Abstract
Background
There are obviously ethnic differences between the UGT1A1 gene polymorphisms. Due to the difference of genetic background and environment, the treatment with colorectal cancer patients of Guangxi Zhuang should not completely follow the Euramerican or Chinese han patients. The study aimed to explore the correlation of UGT1A1 gene polymorphism of Guangxi Zhuang metastatic colorectal cancer (mCRC) with irinotecan -based chemotherapy, in order to develop an individualized irinotecan regimen for mCRC patients of Guangxi Zhuang.
Methods
From June 2013 and June 2015, a total of 406 patients of Guangxi who were histologically diagnosed as metastatic colorectal cancer with 102 patients of this cohort with three generations of Zhuang, and 86 patients that conformed to inclusion and exclusion criteria were competitively enrolled. The distribution of UGT1A1 gene polymorphism was analyzed-retrospectively in all patients. Pyrosequencing method was used to detect the UGT1A1*28 and*6 gene polymorphism in the 86 Guangxi Zhuang mCRC patients. After first-line chemotherapy with FOLFIRI regimen, the relationship between gene polymorphism of UGT1A1 and adverse reactions, and efficacy of Irinotecan were analyzed with χ2 test and Kaplan-Meier method.
Results
UGT1A1*28 wild-type (TA6/6), heterozygous mutant (TA6/7) and homozygous mutant (TA7/7) accounted for 69.8, 30.2 and 0%, respectively. UGT1A1*6 wild type (G/G), heterozygous mutation type (G/A) and homozygous mutant (A/A) accounted for 76.7%, 20.9 and 2.3%, respectively. UGT1A1*28 TA6/7 type could increase the risk of grade 3~4 diarrhea (p = 0.027), which did not increase the risk of grade 3~4 neutropenia (p = 0.092). UGT1A1*6G/A and A/A type could increase the risk of grade 3~4 diarrhea and neutropenia (p = 0.001; p = 0.017). After chemotherapy with FOLFIRI, there was no significant difference in response rate (RR) (p = 0.729; p = 0.745) or in median progression-free survival (mPFS) between the wild-type, mutant treatment of UGT1A1*28 and UGT1A1*6 (7.0 m vs 7.4 m, p = 0.427; 6.9 m vs 7.0 m p = 0.408).
Conclusions
The distribution of UGT1A1*28 and UGT1A1*6 gene polymorphism in Guangxi Zhuang patients were differed from the existing reported of European people and Chinese Han population. The UGT1A1 gene polymorphism with irinotecan chemotherapy-associated diarrhea and neutropenia were closely related. There was no significant association between UGT1A1 gene polymorphism and therapeutic efficacy of irinotecan.
Similar content being viewed by others
Background
Colorectal cancer (CRC) is a common gastrointestinal malignancy, with the third highest incidence and fourth highest mortality among malignant tumors worldwide [1, 2]. Since the early symptoms of CRC are obscure, majority of the patients are diagnosed in the intermediate and late stages, and suffer from metastasis at diagnosis, with a 5-year survival rate < 10%. Chemotherapy is the main treatment method for CRC [3,4,5].
Irinotecan-based chemotherapy is a standard first-line or second-line regimen for the treatment of mCRC. However, irinotecan has dose-limiting toxicities, mainly neutropenia and delayed-onset diarrhea, wherein the incidence of grade 3~4 delayed-onset diarrhea and neutropenia is high. Studies have shown that UGT1A1 gene polymorphism is differently distributed among different ethnicities, which may lead to different toxicities and efficacies of irinotecan [6]. Even with the same ethnicity, the gene frequency differs in varying geographical regions [7]. Moreover, the frequency of gene mutation differs among various ethnic groups due to different genetic backgrounds. Thus, the therapeutic regimen of irinotecan should be individualized for different ethnic groups, rather than referring to the guidelines of Europe or America for Chinese Han patients. This study aimed to analyze the distribution of UGT1A1 gene polymorphisms in mCRC patients of Guangxi Zhuang, as well as the correlation of UGT1A1 gene polymorphisms with the adverse reactions and efficacy of irinotecan chemotherapy, in order to develop an individualized irinotecan regimen for mCRC patients of Guangxi Zhuang.
Methods
Patient selection
A total of 406 mCRC patients receiving initial treatment in four centers of Guangxi between June 01, 2013 and June 01, 2015 were selected. After three generations of strict screening, 102 patients were found to be fully compliant with the criteria of Chinese Zhuang, of which 16 were excluded due to severe diseases and incomplete follow-up data. Eligibility criteria included: 1) Patients were diagnosed as mCRC by pathological and imaging results, and did not previously receive chemoradiotherapy; 2) Patients were Guangxi Zhuang for three generations; 3) Patients had measurable lesions by MRI or CT examination; 4) Patients had a ECOG PS ≤1 point, with an expected survival of ≥3 months; 5) Patients did not have significant contraindications to chemotherapy in routine blood, liver and kidney function, ECG and other examinations prior to chemotherapy; 6) Patients had no history of other malignancies; 7) Patients signed informed consent; 8) Patients were routinely followed-up. The selected patients were aged 21~76 years (median age: 56 years), and their clinical data are listed in Table 1. This study was approved by the local ethics committee. Informed consent was obtained from all individual participants included in the study.
Treatment regimen
UGT1A1 genotyping
Genotyping studies were performed by an independent laboratory (Department of Clinical Pharmacology, Xiangya Hospital, Central South University), and 2 mL peripheral blood was collected in an EDTA containing glass tube about 1 week before the first chemotherapy. Subsequently, genomic DNA was extracted using the phenol-chloroform method, the target gene fragment was amplified using PCR, and the PCR products were sequenced by pyrosequencing. The UGT1A1*28 and *6 polymorphisms were read using the SNP analysis software.
Chemotherapy regimen
The selected patients received a first-line chemotherapy with irinotecan (CPT-11) + 5-Fluorouracil (5-Fu)/LV (FOLFIRI) regimen. The dosage and administration were as follows: patients were given intravenous infusion of irinotecan (180 mg/m2, 90 mins),d1,intravenous infusion of LV (400 mg/m2),d1, and intravenous injection of 5-Fu (400 mg/m2),d1. Then, the patients were given continuous intravenous injection of 5-Fu (2400 mg/m2),46~48 h. All the treatment were repeated every 2 weeks for 4~6 cycles. The efficacy was evaluated after patients were given at least four cycles of treatment.
Response and toxicity evaluation
The follow-up ranged from 31~55 months with a median 40 months as of Jan 2018. Four cases were lost to follow-up, with a follow-up rate of 95.56%. The primary endpoint of this study was toxicity, and the secondary endpoint included short-term efficacy and progression-free survival (PFS). The efficacy was evaluated according to the response evaluation criteria in solid tumors, Revised RECIST guidelines (version 1.1) [8]. The adverse reactions were evaluated according to the evaluation and classification of neutropenia, delayed diarrhea in National Cancer Institute NCI-CTC (version 3.0) [9].
Endpoints and statistical analysis
All SNPs were analyzed for deviation from Hardy-Weinberg equilibrium (HWE),and the genotype and allelic frequencies were calculated. Two-tailed Fisher’s exact test was used to calculate the genotype distribution in controls and patients, and to compare the differences between both groups; χ2 test or Fisher exact probability was used to analyze the adverse reactions and efficacy. Logistic regression was adopted to analyze the relationship between the clinical characteristics of patients and the adverse reactions of irinotecan. Kaplan-Meier method was used to plot the survival curve as well as analyze the relationship between different genotypes and PFS.
Results
Distribution of the UGT1A1 genotype
The genotype distribution of UGT1A1 gene*28 and *6 in 86 patients was consistent with the Hardy-Weinberg equilibrium (HWE) (p > 0.1) in population genetics, as shown in Table 2. For the UGT1A1*28, there were 60 cases (69.8%) of wild-type (TA6/6), 26 cases (30.2%) of heterozygous–type (TA6/7), and no case of homozygous-type (TA7/7). The sequencing results are shown in Fig. 1. For the UGT1A1*6, there were 66 cases (76.7%) of wild-type (G/G), 18 cases (20.9%) of heterozygous–type (G/A) and two cases (2.3%) of homozygous-type (A/A). The sequencing results are shown in Fig. 2.
Toxicity to chemotherapy based on the UGT1A1 genotype
Among the 86 patients, there were 60 cases of UGT1A1*28 TA6/6, of which seven cases (11.7%) experienced grade 3~4 delayed-onset diarrhea and nine cases (15.0%) had grade 3~4 neutropenia. Among the 26 cases of UGT1A1*28 TA6/7, nine cases (34.6%) experienced grade 3~4 delayed-onset diarrhea and eight cases (30.8%) had grade 3~4 neutropenia. Statistical analysis revealed that UGT1A1*28 TA6/7 patients had a higher risk of grade 3~4 delayed-onset diarrhea as compared to UGT1A1*28 TA6/6 patients, and the difference was statistically significant (χ2 = 4.884, p = 0.027). Meanwhile, UGT1A1*28 TA6/7 was unlikely to increase the risk of grade 3~4 neutropenia (χ2 = 2.844, p = 0.092). Among the 86 patients, there were 66 cases of UGT1A1*6 G/G, of which six cases (9.1%) experienced grade 3~4 delayed-onset diarrhea, and 10 cases (15.2%) had grade 3~4 neutropenia. Among the 20 cases of UGT1A1*6 G/A + A/A, nine cases (45.0%) had grade 3~4 delayed-onset diarrhea and eight cases (40.0%) experienced grade 3~4 neutropenia. Statistical analysis showed that UGT1A1*6 G/A + A/A patients had a higher risk of grade 3~4 diarrhea as compared to G/G patients, with statistically significant difference (χ2 = 11.364, p = 0.001), and also a higher risk of grade 3~4 neutropenia as compared to G/G patients (χ2 = 5.727, p = 0.017). In this study, no patient suffered from adverse event-related death. (Table 3). The delayed diarrhea and neutropenia was not associated with gender, age, PS score, primary lesion and the number of metastatic organs(p > 0.05) (Table 4), but associated with genotype((Table 5, Fig. 3; Table 6, Fig. 4). The probability of occurrence of grade 3~4 delayed diarrhea and neutropenia in wild-type patients was significantly lower than that in single-point and double-point mutants (χ2 = 8.802, p = 0.005, χ2 = 23.171, p = 0.000). (Tables 7 and 8).
Response to chemotherapy based on the UGT1A1 genotype
Among the 86 UGT1A1*28 patients, there were two cases of CR (7.7%) (both of TA6/7), 32 cases of PR [including 23 TA6/6 cases (38.3%) and nine TA6/7 cases (34.6%)], 30 cases of SD [including 21 TA6/6 cases (35.0%) and nine TA6/7 cases (34.6%)], and 22 cases of PD [including 16 TA6/6 cases (26.7%) and six TA6/7 cases (23.1%)] (Fig. 5). Among the 86 UGT1A1*6 patients, there were two cases of CR [one G/G case (1.5%) and one G/A + A/A case (5.0%)], 34 cases of PR [including 26 G/G cases (39.4%) and eight G/A + A/A cases (40.0%)], 28 cases of SD [including 22 G/G cases (33.3%) and six G/A + A/A cases (30%)], and 22 cases of PD [including 17 G/G cases (25.8%) and five G/A + A/A cases (25%)] (Tables 4 and 5). Of these, the ORRs of UGT1A1*28 TA6/6 and TA6/7 patients after chemotherapy were 38.3 and 42.3% (χ2 = 0.120, p = 0.729), respectively, while those of UGT1A1*6 G/G and G/A + A/A patients were 40.9 and 45.0% (χ2 = 0.106, p = 0.745), respectively. (Tables 9 and 10).
Progression-free survival based on the UGT1A1 genotype
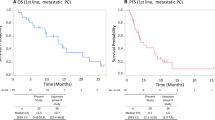
Patients were followed-up until June 01, 2017. The median PFS of UGT1A1*28 TA6/6 and TA6/7 patients were 7.0 and 7.4 months, respectively, with statistically insignificant difference (p = 0.427), and those of UGT1A1*6 G/G and G/A + A/A patients were 6.9 and 7.0 months, respectively, with statistically insignificant difference (p = 0.408). The survival curves plotted using the Kaplan-Meier method are shown in Figs. 6 and 7.
Distribution of the UGT1A1 genotype with irinotecan dose reduction
Among the 86 patients, irinotecan dose was reduced in 15 patients, including six cases of wild-type (TA6/6) and nine cases of mutant-type (TA6/7) UGT1A1*28, and seven cases of wild-type (G/G) and eight cases of mutant-type (G/A) UGT1A1*6. This indicated that a higher ratio of patients with mutant-type UGT1A1*28 and UGT1A1*6 required irinotecan dose reduction as compared to patients with wild-type UGT1A1*28 and UGT1A1*6 (χ2 = 6.019, p = 0.014; χ2 = 7.281, p = 0.007) (Table 11). During subsequent chemotherapy, the irinotecan dose was reduced by about 20%. Fifteen patients could tolerate the reduced dose of irinotecan, and did not require irinotecan dose reduction to the next gradient.
Toxicity of irinotecan dose reduction based on the UGT1A1 genotype
After the irinotecan dose was reduced, the incidences of grade 3~4 diarrhea and grade 3~4 neutropenia were significantly decreased in both the dose reduction group and non-dose reduction group for patients with UGT1A1*28 (p = 1.000; p = 0.613) and UGT1A1*6 (p = 0.442; p = 0.139) in Table 12.
Efficacy of irinotecan dose reduction based on the UGT1A1 genotype
In patients with UGT1A1*28 and UGT1A1*6 genes, there was insignificant difference in the short-term effect between the dose reduction group and non-dose reduction group (p = 0.402, p = 0.368) (Figs. 8 and 9). Also, there was insignificant difference in the median PFS between the dose reduction group and non-dose reduction group (χ2 = 1.946, p = 0.378; χ2 = 1.895, p = 0.388) (Table 13).
Discussion
Irinotecan is a semi-synthetic derivative of natural camptothecin, and has been widely used in the treatment of solid tumors such as gastric cancer, colorectal cancer (CRC), lung cancer, etc. A combination of irinotecan and fluorouracil is a standard first-line regimen for advanced CRC, especially advanced CRC with rapid progression, with an efficacy rate of up to 40% [10,11,12]. However, this regimen has two major adverse reactions of delayed-onset diarrhea and neutropenia, where the incidences of grade 3~4 neutropenia and severe diarrhea are 45% and 20~40% [13], respectively, which limits its clinical application and exhibits inter-individual variations.
Irinotecan is hydrolyzed in vivo by carboxylesterase (CE) into an active metabolite 7-ethyl-10-hydroxycamptothecin (SN-38). The latter is a topoisomerase I inhibitor that inhibits repair of broken single-stranded DNA, disrupts DNA replication and transcription, and exerts cytotoxic effects. SN-38 is inactivated by UGT1A1 as glucuronic acid product SN-38G, which is excreted into the intestine through the bile and is transformed into SN-38 by the intestinal bacterial β-glucuronidase, thereby inducing mucosal injury and delayed-onset diarrhea. UGT1A1 enzyme in the intestine can re-catalyze SN-38 for SN-38G detoxification. Therefore, the adverse reactions of irinotecan are related to its main drug-metabolizing enzyme UGT1A1, whose activity is affected by polymorphism. UGT1A1*28 polymorphism and irinotecan-related adverse reactions have been reported in many studies, but the correlation between UGT1A1*6 polymorphism and adverse reactions of irinotecan remains unclear. Several studies, with conflicting results, have indicated that the UGT1A1 polymorphism has insufficient sensitivity and specificity in predicting the adverse effects of irinotecan. The results of different ethnic groups in the same race are rarely reported. In addition, the role of UGT1A1 polymorphism in predicting the toxicity in patients receiving different doses of irinotecan is clinically controversial.
Due to differences in genetic background, different UGT1A1 mutation sites exist among various ethnic groups, which is responsible for different incidences of toxicity between different populations in eastern and western countries receiving irinotecan treatment. Studies on the distribution of UGT1A1 *28 and *6 polymorphisms have demonstrated that UGT1A1 * 28 homozygous mutant TA7/7 accounts for 10~15% and 12~27% in the Caucasian and African populations, respectively [14, 15], while its mutation rate is only 1.2~4.7% in Asian population [16, 17]. Nakaura et al. [18] showed that the wild-type UGT1A1*28 accounted for 46 and 76% in the Caucasian and Asian populations, respectively, indicating significant inter-ethnic differences. Etienne-Grimald et al. [19] concluded that UGT1A1*28 had the highest expression frequency in Americans and Caucasians, accounting for 38~45% and 29~39%, respectively, while a mutation frequency of about 15~18% in Asians, of which the homozygous mutation type accounted for 3%. Zhang et al. [20] investigated the distribution of UGT1A1*28 polymorphism in 517 Han patients, and found that UGT1A1*28 TA6/6, TA7/6 and TA7/7 accounted for 77.2, 22 and 0.8%, respectively. In this study, the distribution frequencies of wild-type and heterozygous-type UGT1A1*28 were 69.8 and 30.2%, respectively, in 86 patients in Guangxi Zhuang, with no homozygous-type case, which indicated that the homozygous mutation rate was further reduced as compared to the Asian Han ethnicity.
UGT1A1*6 is a unique UGT1A1 mutation type in Asian populations. The frequencies of UGT1A1*6 homozygous mutant A/A in Koreans and Japanese were reported to be 7 and 4%, respectively, while the frequencies of UGT1A1*6 G/G, G/A and A/A in Han Chinese were 66.9, 29.3 and 3.8%, respectively [20]. This study revealed that the frequencies of wild-type, heterozygous-type and homozygous-type UGT1A1*6 in Guangxi Zhuang were 76.7, 20.9 and 2.3%, respectively, which differed from the reported distribution frequencies of genetic polymorphisms in Europeans and Americans, wherein the mutation rate was slightly lower than that in Chinese Han and other Asians. However, this may be due to a small sample size. Equilibrium test of UGT1A1*28 and UGT1A1*6 allelic frequencies in this study revealed that the samples were in accordance with the Hardy-Weinberg equilibrium, derived from a larger and randomized marriage-balanced population, and was representative.
Clinical studies have shown that Caucasians with UGT1A1*28 gene mutants (TA6/7 and TA7/7) have a higher risk of severe granulocytopenia and diarrhea than those with wild-type UGT1A1*28 (TA6 /6) after receiving irinotecan [21]. In our study, Guangxi Zhuang patients with UGT1A1*28 mutations showed a higher risk of 3~4 grade delayed-onset diarrhea as compared to those with wild-type UGT1A1*28 (30.8% vs. 11.7%, p = 0.044), but did not have a higher risk of 3~4 grade neutropenia (34.6% vs. 15.0%, p = 0.112), which was consistent with other domestic reports [22]. Moreover, Guangxi Zhuang patients with UGT1A1*6 mutations had a higher risk of 3~4 grade delayed-onset diarrhea after receiving irinotecan (45% vs. 9.1%, p = 0.001), and an increased risk of 3~4 grade neutropenia (40% vs. 15.2%, p = 0.017), which was consistent with numerous clinical studies in Japan [23,24,25]. The correlation between UGT1A1 genotype and adverse reactions of irinotecan remains controversial. For the effect of UGT1A1* 28 mutant on delayed-onset diarrhea and 3~4 grade granulocytopenia, only one adverse reaction was mentioned in some studies. For example, Miyata et al. only compared the risk of neutropenia, but not the risk of delayed-onset diarrhea [26]. Also, the predictive effects were refuted in some studies, but supported in other studies. Some studies supported the predictive effect on 3~4 grade neutropenia but refuted the risk of delayed-onset diarrhea [17], while others supported the occurrence of delayed-onset diarrhea but refuted the risk of 3~4 grade neutropenia [22]. In addition to the racial differences, we believe it is also related to the dose of irinotecan. In many studies, Caucasians were enrolled and a larger initial dose of irinotecan may have been selected, but the dose of irinotecan was not investigated. Previous studies revealed that UGT1A1*28 and UGT1A1*6 have a predictive effect on the adverse reactions of intermediate or high dose of irinotecan, but not on a low dose of irinotecan [27]. In this study, the homozygous and heterozygous mutations of UGT1A1 showed a predictive effect when the dose of irinotecan was reduced. For UGT1A1*28 and UGT1A1*6, the incidence of 3~4 grade neutropenia and 3~4 grade diarrhea significantly declined in both the dose reduction and non-reduction groups.
The correlation between UGT1A1 gene polymorphism and efficacy of irinotecan chemotherapy remains inconclusive. Toffoli et al. [28] believed that the efficacy was better in patients with mutant UGT1A1*28 as compared to those with wild-type UGT1A1*28. However, others believed that the efficacy was better in wild-type patients as compared to mutant patients [29], or the UGT1A1 gene polymorphism was not correlated with the efficacy [30]. The relationship between UGT1A1 gene polymorphism and adverse reactions of chemotherapy has been reported in several studies, while the efficacy of chemotherapy has been rarely studied. This study showed that the polymorphism of UGT1A1*28 and UGT1A1*6 genes in Guangxi Zhuang patients with CRC was not significantly correlated with the RR and median PFS of irinotecan chemotherapy. Nevertheless, the correlation between UGT1A1 polymorphism and efficacy of irinotecan chemotherapy needs further verification with a large sample size study.
The above results may be affected by differences in population, drug dose, and a small sample size. Whether the gene polymorphism distribution and inter-individual variations in response to irinotecan in Chinese Zhuang are different from that in Han as well as European and American populations remains unknown. The sample size should be increased and the follow-up survival should be analyzed based on the gene distribution characteristics of Chinese and combined with the drug dose, in order to achieve the goal of individualization.
Conclusions
The frequency of gene mutation differs among various ethnic groups due to different genetic backgrounds. In our study, we found the distribution frequencies of wild-type, heterozygous-type and homozygous-type UGT1A1 in Guangxi Zhuang patients of metastatic colorectal cancer differed from the reported distribution frequencies of genetic polymorphisms in European patients people and Chinese Han patients. The UGT1A1 gene polymorphism with irinotecan chemotherapy-associated diarrhea and neutropenia were closely related. Guangxi Zhuang patients of metastatic colorectal cancer with UGT1A1*28 mutations showed a higher risk of 3~4 grade diarrhea as compared to those with wild-type UGT1A1*28 which did not increase the risk of grade 3~4 neutropenia. Moreover, Guangxi Zhuang patients with UGT1A1*6 mutations had a higher risk of 3~4 grade diarrhea and neutropenia those with wild-type. After chemotherapy with FOLFIRI, there was no significant difference in response rate and the median progression-free survival between the wild-type, mutant treatment of UGT1A1 * 28 and UGT1A1 * 6. The above results may be affected by differences in population, or drug dose. The follow-up survival should be analyzed based on the gene distribution characteristics of Chinese and combined with the drug dose, in order to achieve the goal of individualization.
Availability of data and materials
The data sets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- CE:
-
Carboxylesterase
- CRC:
-
Colorectal cancer
- HWE:
-
Hardy-Weinberg equilibrium
- mCRC:
-
Metastatic colorectal cancer
- mPFS:
-
Median progression-free survival
- RR:
-
Response rate
- UGT1A1 :
-
Uridine diphosphate glucuronosyl transferase 1A1
References
Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108.
Jakobsen A, Andersen F, Fischer A, et al. Neoadjuvant chemotherapy in locally advanced colon cancer. A phase II trial. Acta Oncol. 2015;54:1747–53.
Huo YR, Huang Y, Liauw W, Zhao J, Morris DL. Prognostic value of carcinoembryonic antigen (CEA), AFP, CA19-9 and CA125 for patients with colorectal cancer with peritoneal Carcinomatosis treated by Cytoreductive surgery and intraperitoneal chemotherapy. Anticancer Res. 2016;36:1041–9.
Arredondo J, González I, Baixauli J, et al. Tumor response assessment in locally advanced colon cancer after neoadjuvant chemotherapy. J Gastrointest Oncol. 2014;5:104–11.
Suenaga M, Mizunuma N, Matsusaka S, et al. A phase I/II study of biweekly capecitabine and irinotecan plus bevacizumab as second-line chemotherapy in patients with metastatic colorectal cancer. Drug Des Devel Ther. 2015;9:1653–62.
Chen Z, Su D, Ai L, et al. UGT1A1 sequence variants associated with risk of adult hyperbilirubinemia: a quantitative analysis. Gene. 2014;552:32–8.
Yan W, Wang YW, Yang FF, et al. Differences in frequencies of UGT1A19, 1A, and 1A1 genetic polymorphisms in Chinese Tibetan versus Han Chinese populations. Genet Mol Res. 2013;12:6454–61.
Kim JH, Min SJ, Jang HJ, et al. Comparison of RECIST 1.0 and RECIST 1.1 in patients with metastatic cancer: a pooled analysis. J Cancer. 2015;6:387–93.
Trotti A. The need for adverse effects reporting standards in oncology clinical trials. J Clin Oncol. 2004;22:19–22.
Ottaiano A, Nasti G, Cassata A, et al. FOLFOXIRI in metastatic colorectal cancer: a criticism from its native land. Cancer Lett. 2017;408:71–2.
Leal F, Ferreira FP. FOLFOXIRI regimen for metastatic colorectal cancer: a systematic review and meta-analysis. Clin Colorectal Cancer. 2017. https://doi.org/10.1016/j.clcc.2017.03.012.
Wulaningsih W, Wardhana A, Watkins J, Yoshuantari N, Repana D, Van Hemelrijck M. Irinotecan chemotherapy combined with fluoropyrimidines versus irinotecan alone for overall survival and progression-free survival in patients with advanced and/or metastatic colorectal cancer. Cochrane Database Syst Rev. 2016;2:CD008593.
Liu D, Li J, Gao J, Li Y, Yang R, Shen L. Examination of multiple UGT1A and DPYD polymorphisms has limited ability to predict the toxicity and efficacy of metastatic colorectal cancer treated with irinotecan-based chemotherapy: a retrospective analysis. BMC Cancer. 2017;17:437.
Kaniwa N, Kurose K, Jinno H, et al. Racial variability in haplotype frequencies of UGT1A1 and glucuronidation activity of a novel single nucleotide polymorphism 686C> T (P229L) found in an African-American. Drug Metab Dispos. 2005;33:458–65.
Marcuello E, Altés A, Menoyo A, et al. UGT1A1 gene variations and irinotecan treatment in patients with metastatic colorectal cancer. Br J Cancer. 2004;91:678–82.
Hirose K, Kozu C, Yamashita K, et al. Correlation between plasma concentration ratios of SN-38 glucuronide and SN-38 and neutropenia induction in patients with colorectal cancer and wild-type UGT1A1 gene. Oncol Lett. 2012;3:694–8.
Gao J, Zhou J, Li Y, et al. UGT1A1 6/28 polymorphisms could predict irinotecan-induced severe neutropenia not diarrhea in Chinese colorectal cancer patients. Med Oncol. 2013;30:604.
Nakamura Y, Soda H, Oka M, et al. Randomized phase II trial of irinotecan with paclitaxel or gemcitabine for non-small cell lung cancer: association of UGT1A1*6 and UGT1A1*27 with severe neutropenia. J Thorac Oncol. 2011;6:121–7.
Etienne-Grimaldi MC, Boyer JC, Thomas F, et al. UGT1A1 genotype and irinotecan therapy: general review and implementation in routine practice. Fundam Clin Pharmacol. 2015;29:219–37.
Zhang A, Xing Q, Qin S, et al. Intra-ethnic differences in genetic variants of the UGT-glucuronosyltransferase 1A1 gene in Chinese populations. Pharmacogenomics J. 2007;7:333–8.
Martinez-Balibrea E, Abad A, Martínez-Cardús A, et al. UGT1A and TYMS genetic variants predict toxicity and response of colorectal cancer patients treated with first-line irinotecan and fluorouracil combination therapy. Br J Cancer. 2010;103:581–9.
Zhou CF, Ma T, Su Y, et al. UGT1A1 gene polymorphisms and the toxicities of FOLFIRI in Chinese Han patients with gastrointestinal cancer. Anti Cancer Agents Med Chem. 2013;13:235–41.
Onoue M, Terada T, Kobayashi M, et al. UGT1A1*6 polymorphism is most predictive of severe neutropenia induced by irinotecan in Japanese cancer patients. Int J Clin Oncol. 2009;14:136–42.
Takano M, Kato M, Yoshikawa T, et al. Clinical significance of UDP-glucuronosyltransferase 1A1*6 for toxicities of combination chemotherapy with irinotecan and cisplatin in gynecologic cancers: a prospective multi-institutional study. Oncology. 2009;76:315–21.
Hu ZY, Yu Q, Pei Q. Dose-dependent association between UGT1A1*28 genotype and irinotecan-induced neutropenia: low doses also increase risk. Clin Cancer Res. 2010;16:3832–42.
Miyata Y, Touyama T, Kusumi T, et al. Erratum to: UDP-glucuronosyltransferase 1A1*6 and *28 polymorphisms as indicators of initial dose level of irinotecan to reduce risk of neutropenia in patients receiving FOLFIRI for colorectal cancer. Int J Clin Oncol. 2016;21:704.
Moriya H, Saito K, Helsby N, et al. Association between the low-dose irinotecan regimen-induced occurrence of grade 4 neutropenia and genetic variants of UGT1A1 in patients with gynecological cancers. Oncol Lett. 2014;7:2035–40.
Toffoli G, Cecchin E, Corona G, et al. The role of UGT1A1*28 polymorphism in the pharmacodynamics and pharmacokinetics of irinotecan in patients with metastatic colorectal cancer. J Clin Oncol. 2006;24:3061–8.
Shulman K, Cohen I, Barnett-Griness O, et al. Clinical implications of UGT1A1*28 genotype testing in colorectal cancer patients. Cancer. 2011;117:3156–62.
Sugiyama T, Hirose T, Kusumoto S, et al. The UGT1A1*28 genotype and the toxicity of low-dose irinotecan in patients with advanced lung cancer. Oncol Res. 2010;18:337–42.
Acknowledgements
Not Applicable.
Funding
This study was supported by the National Natural Science Foundation of China (NSFC:81260340) and Guangxi Natural Science Foundation (2013GXNSFAA019263). The sponsor had no role in:study design;collection,analyses,or interpretation of data;manuscript writing;or in the decision to publish the results.
Author information
Authors and Affiliations
Contributions
SC, LH, CF, QM and MW contributed to the study design, literature research, interpretation of findings and writing of the manuscript. YS and ZL contributed to the follow-up, data collection and analyses. GL provided suggestion of statistics. XJ, CG and HH contributed to critical review of data analyses and critical edit of the manuscript. All authors reviewed and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
This study was approval by the Ethics Committee of Guangxi Medical University and the Forth Affiliated Hospital of Guangxi Medical University, written informed consent was obtained from each patient.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Chen, S., Hua, L., Feng, C. et al. Correlation between UGT1A1 gene polymorphism and irinotecan chemotherapy in metastatic colorectal cancer: a study from Guangxi Zhuang. BMC Gastroenterol 20, 96 (2020). https://doi.org/10.1186/s12876-020-01227-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12876-020-01227-w