human brain. NeuroImage. 2012;59(3):2208–16.
Wu EX, Cheung MM. MR diffusion kurtosis imaging for neural tissue characterization. NMR Biomed. 2010;23(7):836–48.
Sun SW, Liang HF, Le TQ, Armstrong RC, Cross AH, Song SK. Differential sensitivity of in vivo and ex vivo diffusion tensor imaging to evolving optic nerve injury in mice with retinal ischemia. NeuroImage. 2006;32(3):1195–204.
Song SK, Sun SW, Ju WK, Lin SJ, Cross AH, Neufeld AH. Diffusion tensor imaging detects and differentiates axon and myelin degeneration in mouse optic nerve after retinal ischemia. NeuroImage. 2003;20(3):1714–22.
Lin F, Yu C, Jiang T, Li K, Chan P. Diffusion tensor tractography-based group mapping of the pyramidal tract in relapsing-remitting multiple sclerosis patients. AJNR Am J Neuroradiol. 2007;28(2):278–82.
Henry RG, Oh J, Nelson SJ, Pelletier D. Directional diffusion in relapsing-remitting multiple sclerosis: a possible in vivo signature of Wallerian degeneration. Journal of magnetic resonance imaging : JMRI. 2003;18(4):420–6.
Acknowledgements
We would like to gratefully thank Jia Jia Zhu of Department of Radiology Affiliated Hospital, Anhui Medical University for his support and assistance.
We also would like to gratefully thank Pro. Chi-Shing ZEE, Keck hospital of USC for his kindly revised the manuscript finally.
Funding
This work was supported by grants from the National Natural Science Foundation of China (81301203, 81471627 and 81200919), Science and Technology Commission of Shanghai Municipality (17411953700), Specialized Research Fund for the Doctoral Program of Higher Education of China (20130071130011) and Shanghai Health System Important Disease Joint Research Project (2013ZYJB009).
Availability of data and materials
The datasets used and analysed during the current study are available from the corresponding author on reasonable request.
Author information
Authors and Affiliations
Contributions
Study concept and design: YX L, J L and DY G. Acquisition of data: HQ L, B Y, C Q, H Y and YF B. Analysis and interpretation of data: HQ L, B Y, C Q, H Y and YF B. Clinical assessments: C Q and H Y. Drafting of the manuscript: HQ L, B Y and C Q. Critical revision of the manuscript for important intellectual content: YX L, J L and DY G. Statistical analysis: HQ L, B Y, C Q, H Y and YF B. Study supervision: DY G. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Approval for this study was obtained from Ethics Committee of Huashan Hosptial, Fudan University (NO.2013–062) and informed consents had been written by all the subjects.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Li, H.Q., Yin, B., Quan, C. et al. Evaluation of patients with relapsing-remitting multiple sclerosis using tract-based spatial statistics analysis: diffusion kurtosis imaging. BMC Neurol 18, 108 (2018). https://doi.org/10.1186/s12883-018-1108-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12883-018-1108-2