Abstract
Background
Our aim was to compare CT images from native, nephrographic and excretory phases using image quality criteria as well as the detection of positive pathological findings in CT Urography, to explore if the radiation burden to the younger group of patients or patients with negative outcomes can be reduced.
Methods
This is a retrospective study of 40 patients who underwent a CT Urography examination on a 192-slice dual source scanner. Image quality was assessed for four specific renal image criteria from the European guidelines, together with pathological assessment in three categories: renal, other abdominal, and incidental findings without clinical significance. Each phase was assessed individually by three radiologists with varying experience using a graded scale. Certainty scores were derived based on the graded assessments. Statistical analysis was performed using visual grading regression (VGR). The limit for significance was set at p = 0.05.
Results
For visual reproduction of the renal parenchyma and renal arteries, the image quality was judged better for the nephrogram phase (p < 0.001), whereas renal pelvis/calyces and proximal ureters were better reproduced in the excretory phase compared to the native phase (p < 0.001). Similarly, significantly higher certainty scores were obtained in the nephrogram phase for renal parenchyma and renal arteries, but in the excretory phase for renal pelvis/calyxes and proximal ureters. Assessment of pathology in the three categories showed no statistically significant differences between the three phases. Certainty scores for assessment of pathology, however, showed a significantly higher certainty for renal pathology when comparing the native phase to nephrogram and excretory phase and a significantly higher score for nephrographic phase but only for incidental findings.
Conclusion
Visualisation of renal anatomy was as expected with each post-contrast phase showing favourable scores compared to the native phase. No statistically significant differences in the assessment of pathology were found between the three phases. The low-dose CT (LDCT) seems to be sufficient in differentiating between normal and pathological examinations. To reduce the radiation burden in certain patient groups, the LDCT could be considered a suitable alternative as a first line imaging method. However, radiologists should be aware of its limitations.
Similar content being viewed by others
Background
CT Urography (CTU) has emerged as the modality of choice in imaging of the abdomen in patients with urinary tract diseases due to its high sensitivity and specificity [1]. Although it comes with a high radiation dose penalty, the benefits of CT imaging outweigh the risk for many of these patients. Optimisation is not only about patient dose and image quality but also about the diagnostic task at hand, i.e. the correct examination technique for a specific diagnostic enquiry in accordance to the ALARA (radiation dose as low as reasonably achievable) and AHARA (image quality as high as reasonably achievable) principles [2, 3]. Patients presented with haematuria or acute flank pain usually undergo diagnostic imaging to rule out any serious conditions underlining upper urinary tract disease such as urolithiasis, renal cell cancer or upper urinary tract urothelial cell carcinoma (UUT-UCC) [4]. Urolithiasis is a common health problem with a high recurrent rate requiring considerable radiological imaging resources for this population, many of which are younger than 50 years of age [5].
The standardized care pathway (SCP) led to general recommendations of the use of medical imaging in diagnostics of urinary tract disease for patients with macroscopic hematuria who are ≥40 years (revised to ≥50 years in 2018), but even younger patients with risk factors are investigated [6]. The majority of the SCP population consist of malignant cancer diagnoses of the urinary tract and a third of the patients present with benign causes of hematuria [6]. Approximately 20–30% of these patients with symptoms of visible blood have negative outcomes and are being subjected to diagnostic imaging tests and radiation related risks based on the presence of macro-hematuria [6].
Macroscopic hematuria is a common symptom in other treatable benign diseases such as urinary tract infection and targets younger women of child bearing age, among others [7].
CTU is a multiphase examination associated with a relatively high radiation dose and can be justified as a first line investigation in hematuria patients if important risk factors are present and clinical tests indicate high risk probability of cancer [8]. In the emergency department, where the short examination times as well as the detection of alternative diagnoses is paramount, CT is the preferred modality with a selection of scan protocols depending on the indication. The detrimental effects of ionizing radiation and the expanding use of CT technique have been the driving factors in optimization of clinical practices regarding appropriateness criteria and dose reduction [9]. These can be achieved foremost by selective use of nonradiative modalities such as ultrasound and magnetic resonance imaging (MRI) and referrals based on proper clinical indications, preliminary tests and patient groups. Dose reduction is achieved in a number of ways, such as, limiting scan lengths and number of phases, and the use of lower exposure settings. Dose reduction is additionally seen in the use of features such as automatic dose modulation, iterative reconstruction [10,11,12] and split-bolus techniques especially in diagnostic imaging of younger, high risk patients [13, 14]. Recent advancements show a trend towards use of low-dose CT (LDCT) in several diagnostic indications such as the investigation of acute flank pain and acute abdomen for diverticulitis, appendicitis and renal stone disease [15, 16].
Both the Bonn call for action and the triple AAA campaign were introduced to strengthen the need for stringent measures in radiation protection for safe and appropriate use of ionizing radiation in medical imaging [17,18,19]. Published literature on renal stone evaluation have validated the trend towards use of low-dose CT (LDCT) due to the high contrast between urinary stones and the surrounding soft tissue [15, 20] as well as in investigation of acute abdomen [16]. However, implementation of the LDCT protocol in the clinical setting has been very slow partly due to the low quality of the images and lack of confidence in interpreting reduced-dose images [21]. But with practice and growing experience it is possible to increase diagnostic confidence and acceptance of lower quality images [22].
The diagnostic performance of non-enhanced CT compared to intravenous urography (IVU) [23] and plain abdominal radiography [24] has been evaluated but there are, to our knowledge, no studies that have compared the image quality and pathology assessment between phases as the present study.
The aim was to compare CT images from native, nephrographic and excretory phases using image quality criteria as well as the detection of positive pathological findings in CT Urography to explore if the radiation burden to the younger group of patients, patients undergoing repetitive imaging or patients with negative outcomes can be reduced.
Materials & methods
This is a retrospective study approved by the regional ethical board. Of the 50 patients referred for a clinical CTU between 2016-03-14 and 2016-11-22 and examined on a 192-slice dual source scanner in single source mode (Siemens Healthineers, Erlangen, Germany), forty patients were included in the study. The acquisition data are presented in Table 1.
Ten patients were excluded due to motion artefacts, difference in scan protocol and scan range. The standard CTU protocol was used with intravenous administration of contrast medium, Iopromide (Ultravist 370mgI/ml, Bayer, Dublin, Ireland), the rate and dose tailored to patient body weight using OMNIVIS calculator (GE healthcare). Computed Tomography Dose Index (CTDIvol) and, Dose Length Product (DLP) were recorded and Size Specific Dose Estimate (SSDE) was calculated based on the antero-posterior (AP) and lateral (LAT) dimensions of each patient at the level of the kidneys using the center slice approach as described in Boos et al. [25, 26].
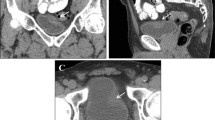
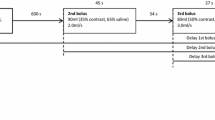
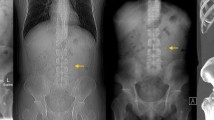
The standard protocol for CTU consists of three phases: the native or unenhanced phase (low-dose), a nephrographic phase (standard dose) and an excretory phase (low-dose), all of which are reconstructed with multi-planar reconstruction (MPR) in three planes: axial, coronal and sagittal (Fig. 1). In patients < 50 years of age, the nephrographic phase is limited to the upper abdomen. Patients > 50 years of age are examined with all three phases from dome of diaphragm to symphysis pubis arch.
During the reading sessions the readers were asked to grade four anatomical structures (renal parenchyma, renal pelvis and calyxes, proximal ureters and renal arteries) obtained from European guidelines for quality criteria on a five-point Likert scale with numerical scores from one to five allocated to response alternatives; criterion was fulfilled, criterion was probably fulfilled, indecisive, criterion was probably not fulfilled and criterion was not fulfilled (Table 2).
The readers were also asked to assess the presence of pathology in three categories; renal, abdominal and incidental findings. These were also graded on a five-point Likert-type scale based on numerical scores from one to five with response alternatives; normal examination, probably normal examination, indecisive, probably pathological examination and pathological examination (Table 3). To determine how certain the readers were in their scoring of the image criteria and pathological findings, variation in certainty scores between the three phases were also calculated by grouping the score options as shown in Table 4.
Statistical analysis within the Visual Grading Regression (VGR) framework [27] was performed with the software Stata 13.1 (Stata Corporation LP, College Station, TX, USA) using the multi-level mixed-effects ordered logistic regression (meologit) command for image quality scores [28]. Thus, ordinal logistic regression was applied to scores from observer ratings whilst controlling for dependencies between observers and patients, where the regression coefficients describe the combined effect of dose and intravenous contrast on image quality, related to each of the phases of the CTU. The certainty scores were analyzed using the same statistic model as the original scores.
Results
Of the 40 patients included in the study, 12 were women, age range 27–74 years (mean 58.9 ± 14 (SD)) with a body mass index (BMI) of 20.7–35.7 kg/m2 (mean 27.7 ± 5.0) and 28 men, age range 29–85 years (mean 63.8 ± 15.0) with a BMI of 19.3–38.9 kg/m2 (mean 27.0 ± 3.7). The mean values, SD and ranges of DLP, CTDIvol, SSDE for the native, nephrogram and excretory phases are displayed in Table 5.
Assessment of anatomical image criteria
When comparing the native phase with nephrogram and excretory phase respectively, criteria C1 and C4 (renal parenchyma and renal arteries) were better reproduced in the nephrographic phase, whereas criteria C2 and C3 (renal pelvis/calyxes, proximal ureters), were visually better reproduced in the excretory phase, (p < 0.001). Similar results were obtained when comparing the nephrogram to excretory phase, where the nephrographic phase was favorable for criteria C1 (renal parenchyma) and C4 (renal arteries) (p < 0.001). For the delineation of criteria C2 (renal pelvis/calyxes) and C3 (proximal ureters), the excretory phase was preferred (p < 0.001 and p = 0.034, respectively) (Fig. 2).
Distribution of number of scores (annotation above each bar) allocated to each criterium assessed by readers in a Computed Tomography Urography (CTU). All differences between phases are significant for Criterion 1 to 4. All differences between phases were non-significant for Criterion C5 to C7 (Additional file 1: Table S1)
Assessment of pathology
For the detection of renal and other abdominal pathologies, only marginal and not statistically significant differences in scores were found when comparing the native phase with nephrographic and excretory phases. Similarly, the scores for incidental findings in all three phases were not significantly different. For all three pathology categories, the number of inconclusive scores were very low suggesting that normality and pathology could be assessed in all three phases with marginal differences in scores. The native phase had a higher number of probably normal examination scores compared to the other two phases. None of these differences were statistically significant.
Certainty scores
Image criteria
High certainty scores are seen for criteria C1 (renal parenchyma) and C4 (renal arteries) in favor of the nephrographic phase, when comparing native to nephrographic phase (p < 0.001). Similar results are seen for comparison between nephrographic and excretory phase with significant differences in favor of the nephrographic phase (p < 0.001). However, when comparing native and excretory phases, certainty for criterion C1 (renal parenchyma) was statistically significantly higher for the native phase whereas no significant difference was found for criterion C4 (renal arteries). Criteria C2 (renal pelvis/calyxes) and C3 (proximal ureters) showed significantly higher certainty scores in the contrast-enhanced phases when comparing native phase to nephrographic and excretory phases (p < 0.001). Comparisons between nephrographic and excretory phases show a highly significant difference in scores in favor of excretory phase for criterion C2 (renal pelvis/calyxes) (p < 0.001) and a significant difference in scores for criterion C3 (proximal ureters) (p = 0.021).
Pathology
Significantly high certainty scores in favor of the contrast enhanced phases are obtained for C5 (renal pathology) (p < 0.05), when comparing native to nephrographic and excretory phases, however, there was no significant difference between the two contrast-enhanced phases. Marginal not significant differences in medium and high certainty scores are observed for Criteria C6 (abdominal pathology) and C7 (incidental findings) when comparing all three phases with each other. Except for determination of C7 (incidental findings), which was statistically significant in favor of the nephrographic phase (p < 0.01), when native and nephrographic phases were compared, all other differences in scores between phases were not significant.
Discussion
The present study showed that the contrast-enhanced phases were considered significantly better for determination of renal pathology. This is not an unusual finding as the acquisition delays after contrast injection are designed to render anatomical features in the best possible way. However, the marginal differences in scores when assessing pathology in the three categories were not significant, with very few inconclusive scores suggesting that it was possible to determine whether the examination was normal or pathological in all three phases. This is also demonstrated by the larger number of high and medium certainty scores for all three categories (Fig. 3). The LDCT is one of many alternatives, that could possibly be used to differentiate between normal and pathological in order to reduce the radiation burden in patients with negative outcomes and those who are more sensitive to ionising radiation.
Distribution of number of certainty scores (annotation above each bar) allocated to each criterion assessed by readers in a Computed Tomography Urography (CTU) protocol. The scores 1 and 5 were grouped for high certainty, scores 2 and 4 for medium certainty and a score of 3 for low certainty. Significance values are presented in supplementary data (Additional file 2: Table S2)
There are studies that have evaluated the effect of iterative reconstruction on dose reduction in patients presented with acute abdominal pain [11, 12]. Lee et al. [12] concluded that despite subjective differences in image quality between full-dose and half-dose images, the diagnostic performance is maintained for the low-dose images with the exception of lesion detection at sub-centimeter size. Poletti et al. [11] concluded that low-dose imaging can be achieved in non-obese patients. However, these studies were performed with contrast enhancement which, combined with dose reduction properties of IR, maintains the diagnostic performance of the low-dose protocol when compared to contrast-enhanced standard-dose protocol.
In order to optimize the use of CT in urinary tract investigations, there are several methods that can be used to reduce dose in abdominal CT such as modifying scanning parameters [29], using a combination of standard-dose and low-dose phases in a CTU protocol [30] and reducing the number of acquisitions and scan lengths [14]. As the implementation of the LDCT has been very slow, this study provides an insight into the possible applicability of this protocol in clinical practice to reduce radiation dose for adolescents and children, as well as patients that require repetitive imaging.
Our CTU protocol is optimized using automatic dose modulation as well as iterative reconstruction and combination of low-dose and standard-dose series in concurrence with Dahlman et al. [30] who obtained significant dose reductions, in the unenhanced and excretory phases, achieved when combined with one normal-dose phase. However, the dilemma of reducing the radiation burden in certain patient groups still remains. Some institutions have adopted a work-flow routine in order to minimize the number of phases required by viewing the low-dose series to determine further need for imaging (Magnusson A, CT lecture, Larvik, 2018, personal communications). However due to logistics this is not always possible especially for referrals from the emergency department where time is of essence.
One of the sites at our institution extensively adopted the LDCT in 2012, to meet the urologists needs for imaging after Extracorporal Shockwave Lithotripsy (ESWL) as well as follow-up imaging of patients with nephro- and uro-lithiasis. With growing expertise and increasing diagnostic confidence, the comfort zone boundaries were broadened and the LDCT use was extended to diagnostics of acute flank pain and acute abdomen for several indications such as diverticulitis, appendicitis and renal calculi [22] in concurrence with Hamimi et al. [31] who studied the efficiency of low-dose technique using an effective mAs of 50 (very similar to our LDCT protocol), in diagnosis of renal calculi and concluded that it was crucial in the management of renal stone disease in the acute setting. Both Lee et al. [12] and Poletti et al. [11] demonstrated the use of LDCT in acute abdominal pain diagnoses. An LDCT protocol can be considered as a valuable tool in acute abdominal pain evaluation as it allows for many possible differential diagnoses, but radiologists should also be aware of its limitations [22].
The statistical method (VGR) that we used, allowed us to analyze the three phases in the same analysis, with subsequent pair-wise comparisons. VGR also has the ability to let the researcher estimate the potential dose reduction resulting from changes in the imaging protocol. However, the design of this retrospective study does not permit such an analysis.
There is one major study limitation: due to the retrospective nature of our study we failed to separate the individual effects of dose and contrast enhancement on image quality. Ideally to determine these effects individually, all three phases should have been compared at different dose levels which would also allow for estimation of the potential dose reduction for each individual phase [32]. However, this would have increased the radiation burden for these patients.
Conclusion
Visualisation of renal anatomy in the three phases were as expected with each post-contrast phase showing favourable scores compared to the native phase. No statistically significant differences in the assessment of pathology were found between the three phases.
Since many certainty scores were in the high and medium categories, the LDCT seems to be sufficient to differentiate between normal and pathological examinations. In order to reduce the radiation burden in adolescents and children, as well as patients with negative outcomes and those that require repetitive imaging, the LDCT could be considered a suitable alternative as a first line imaging method. However, radiologists should be aware of its limitations.
Availability of data and materials
The datasets used and/ or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- AHARA:
-
As high as reasonably achievable
- ALARA:
-
As low as reasonably achievable
- CTDIvol :
-
Computed Tomography Dose Index
- CTU:
-
Computed Tomography Urography
- DLP:
-
Dose Length Product
- ESWL:
-
Extracorporal Shock Wave Lithotripsy
- kV:
-
kilovolt
- LDCT:
-
Low-dose Computed Tomography
- mAs:
-
milliampere seconds
- mGy:
-
milligray
- MRI:
-
Magnetic Resonance Imaging
- mSv:
-
millisievert
- SCP:
-
Standardized Care Pathway
- SD:
-
Standard deviation
- SSDE:
-
Size Specific Dose Estimate
References
Sudah M. Modern Imaging of the Upper Urinary Tract [Dissertations in Health Sciences]: Publications of the University of Eastern Finland. Kuopio: University of Eastern Finland; 2016. http://epublications.uef.fi/pub/urn_isbn_978-952-61-2083-6/urn_isbn_978-952-61-2083-6.pdf.
IAEA Safety standards - Radiation protection and safety of radiation sources - International Basic Safety standards. Vienna: International Atomic Energy Agency; 2014. https://www-pub.iaea.org/MTCD/Publications/PDF/Pub1578_web-57265295.pdf.
Kalra M, Sodickson AD, Mayo-Smith WW. CT radiation key concepts for gentle and wise use. Radiographics. 2015;35:1706–21.
Cowan NC. CT urography for hematuria. Nat Rev Urol. 2012;9(4):218–26.
Bhatt K, Monga M, Remer EM. Low-dose computed tomography in the evaluation of urolithiasis. J Endourol. 2015;29(5):504–11.
Standardiserade vårdförlopp cancer-urinblasa-ovre-urinvagarna www.cancercentrum.se Regionala cancercentrum; 2018 [updated 27th February 2018]. https://www.cancercentrum.se/globalassets/cancerdiagnoser/urinvagar/urinblase%2D%2Doch-urinrorscancer/vardforlopp/svf-cancer-urinblasa-ovre-urinvagarna.pdf. Accessed 15 Dec 2018.
Muthulakshmi M, Gopalakrishnan S. Study on urinary tract infection among females of reproductive age group in a rural area of Kancheepuram district, Tamil Nadu. Int J Community Med Public Health. 2017;4(10):3915-21.
Van Der Molen AJ, Cowan NC, Mueller-Lisse UG, Nolte-Ernsting CC, Takahashi S, Cohan RH. CT urography: definition, indications and techniques. A guideline for clinical practice. Eur Radiol. 2008;18:4–17.
Mathews JD, Forsythe AV, Brady Z, Butler MW, Goergen SK, Byrnes GB, et al. Cancer risk in 680,000 people exposed to computed tomography scans in childhood or adolescence: data linkage study of 11 million Australians. BMJ. 2013;346:f2360.
Kaza RK, Platt JF, Goodsitt MM, Al-Hawary MM, Maturen KE, Wasnik AP, et al. Emerging techniques for dose optimization in abdominal CT. Radiographics. 2014;34:4–17.
Poletti PA, Becker M, Becker CD, Halfon Poletti A, Rutschmann OT, Zaidi H, et al. Emergency assessment of patients with acute abdominal pain using low-dose CT with iterative reconstruction: a comparative study. Eur Radiol. 2017;27:3300–9.
Lee KH, Shim YS, Park SH, Choi SJ, Pak SY, Cheong H. Comparison of standard-dose and half-dose dual-source abdominopelvic CT scans for evaluation of acute abdominal pain. Acta Radiol. 2018;0:1–9.
Safety and efficacy of computed tomography (SECT): A broad perspective (CT Safety and Efficacy) Euratom call 2003. 2008 http://www.biophysicssite.com/Documents/SECT2008/DeliverableWP1D5F.pdf. Accessed 5 Feb 2019.
Sung MK, Singh S, Kalra MK. Current status of low dose multi-detector CT in the urinary tract. World J Radiol. 2011;3:256–65.
Rodger F, Roditi G, Aboumarzouk OM. Diagnostic accuracy of low and ultra-low dose CT for identification of urinary tract stones: a systematic review. Urol Int. 2018;100:375–85.
Kim K, Kim YH, Kim SY, Kim S, Lee YG, Kim KP, et al. LDCT for evaluating appendicitis. N Engl J Med. 2012;366:1596–605.
Bonn-call-for-action 10 actions to improve Radiation Protection. Vienna: IAEA; 2014. https://www.who.int/ionizing_radiation/medical_exposure/bonncallforaction2014.pdf?ua=1. Accessed 15 Dec 2018.
IAEA Bulletin 52-2-2011 Division of Public Information: IAEA; 2011 https://www.iaea.org/sites/default/files/bull52-2-february2011.pdf. Accessed 10 Feb 2019.
Dose reduction in CT while maintaining diagnostic confidence: a feasibility/demonstration study. Vienna: IAEA; 2009. ISNN 1011-4289 https://www-pub.iaea.org/MTCD/Publications/PDF/te_1621_web.pdf. Accessed 7 Feb 2019.
Gervaise A, Gervaise-Henry C, Pernin M, Naulet P, Junca-Laplace C, Lapierre-Combes M. How to perform low-dose computed tomography for renal colic in clinical practice. Diagn Interv Imaging. 2016;97:393–400.
Weisenthal K, Karthik P, Shaw M, Sengupta D, Bhargavan-Chatfield M, Burleson J, et al. Evaluation of kidney stones with reduced-radiation dose CT: Progress from 2011-2012 to 2015-2016-not there yet. Radiology. 2018;286:581–9.
Halilic S and Kämmerling N. Värdet av DT-buköversikt som primär undersökning vid akut buk smärta. Linköping University Electronic Press, Linköping, 2016. http://liu.diva-portal.org/smash/get/diva2:1304503/FULLTEXT01.pdf. Accessed 12 Apr 2019.
Wang J-H, Shen S-H, Huang S-S, Chang C-Y. Prospective comparison of unenhanced spiral computed tomography and intravenous urography in the evaluation of acute renal colic. J Chin Med Assoc. 2008;71(1):30–6.
Alshamari M, Norrman E, Geijer M, Jansson K, Geijer H. Diagnostic accuracy of low-dose CT compared with abdominal radiography in non-traumatic acute abdominal pain: prospective study and systematic review. Eur Radiol. 2016;26(6):1766–74.
The report of AAPM task group 204. Size-specific dose estimates in paediatric and adult body CT examinations, 2011. https://www.aapm.org/pubs/reports/RPT_204.pdf. Accessed 6 June 2019.
Boos J, Kropil P, Bethge OT, Aissa J, Schleich C, Sawicki LM, et al. Accuracy of size-specific dose estimate calculation from center slice in computed tomography. Radiat Prot Dosim. 2018;178(1):8–19.
Smedby Ö, Fredrikson M. Visual grading regression: analysing data from visual grading experiments with regression models. Br J Radiol. 2010;83(993):767–75.
Saffari SELA, Fredrikson M, Smedby O. Regression models for analyzing radiological visual grading studies--an empirical comparison. BMC Med Imaging. 2015;15:49):1–10.
Dyakov I, Alamin M, Groudeva V, Vassileva J, Stoinova V, Hadjidekov V. Optimisation of CT procedures in two radiology departments. Phys Med. 2014;30:e17.
Dahlman P, Van der Molen AJ, Magnusson M, Magnusson A. How much dose can be saved in three-phase CT urography? A combination of normal-dose corticomedullary phase with low-dose unenhanced and excretory phases. AJR Am J Roentgenol. 2012;199:852–60.
Hamimi A, El Azab M. MSCT renal stone protocol; dose penalty and influence on management decision of patients: is it really worth the radiation dose? Egypt J Radiol Nucl Med. 2016;47:319–24.
Kataria B, Althen JN, Smedby O, Persson A, Sokjer H, Sandborg M. Assessment of image quality in abdominal CT: potential dose reduction with model-based iterative reconstruction. Eur Radiol. 2018;28(6):2464–73.
Acknowledgements
We thank our participating radiologists, Anki Pozson, Jenny Öman and Senija Halilic for grading the images.
Funding
This work was supported by ALF- and LFoU-grants from Region Östergötland and the Medical Faculty at Linköping University. The funding bodies have had no role in the design of the study and collection, analysis, interpretation of data and in writing the manuscript.
Author information
Authors and Affiliations
Contributions
All authors (BK, JNA, ÖS, AP, HS and MS) have contributed to the conception, design of the work and interpretation of the data. BK has been responsible for acquisition, drafting and revision of the manuscript in collaboration with the other authors and ÖS for performing the statistical analysis. All authors have contributed to the final manuscript and approved it.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional research committee (Linköping University) and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Our regional Ethical Review Board (EPN, Linköping) approved this retrospective study, and the requirement for informed consent was waived.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Additional files
Additional file 1:
Table S1. Scores for each of the criteria. Significance of differences between phases tested with mixed-effects ordinal logistic regression, with pairwise comparisons using Bonferroni correction. (DOCX 63 kb)
Additional file 2:
Table S2. Certainty scores for each of the criteria. Significance of differences between phases tested with mixed-effects ordinal logistic regression in phase comparisons using Bonferroni correction. (DOCX 55 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Kataria, B., Nilsson Althén, J., Smedby, Ö. et al. Image quality and pathology assessment in CT Urography: when is the low-dose series sufficient?. BMC Med Imaging 19, 64 (2019). https://doi.org/10.1186/s12880-019-0363-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12880-019-0363-z