Abstract
Background
Mycobacterium fortuitum complex is a group of rapidly growing nontuberculous mycobacteria (NTM) associated with skin and soft-tissue infections after surgery or trauma. Treatment of NTM is challenging, due to resistance to multiple antimycobacterial agents. Bedaquiline is a diarylquinoline that inhibits mycobacterial ATP-synthase. The drug has recently been approved for the treatment of multidrug-resistant tuberculosis and evidence of its in vitro efficacy against NTM, including Mycobacterium fortuitum complex, has been published.
Case presentation
A 20-year-old Caucasian woman with chronic skin and soft tissue infection in the lower leg following a traffic accident in Vietnam underwent a tedious journey of healthcare visits, hospital admissions, empiric antimicrobial treatments, surgical debridement and plastic reconstruction before definite diagnosis of Mycobacterium fortuitum complex-infection was established by culture from a tissue biopsy and targeted antimycobacterial therapy was administered. Histopathological examination revealed granulomatous purulent inflammation, which strongly supported the diagnosis. Genotypic identification was performed and broth microdilution for susceptibility testing showed macrolide resistance. Five weeks of induction treatment with intravenous amikacin, imipenem / cilastin, and oral levofloxacin was administered, followed by all-oral treatment with bedaquiline combined with levofloxacin for four months, which was well-tolerated and led to persistent healing with scars but without signs of residual infection.
Conclusions
Bedaquiline is a promising novel agent for NTM treatment, although clinical data are limited and trials evaluating efficacy, safety, and resistance of bedaquiline are required. To our knowledge, this is the first reported case of successful in vivo use of bedaquiline for a skin and soft tissue infection caused by Mycobacterium fortuitum complex.
Similar content being viewed by others
Background
Nontuberculous mycobacteria (NTM) are mycobacteria other than Mycobacterium tuberculosis or Mycobacterium leprae that can ubiquitously be found in the environment [1,2,3]. Depending on the growth rate, NTM can be divided into slowly and rapidly growing mycobacteria (RGM) [1, 4]. The incidence of NTM-related infections is increasing, slowly emerging as a global public health problem [5, 6]. Amongst these RGM, Mycobacterium fortuitum complex (MFC) species are predominantly associated with extrapulmonary disease, causing localized skin-, soft tissue-, wound-, or bone-infections following traumatic injuries or surgery [2]. Clinically, the infection may present with subcutaneous nodules and localized recurrent abscesses that often drain spontaneously. Disseminated infections rarely appear and affect predominantly immunocompromised patients [7]. Diagnosis is often delayed as strong suspicion of RGM-infection is inevitable to initiate special culturing from drainage material or ideally a tissue specimen [1]. Accurate genotypic identification of the isolated RGMs to species level is highly recommended, making molecular methods such as DNA hybridization strip assays and gene sequencing essential [1, 8, 9]. As clinical studies of treatment regimens for RGM-infections have not been performed yet [4, 10], no definite standard therapy for MFC-infections has been established to date [4]. Published treatment recommendations and guidelines are mainly based on case studies, clinical experience of experts and antimicrobial susceptibility testing [1, 10]. As with all other NTM-species, MFC-isolates do generally not respond to first-line antituberculous drugs and may be naturally or secondarily resistant to several antimicrobial agents [11,12,13]. MFC-susceptibility to amikacin, fluoroquinolones, sulfonamides, imipenem, linezolid, cefoxitin, doxycycline, minocycline, and clarithromycin [1] has been reported. However, there is evidence of a high variability in susceptibility to these antimicrobials among different isolates [12]. While macrolides are key drugs for treatment of NTM-related pulmonary disease, in MFC-isolates macrolide resistance via an inducible erythromycin methylase gene (erm) was identified despite in vitro susceptibility. Thus, the use of macrolides needs careful evaluation [1, 14]. Due to the intrinsic resistance to various antibiotics, clinical treatment of NTM-related infections is challenging [11, 15, 16]. Especially drug-resistant isolates require potent, well-tolerated and possibly novel antimicrobial agents [11]. Bedaquiline is a diarylquinoline that acts by inhibiting mycobacterial ATP synthase [17, 18]. The agent was FDA-approved for the treatment of multidrug-resistant tuberculosis (MDR-TB) in 2012 [17, 19] and was shown to exhibit activity against several NTM species, including MFC, in vitro [11, 17, 20,21,22]. Consequently, the use of bedaquiline in combination regimens has been proposed to be a promising therapeutic strategy for NTM-related diseases [11, 15, 23].
Case presentation
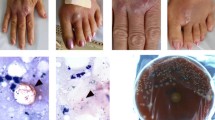
This case presents a 20-year-old German female patient diagnosed with a chronic wound infection caused by MFC following a traffic accident in Vietnam (see Fig. 1 for a timeline). The patient was travelling through Vietnam when she was involved in a motorbike accident in February 2019. She was taken to the emergency department of a hospital in Hanoi where she presented with a large wound on the right leg. At the time of admission, she was complaining of severe pain in her back and right foreleg, as well as headache and nausea. The patient reported no other pre-existing medical conditions. Radiograph of the lower leg did not show any signs of fracture, and cranio-encephalic traumatic lesions were excluded in the cranial computed tomography (CT) scan. However, a lumbosacral spine CT scan revealed stable fractures of the thoracic vertebrae (T4 / 5). The patient underwent surgical debridement and suture of the leg wound the next day (see Fig. 2a for a representative photograph) and was treated with metronidazole and ceftriaxone over four days. She was discharged eight days after admission, transported to Germany and immediately admitted to a local hospital. At that time, her right lower leg was swollen, but lacked signs of surgical site infection. The patient was discharged three days afterwards, and dressing changes were subsequently performed in an outpatient-setting by her general practitioner and in local hospitals. Approximately five weeks after the accident, wound inflammation was observed for the first time, with redness and warmth around the suture site and dehiscent areas of the wound edges. However, no signs of systemic infection such as fever, increased leukocyte count, or C-reactive protein levels were apparent. Three days later, she was re-admitted to a local hospital with the diagnosis of wound infection. Surgeons described purulent exudate and a subcutaneous firm tumor measuring 2 × 4 cm in size (Fig. 2b) with spontaneous drainage (Fig. 2c). The patient was discharged, and oral antibiotic treatment with amoxicillin / clavulanic acid was continued for two weeks on suspicion of soft tissue infection. In the next follow-up presentation, two new lesions with serous drainage (Fig. 2d) were documented. Of note, several swabs failed to show growth of any pathogenic bacteria on standard microbiological examination. Magnetic resonance imaging (MRI) was performed, showing neither bone involvement, nor a remaining foreign body underlying the chronic infection. The patient was transferred to our university hospital where plastic surgical debridement was performed. On histopathological examination, chronic granulomatous purulent inflammation was documented. The lesion was covered by skin grafting and local flap surgery three days later, while intravenous antibiotic treatment with ampicillin / sulbactam was continued over ten days (Fig. 2e). However, the wound failed to heal, and persistent infected cutaneous lesions were noted on follow-up outpatient appointments (Fig. 2f). MFC could then be grown from a biopsy on Columbia agar on culture day two. Species identification via MALDI-TOF and subsequent genotypic identification using a DNA hybridization strip assay (GenoType Mycobacterium CM, Hain Lifescience / Bruker) [9] was performed and Infectious Diseases specialist consultation was initiated. Minimal inhibitory concentration (MIC)-determination via broth microdilution revealed susceptibility to amikacin and fluoroquinolones, and intermediate sensitivity to imipenem, while the isolated strain exhibited resistance toward linezolid, doxycycline / minocycline, trimethoprim-sulfamethoxazole, clarithromycin, and cefoxitin (based on CLSI-breakpoints, M24-A2 2011). In addition, susceptibility testing for the second line antimycobacterial agents clofazimine, bedaquiline, and delamanid was performed (Table 1). Though official clinical breakpoints for susceptibility testing of NTM for those agents have not been defined yet [4, 15], the results pointed towards resistance against delamanid and susceptibility for bedaquiline considering proposed European Committee on Antimicrobial Susceptibility Testing (EUCAST) breakpoints for Mycobacterium tuberculosis (version 10.0) and previous studies [24].
Timecourse of the antimicrobial treatments, surgical procedures, diagnostics and hospital admissions. Links to the respective photos depicted in Fig. 2 have been included. CT, computer tomography; d, day; GER, Germany; LH, local hospital; metron., metronidazole; MRI, magnet resonance imaging; UH, university hospital; US, ultrasound; Nov., November; w, weeks; XR, Xray
Serial images of the patient’s right foreleg. (a) Photograph taken four days after the traffic accident at a hospital in Hanoi following surgical debridement and suture of the wound. (b, c) Six weeks after the accident a firm subcutaneous nodule was noted (b), which drained spontaneously the next day (c). The soft tissue infection persisted despite four weeks of empiric antimicrobial treatment (sixth to tenth week after the accident) (d) leading to the decision to perform surgical debridement and subsequent skin grafting as well as local flap surgery 11 weeks after the trauma (e). (f) A tissue biopsy was obtained 19 weeks after the accident when abscesses and nodules reappeared within a few weeks upon surgical debridement and intravenous empiric antibiotic therapy. (g-i) After 20 weeks, antimycobacterial treatment was initiated following susceptibility testing of the identified Mycobacterium fortuitum: Photographs show the status before (g), eight weeks after antimycobacterial therapy (h) and upon completion of the four-month therapy regimen (i). The patient was followed-up, continuous improvement was noticed nine months (j), 12 months (k) and 14 months after the initial accident (l)
The table lists Minimal inhibitory concentrations (MIC) of fourteen distinct antimicrobial substances tested on the clinical MFC isolate. Susceptibility was interpreted using CLSI breakpoints (M24-A2 2011), if available. S = sensitive; R = resistant; I = intermediate.
The patient was transferred to the Infectious Diseases department for further diagnostics and treatment. Due to persistent back pain and mild paresthesia in the peripheral extremities, a whole spine MRI was performed, which showed no sign of disseminated infection. In addition, chest radiograph and abdominal ultrasound did not reveal any abnormal findings and the patient tested negative for Human Immunodeficiency Virus (HIV)-infection. Based on the American Thoracic Society / Infectious Diseases Society of America (ATS / IDSA) guideline and susceptibility testing results, antibiotic therapy was initiated with amikacin (initially 10–15 mg / kg of body weight once a day, intravenously, subsequently adjusted to serum level), levofloxacin (500 mg once a day, orally), and imipenem / cilastatin (0.5 g / 0.5 g twice a day, intravenously) (Fig. 2g). Treatment was generally tolerated well, however, nausea occasionally required symptomatic antiemetic therapy. Also, the patient reported to feel dizzy when exposed to sunlight. After five weeks and upon clinical improvement as well as approval of health insurance coverage and written informed consent on off-label use, the treatment was changed to an oral regimen consisting of levofloxacin (500 mg once a day, orally) combined with bedaquiline used at doses that have been recommended for MDR TB [25] (14 days of 400 mg once a day orally, followed by 200 mg three times per week). The patient could be discharged and was followed up as an outpatient in our Infectious Diseases clinic. Continuous clinical improvement was noted (Fig. 2h). Apart from persisting nausea, the patient did not report any notable treatment-related side-effects. Liver enzymes, renal function and blood count were regularly checked and remained unremarkable. Upon completion of four months of treatment the former wound showed persistent scarring without clinical or radiological signs of infection (Fig. 2i). Since then, the appearance of the scar has continuously improved (Fig. 2j, k) and five months after completion of therapy, which is 14 months after the initial traffic accident, there is no evidence of infection (Fig. 2l).
Discussion and conclusions
NTM-related infections remain difficult to treat as the range of effective agents is limited and resistance may develop despite combination therapy [15, 16]. The international ATS / IDSA-guidelines recommend at least four months of therapy with a minimum of two drugs with in vitro activity for severe soft-tissue infection caused by MFC, if bone involvement or disseminated disease is absent [1]. For treatment induction, a parenteral regimen consisting of at least two active agents should be administered for two to six weeks. The guidelines recommend to combine an aminoglycoside (preferably amikacin in MFC-infection) with two of the following antibiotics: cefoxitin, imipenem, or levofloxacin [26]. In our case, amikacin and levofloxacin had to be combined with imipenem, as imipenem was categorized intermediate in susceptibility testing (MIC 8 mg / L, Table 1). Prolonged use of amikacin should be avoided, as cumulative dose and treatment duration are risk factors for adverse effects, most predominantly ototoxicity [27,28,29]. Parenteral treatment induction might be switched to oral combination therapy if compatible with the susceptibility patterns [26], which also facilitates the management of the disease in an outpatient setting [1]. Antimycobacterial agents with sufficient oral bioavailability are limited, particularly in macrolide-resistant MFC-isolates [1]. In the presented case, fluoroquinolones were the only licensed oral treatment option the pathogen was sensitive to. This highlights the importance of sensitivity screening for novel agents, as there appears to be an unmet demand for antimicrobial drugs against multidrug-resistant NTM-isolates [10]. For MDR-TB, several novel and repurposed drugs have successfully been tested and show promising therapeutic effects [30]. Among these drugs, bedaquiline, delamanid and clofazimine have shown activity against NTM in vitro [11, 15, 23, 31,32,33]. Clofazimine and delamanid were found to be effective against slowly growing mycobacteria (SGM) in vitro [31, 32, 34]. However, they seem to be less active against RGM [11, 34, 35]. In contrast, bedaquiline exhibited clear inhibitory effects against various species of SGM and RGM, including MFC in different studies [11, 12, 17, 20, 33, 36]. Bedaquiline has mostly been described as bacteriostatic but bactericidal activity for several MFC-strains has been reported [20, 22, 36]. Data on MIC-distribution of different NTM species are limited and there are no established clinical breakpoints. For MFC, MICs between 0.007 and 0.25 have been described [20, 36, 37].
In vivo data and clinical experience of bedaquiline in treatment regimens for NTM-related diseases are scarce and restricted to distinct species [11]. Though not consistent, significant activity of bedaquiline has been reported in mice and zebrafish models for Mycobacterium abscessus, another species of RGM [37,38,39]. Human data on bedaquiline is scarce and explicitly limited to a few case studies on the use of bedaquiline in pulmonal disease with Mycobacterium abscessus, Mycobacterium avium complex and Mycobacterium intracellulare [23, 39, 40]. However, due to the paucity of data on MFC-infection and novel antimycobacterial drugs, we sought to extrapolate some knowledge from closely related species in order to optimize the oral treatment-regimen for the patient.
Although bedaquiline has not been extensively studied in NTM disease, the drug’s adverse effects have been investigated in various clinical trials for MDR-TB and it has been safely used for even over 18 months [41,42,43]. However, the long plasma half-life of four to five months and known QTc interval prolongation require careful clinical evaluation [41, 44].
Emergence of resistance is a key problem in NTM treatment. Zweijpfenning et al. have recently published a case where an increase in the bedaquiline MIC led to a resistant phenotype (or sub-population) of Mycobacterium avium complex resulting in failure of a bedaquiline-based treatment regimen [45]. We therefore believe that the combination with a highly active flourochinolon to efficiently suppress mycobacterial growth and the preceding intravenous induction therapy are key elements of successful treatment. This combination, however, needs careful evaluation and monitoring of drug interactions, such as QTc interval prolongation. Particularly in individual NTM-drug-regimens based on susceptibility testing a complete check of drug-interactions is important. Further, it will be warranted to monitor bedaquiline resistance through multiple MIC testing in future studies, especially in patients with treatment failure or relapse [46]. In light of preclinical and clinical data and considering the rising incidence of NTM-infections and emerging MDR, bedaquiline may be a promising therapeutic agent as part of combination-regimens to treat NTM infections [15, 23]. However, studies on clinical efficacy are required to confirm this option in the management of MFC-infections. Furthermore, additional research will be needed to potentially establish valid breakpoints for susceptibility testing, to understand resistance mechanisms and to design bedaquiline-containing treatment regimens as synergy for combined treatment with clofazimine and bedaquiline against the RGM Mycobacterium abscessus has been shown in vitro [22]. Thus, it will be remarkably interesting to test bedaquiline in combination-therapy to identify promising, potentially synergistically acting combination regimens to maximize its effect.
In summary, to our knowledge, we report the first case of safe and successful in vivo use of oral bedaquiline-fluoroquinolone-combination-therapy in a chronic wound infection caused by MFC. Bearing in mind that clinical studies will be required to gain data on efficacy, safety, and outcome, this case report encourages testing of MFC isolates for susceptibility to bedaquiline and, upon careful clinical evaluation, use of the agent in combination regimens as oral treatment option for MFC and other NTM-associated diseases.
Availability of data and materials
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
Abbreviations
- ATS:
-
American Thoracic Society
- CLSI:
-
Clinical Laboratory and Standards Institute
- CT:
-
Computed tomography
- EUCAST:
-
European committee on antimicrobial susceptibility
- FDA:
-
Food and Drug Administration
- HIV:
-
Human immunodeficiency virus
- IDSA:
-
Infectious Diseases Society of America
- MFC:
-
Mycobacterium fortuitum complex
- MDR-TB:
-
Multidrug-resistance tuberculosis
- MIC:
-
Minimal inhibitory concentration
- MRI:
-
Magnetic Resonance Imaging
- NTM:
-
Nontuberculous mycobacteria
- QTc:
-
Corrected QT interval
- RGM:
-
Rapidly growing mycobacteria
- SGM:
-
Slowly growing mycobacteria
References
Griffith DE, Aksamit T, Brown-Elliott BA, Catanzaro A, Daley C, Gordin F, Holland SM, Horsburgh R, Huitt G, Iademarco MF, et al. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med. 2007;175(4):367–416.
Brown-Elliott BA, Wallace RJ Jr. Clinical and taxonomic status of pathogenic nonpigmented or late-pigmenting rapidly growing mycobacteria. Clin Microbiol Rev. 2002;15(4):716–46.
Covert TC, Rodgers MR, Reyes AL, Stelma GN Jr. Occurrence of nontuberculous mycobacteria in environmental samples. Appl Environ Microbiol. 1999;65(6):2492–6.
Brown-Elliott BA, Philley JV. Rapidly Growing Mycobacteria. Microbiol Spectrum. 2017;5(1):TNMI7-0027-2016.
Donohue MJ. Increasing nontuberculous mycobacteria reporting rates and species diversity identified in clinical laboratory reports. BMC Infect Dis. 2018;18(1):163.
Wentworth AB, Drage LA, Wengenack NL, Wilson JW, Lohse CM. Increased incidence of cutaneous nontuberculous mycobacterial infection, 1980 to 2009: a population-based study. Mayo Clin Proc. 2013;88(1):38–45.
Chetchotisakd P, Mootsikapun P, Anunnatsiri S, Jirarattanapochai K, Choonhakarn C, Chaiprasert A, Ubol PN, Wheat LJ, Davis TE. Disseminated infection due to rapidly growing mycobacteria in immunocompetent hosts presenting with chronic lymphadenopathy: a previously unrecognized clinical entity. Clin Infect Dis. 2000;30(1):29–34.
van Ingen J. Diagnosis of nontuberculous mycobacterial infections. Semin Respir Crit Care Med. 2013;34(1):103–9.
Richter E, Rusch-Gerdes S, Hillemann D. Evaluation of the GenoType Mycobacterium assay for identification of mycobacterial species from cultures. J Clin Microbiol. 2006;44(5):1769–75.
Misch EA, Saddler C, Davis JM. Skin and soft tissue infections due to Nontuberculous mycobacteria. Curr Infect Dis Rep. 2018;20(4):6.
Yu X, Gao X, Li C, Luo J, Wen S, Zhang T, Ma Y, Dong L, Wang F, Huang H. In Vitro Activities of Bedaquiline and Delamanid against Nontuberculous Mycobacteria Isolated in Beijing, China. Antimicrob Agents Chemother. 2019;63(8):00031–19.
Shen Y, Wang X, Jin J, Wu J, Zhang X, Chen J, Zhang W. In vitro susceptibility of Mycobacterium abscessus and Mycobacterium fortuitum isolates to 30 antibiotics. Biomed Res Int. 2018;2018:4902941.
Woods GL, Brown-Elliott BA, Conville PS, Desmond EP, Hall GS, Lin G, Pfyffer GE, Ridderhof JC, Siddiqi SH, Wallace RJ, Jr. et al: CLSI Standards: Guidelines for Health Care Excellence. In: Susceptibility Testing of Mycobacteria, Nocardiae, and Other Aerobic Actinomycetes. edn. Edited by nd. Wayne: Clinical and Laboratory Standards Institute Copyright (c)2011 Clinical and Laboratory Standards Institute; 2011.
Nash KA, Zhang Y, Brown-Elliott BA, Wallace RJ Jr. Molecular basis of intrinsic macrolide resistance in clinical isolates of Mycobacterium fortuitum. J Antimicrob Chemother. 2005;55(2):170–7.
Pang Y, Zheng H, Tan Y, Song Y, Zhao Y. In Vitro Activity of Bedaquiline against Nontuberculous Mycobacteria in China. Antimicrob Agents Chemother. 2017:61(5):02627–16.
Kasperbauer SH, De Groote MA. The treatment of rapidly growing mycobacterial infections. Clin Chest Med. 2015;36(1):67–78.
Brown-Elliott BA, Woods GL. Antimycobacterial Susceptibility Testing of Nontuberculous Mycobacteria. J Clin Microbiol. 2019:57(10):00834–19.
Huitric E, Verhasselt P, Koul A, Andries K, Hoffner S, Andersson DI. Rates and mechanisms of resistance development in Mycobacterium tuberculosis to a novel diarylquinoline ATP synthase inhibitor. Antimicrob Agents Chemother. 2010;54(3):1022–8.
Mahajan R. Bedaquiline: first FDA-approved tuberculosis drug in 40 years. Int J Appl Basic Med Res. 2013;3(1):1–2.
Aguilar-Ayala DA, Cnockaert M, Andre E, Andries K, Gonzalez YMJA, Vandamme P, Palomino JC, Martin A. In vitro activity of bedaquiline against rapidly growing nontuberculous mycobacteria. J Med Microbiol. 2017;66(8):1140–3.
Brown-Elliott BA, Wallace RJ Jr. In Vitro Susceptibility Testing of Bedaquiline against Mycobacterium abscessus Complex. Antimicrob Agents Chemother. 2019;63(2):01919–18.
Ruth MM, Sangen JJN, Remmers K, Pennings LJ, Svensson E, Aarnoutse RE, Zweijpfenning SMH, Hoefsloot W, Kuipers S, Magis-Escurra C, et al. A bedaquiline/clofazimine combination regimen might add activity to the treatment of clinically relevant non-tuberculous mycobacteria. J Antimicrob Chemother. 2019;74(4):935–43.
Philley JV, Wallace RJ Jr, Benwill JL, Taskar V, Brown-Elliott BA, Thakkar F, Aksamit TR, Griffith DE. Preliminary results of Bedaquiline as salvage therapy for patients with Nontuberculous mycobacterial lung disease. Chest. 2015;148(2):499–506.
Jagannath C, Reddy MV, Kailasam S, O'Sullivan JF, Gangadharam PR. Chemotherapeutic activity of clofazimine and its analogues against Mycobacterium tuberculosis. In vitro, intracellular, and in vivo studies. Am J Respir Crit Care Med. 1995;151(4):1083–6.
Bedaquiline (Sirturo) for multidrug-resistant tuberculosis. Medical Lett Drugs Ther. 2013;55(1423):66–8.
Wallace RJ Jr, Swenson JM, Silcox VA, Bullen MG. Treatment of nonpulmonary infections due to Mycobacterium fortuitum and Mycobacterium chelonei on the basis of in vitro susceptibilities. J Infect Dis. 1985;152(3):500–14.
Peloquin CA, Berning SE, Nitta AT, Simone PM, Goble M, Huitt GA, Iseman MD, Cook JL, Curran-Everett D. Aminoglycoside toxicity: daily versus thrice-weekly dosing for treatment of mycobacterial diseases. Clin Infect Dis. 2004;38(11):1538–44.
Aznar ML, Marras TK, Elshal AS, Mehrabi M, Brode SK. Safety and effectiveness of low-dose amikacin in nontuberculous mycobacterial pulmonary disease treated in Toronto, Canada. BMC Pharmacol & Toxicol. 2019;20(1):37.
Kahlmeter G, Dahlager JI. Aminoglycoside toxicity - a review of clinical studies published between 1975 and 1982. J Antimicrob Chemother. 1984;13(Suppl A):9–22.
Lange C, Aarnoutse RE, Alffenaar JWC, Bothamley G, Brinkmann F, Costa J, Chesov D, van Crevel R, Dedicoat M, Dominguez J, et al. Management of patients with multidrug-resistant tuberculosis. Int J Tuberc Lung Dis. 2019;23(6):645–62.
van Ingen J, Totten SE, Helstrom NK, Heifets LB, Boeree MJ, Daley CL. In vitro synergy between clofazimine and amikacin in treatment of nontuberculous mycobacterial disease. Antimicrob Agents Chemother. 2012;56(12):6324–7.
Luo J, Yu X, Jiang G, Fu Y, Huo F, Ma Y, Wang F, Shang Y, Liang Q, Xue Y, et al. In Vitro Activity of Clofazimine against Nontuberculous Mycobacteria Isolated in Beijing, China. Antimicrob Agents Chemother. 2018;62(7):00072–18.
Kim DH, Jhun BW, Moon SM, Kim SY, Jeon K, Kwon OJ, Huh HJ, Lee NY, Shin SJ, Daley CL, et al. In Vitro Activity of Bedaquiline and Delamanid against Nontuberculous Mycobacteria, Including Macrolide-Resistant Clinical Isolates. Antimicrob Agents Chemother. 2019;63(8):00665–19.
van Ingen J, van der Laan T, Dekhuijzen R, Boeree M, van Soolingen D. In vitro drug susceptibility of 2275 clinical non-tuberculous Mycobacterium isolates of 49 species in the Netherlands. Int J Antimicrob Agents. 2010;35(2):169–73.
Shen GH, Wu BD, Hu ST, Lin CF, Wu KM, Chen JH. High efficacy of clofazimine and its synergistic effect with amikacin against rapidly growing mycobacteria. Int J Antimicrob Agents. 2010;35(4):400–4.
Huitric E, Verhasselt P, Andries K, Hoffner SE. In vitro antimycobacterial spectrum of a diarylquinoline ATP synthase inhibitor. Antimicrob Agents Chemother. 2007;51(11):4202–4.
Andries K, Verhasselt P, Guillemont J, Gohlmann HW, Neefs JM, Winkler H, Van Gestel J, Timmerman P, Zhu M, Lee E, et al. A diarylquinoline drug active on the ATP synthase of Mycobacterium tuberculosis. Science. 2005;307(5707):223–7.
Lerat I, Cambau E, Roth Dit Bettoni R, Gaillard JL, Jarlier V, Truffot C, Veziris N. In vivo evaluation of antibiotic activity against Mycobacterium abscessus. J Infect Dis. 2014;209(6):905–12.
Dupont C, Viljoen A, Thomas S, Roquet-Baneres F, Herrmann JL, Pethe K, Kremer L. Bedaquiline Inhibits the ATP Synthase in Mycobacterium abscessus and Is Effective in Infected Zebrafish. Antimicrob Agents Chemother. 2017;61(11):01225–17.
Alexander DC, Vasireddy R, Vasireddy S, Philley JV, Brown-Elliott BA, Perry BJ, Griffith DE, Benwill JL, Cameron AD, Wallace RJ Jr. Emergence of mmpT5 variants during Bedaquiline treatment of Mycobacterium intracellulare lung disease. J Clin Microbiol. 2017;55(2):574–84.
Pontali E, Sotgiu G, D'Ambrosio L, Centis R, Migliori GB. Bedaquiline and multidrug-resistant tuberculosis: a systematic and critical analysis of the evidence. Eur Respir J. 2016;47(2):394–402.
Lewis JM, Hine P, Walker J, Khoo SH, Taegtmeyer M, Squire SB, Sloan DJ. First experience of effectiveness and safety of bedaquiline for 18 months within an optimised regimen for XDR-TB. Eur Respir J. 2016;47(5):1581–4.
Conradie F, Diacon AH, Ngubane N, Howell P, Everitt D, Crook AM, Mendel CM, Egizi E, Moreira J, Timm J, et al. Treatment of highly drug-resistant pulmonary tuberculosis. N Engl J Med. 2020;382(10):893–902.
Li Y, Sun F, Zhang W. Bedaquiline and delamanid in the treatment of multidrug-resistant tuberculosis: promising but challenging. Drug Dev Res. 2019;80(1):98–105.
Zweijpfenning SMH, Schildkraut JA, Coolen JPM, Ruesen C, Koenraad E, Janssen A, Ruth MM, de Jong AS, Kuipers S, Aarnoutse RE, et al. Failure with acquired resistance of an optimised bedaquiline-based treatment regimen for pulmonary Mycobacterium avium complex disease. Eur Respir J. 2019;54(1):00118–2019.
Nguyen TVA, Anthony RM, Banuls AL, Nguyen TVA, Vu DH, Alffenaar JC. Bedaquiline resistance: its emergence, mechanism, and prevention. Clin Infect Dis. 2018;66(10):1625–30.
Acknowledgements
The authors are indebted to the patient who consented to having her data published in this case report.
Funding
No funding was received for this work.
Author information
Authors and Affiliations
Contributions
J.E. was involved in literature review and wrote the initial draft of the manuscript. J.E., S.W., T.T. and J.S. were involved in the clinical care of the patient and revised the manuscript. K.R. provided data on microbiological analyses and revised the manuscript. C.D.S. was involved in case identification, served as consultant in charge of the case, and contributed to writing and editing of the manuscript. K.R., R.M.S., J.S., and C.D.S. supervised clinical and laboratory diagnostics and therapy. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Written informed consent was obtained from the patient for publication of this case report as well as any accompanying images. A copy of the written consent form is available for review by the editor of this journal.
Competing interests
Christoph D. Spinner has received research grants from and served on advisory boards and speaker bureaus for Janssen-Cilag. All other authors declare no conflicts of interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Erber, J., Weidlich, S., Tschaikowsky, T. et al. Successful bedaquiline-containing antimycobacterial treatment in post-traumatic skin and soft-tissue infection by Mycobacterium fortuitum complex: a case report. BMC Infect Dis 20, 365 (2020). https://doi.org/10.1186/s12879-020-05075-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12879-020-05075-7