Abstract
Background
Detection of ceftazidime/avibactam (CAZ/AVI) antibacterial activity is absolutely vital with the rapid growth of carbapenem resistant Enterobacteriaceae (CRE). But now, there is no available automated antimicrobial susceptibility testing card for CAZ/AVI, so Kirby-Bauer has become an economical and practical method for detecting CAZ/AVI antibacterial activity against Enterobacteriaceae.
Result
In this study, antimicrobial susceptibility testing of CAZ/AVI against 386 Enterobacteriaceae (188 Klebsiella pneumoniae, 122 Escherichia coli, 76 Enterobacter cloacae) isolated from clinical patients was performed by broth microdilution. Of the 386 strains, 54 extended spectrum β lactamases negative (ESBL(−)), 104 extended spectrum β lactamases positive (ESBL(+)), 228 CRE. 287 isolates were susceptible to CAZ/AVI and 99 isolates were resistant to CAZ/AVI. At the same time, to obtain optimal content avibactam (AVI) disk containing ceftazidime (30 μg), inhibition zone diameter of four kinds of ceftazidime (30 μg) disk containing different AVI content (0 μg, 10 μg, 25 μg, 50 μg) were tested by Kirby-Bauer method. The microdilution broth method interpretation was used as the standard to estimate susceptible or resistance and then coherence analysis was carried out between Kirby-Bauer and broth microdilution. The result shows the inhibition zone diameter of 30 μg/50 μg disk, susceptible isolates: 20.5 mm–31.5 mm, resistance isolates: 8.25 mm–21.5 mm. The inhibition zone diameter of 30 μg/25 μg disk, susceptible isolates: 19.7 mm–31.3 mm, resistance isolates: 6.5 mm–19.2 mm. The inhibition zone diameter of 30 μg/10 μg disk, susceptible isolates: 19.5 mm–31 mm, resistance isolates: 6.5 mm–11 mm. The inhibition zone diameter of ceftazidime (30 μg), susceptible isolates: 6.5 mm–27.5 mm, resistance isolates 6.5 mm.
Conclusion
Our results show that 30 μg/50 μg, 30 μg/25 μg, 30 μg/10 μg CAZ/AVI disk have significant statistical differences to determinate CAZ/AVI antibacterial activity, but for 30 μg/50 μg disk, there has a cross section between susceptible isolates (minimum 20.5 mm) and resistance isolates (maximum 21.5 mm). For 30 μg/25 μg disk, it is hard to distinguish the difference between susceptible isolates (minimum 19.7 mm) and resistance isolates (maximum 19.2 mm), so 30 μg/10 μg CAZ/AVI disk is more conducive to determinate antibacterial activity.
Similar content being viewed by others
Background
Multidrug-resistant gram-negative bacteria, such as extended spectrum β lactamases (ESBL)-producing and carbapenemase-producing Enterobacteriaceae, are rapidly prevalent worldwide and cause huge medical burden and mortality from these organism infections [1, 2]. Carbapenem antibiotics have been hailed as the most effective drug for the treatment of ESBL-producing Enterobacteriaceae [3], but for now, the numbers of carbapenem-resistant Enterobacteriaceae (CRE) have increased dramatically [4,5,6], so it is imminent to develop new antibiotics against carbapenemase-producing Enterobacteriaceae all around the world. Avibactam (AVI) is a potent, novel diazabicyclooctane β-lactamase inhibitor with in vitro activity against classes A, C and some class D β-lactamases [7, 8]. When used in combination with ceftazidime (CAZ), AVI restores the activity of CAZ against Gram-negative organisms producing these carbapenemases, as well as any co-carried ESBLs [9, 10]. Unfortunately, up to now, there is no available automated antimicrobial susceptibility testing card for ceftazidime/avibactam (CAZ/AVI), so Kirby-Bauer has become an economical and practical method for detection of CAZ/AVI activity against Enterobacteriaceae. Here, we aimed to evaluate the effect of different content AVI on CAZ/AVI activity against Enterobacteriaceae, to obtain optimal content AVI and to determine the breakpoint of CAZ/AVI, so as to provide guidance for clinical use of CAZ/AVI.
Results
Resistant type of strains in this study
Table 1 shows 386 strains resistant type, 188 Klebsiella pneumonia (18 ESBL(−)(4.7%), 37 ESBL(+)(9.6%), 133 CRE (34.5%)), 122 Escherichia coli, (27 ESBL(−)(7.0%), 37 ESBL(+)(9.6%), 58 CRE (15.0%)), 76 Enterobacter cloacae (9 ESBL(−)(2.3%), 30 ESBL(+)(7.8%), 37 CRE (9.6%)).
Broth microdilution MICs for CAZ/AVI against Enterobacteriaceae
Table 2 indicates the isolates broth microdilution minimum inhibitory concentration (MIC) for CAZ/AVI. Using the Clinical & Laboratory Standards Institute (CLSI) breakpoint (CLSI, M100, 28th Edition, 2018) for CAZ/AVI, susceptible: MIC≤8/4 μg/ml, resistant: MIC≥16/4 μg/ml, percentage susceptibility for CAZ/AVI: Klebsiella pneumonia 85.6% (161/188), Escherichia coli 66.4% (81/122), Enterobacter cloacae 59.2% (45/76). Together with Table 1 results, we find that the CAZ/AVI resistant strains are all carbapenem-resistant Enterobacteriaceae.
Disk diffusion inhibition zones analysis
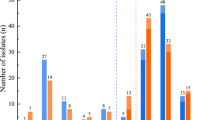
Figure 1 shows the disk diffusion inhibition zone diameter, for CAZ/AVI (30 μg/50 μg) disk, susceptible isolates inhibition zone diameter 20.5 mm–31.5 mm, resistant isolates inhibition zone diameter 8.25 mm–21.5 mm, for CAZ/AVI (30 μg/25 μg) disk, susceptible isolates inhibition zone diameter 19.7 mm–31.3 mm, resistant isolates inhibition zone diameter 6.5 mm–19.2 mm, for CAZ/AVI (30 μg/10 μg) disk, susceptible isolates inhibition zone diameter 19.5 mm–31 mm, resistant isolates inhibition zone diameter 6.5 mm–11 mm. From our result, for CAZ/AVI (30 μg/50 μg) disk, the inhibition zone diameter has a cross section between susceptible isolates (minimum 20.5 mm) and resistance isolates (maximum 21.5 mm). Although there is no cross section between susceptible isolates (minimum 19.7 mm) and resistance isolates (maximum 19.2 mm) in inhibition zone diameter of CAZ/AVI (30 μg/25 μg) disk, it is hard to distinguish the difference between minimum (19.7 mm) and maximum (19.2 mm). While, the 30 μg/10 μg CAZ/AVI disk inhibition zone diameter has significantly difference between susceptible isolates and resistant isolates. To further stratify the inhibition zone diameter distribution, we analysis the inhibition zone diameter range of the isolates with MIC, as shows in the Additional file 1: Table S1, when the MIC value ≥256, the inhibition zone diameter is 6.5 mm (the diameter of disk). When the MIC value ≤0.25, inhibition zone diameter: 25.4 mm–31 mm. When 0.5 ≤ MIC≤8, inhibition zone diameter falls 19.3 mm–27.6 mm. When the MIC is between 16 and 128, the diameter of inhibition zone is fixed at 6.5 mm–11 mm.
The disk diffusion inhibition zones diameter. S: susceptible isolates for ceftazidime/avibactam. R: Resistant isolates for ceftazidime/avibactam. 50: ceftazidime/avibactam disk content 30 μg /50 μg, 25: ceftazidime/avibactam disk content 30 μg /25 μg, 10: ceftazidime/avibactam disk content 30 μg /10 μg, 0: ceftazidime disk content 30 μg, ***P < 0.001 indicate highly statistically significant differences. no: no statistical analysis
Recommended ceftazidime/avibactam disk content and breakpoint for Enterobacteriaceae, and sensitivity and specificity
The Table 3 indicate our recommended CAZ/AVI disk content: CAZ 30 μg, AVI 10 μg. CAZ/AVI disk breakpoint: susceptible≥20 mm, resistant≤11 mm, 11 mm–20 mm interpret as susceptible-dose dependent or intermediate. As reported in Additional file 2: Table S2, the inhibition zone diameter cut-off we recommended is more conducive to evaluate the antibacterial activity of CAZ/AVI against Enterobacteriaceae and achieves perfect specificity (100%) and excellent sensitivity (Klebsiella pneumonia:96.9%, Escherichia coli:97.5%, Enterobacter cloacae:95.6%, overall:96.9%).
Discussion
Increasing carbapenem-resistant Enterobacteriaceae (CRE) have drawn great attention because of their broad resistance spectra and outbreak epidemics [11, 12], as well as have shown a high potential of rapid disseminations [13]. In 2012, a study reported that 3% of ICU patients in Chicago were infected with CRE, among ICU medical staff, the colonization rate of CRE was as high as 30% [14]. In 2016, seven children were infected with non-clonal CRE in pediatric hospital in Mexico [15]. Between 2005 and 2010, carbapenem-resistant Klebsiella pneumoniae invasive infections occurred successively in Greece, Italy, Hungary and Cyprus [16]. European infection control experts reported the interregional transmission or epidemic of CRE in 13/38 countries in 2015, compared with in 6/38 countries in 2013 [17]. Between 2004 and 2013, CRE has gradually increased in medical centers and major medical teaching centers, and prevalence rates of CRE isolated from ICU rose from 3.7% in 2008 to 15.3% in 2017 in Taiwan [18]. In addition, in ICU, the isolation rate of CRE Escherichia coli increased from 1.2% in 2008 to 4.0% in 2017 in medical centers, while the CRE isolation rate in major teaching centers increased from 1.0% in 2008 to 2.8% in 2017 [18]. Because of high mortality (> 30%), infection caused by CRE has become the major worrying health event all around the world [19,20,21]. In 2017, the World Health Organization listed CRE as the first “critical priority pathogens” [22]. These scattered findings in different parts of the world emphasize the fact that the development of new antibiotics against CRE is imminent. Currently, CRE treatment mainly depends on older agents, such as polymyxins, fosfomycin, tigecycline and aminoglycosides, which have been rarely used due to efficacy and/or toxicity concerns [23]. Recently, several drugs were tested to gauge their effectiveness against CRE infections, such as ceftazidime/avibactam (CAZ/AVI), CAZ/AVI is a fixed-dose combination drug containing an antibiotic-third generation cephalosporin ceftazidime and a novel non-β-lactam β-lactamase inhibitor avibactam [24, 25]. Previously approved β-lactamase inhibitors such as tazobactam and clavulanic acid do not inhibit important classes of β-lactamases, including Klebsiella pneumoniae carbapenemases (KPCs), New Delhi metallo-β-lactamase 1 (NDM-1), and AmpC-type β-lactamases [26]. Avibactam can inhibit KPCs, AmpC, and some Class D β-lactamases [25]. Therefore, CAZ/AVI has preferable antibacterial activity against the majority CRE. Unfortunately, there is no available automated antimicrobial susceptibility testing card for CAZ/AVI, so Kirby-Bauer has become an economical and practical method to evaluate CAZ/AVI activity against Enterobacteriaceae. Here, we estimate the disk diffusion of CAZ/AVI cut-off zones and disk content.
In this study, from two different regions of China (Chengdu and Chongqing), a total of 386 clinical isolates of Enterobacteriaceae isolated from patient were collected, 54 ESBL(−) isolates (27 Escherichia coli, 18 Klebsiella pneumonia, 9 Enterobacter cloacae), 104 ESBL(+) isolates (37 Escherichia coli, 37 Klebsiella pneumonia, 30 Enterobacter cloacae), 228 CRE isolates (58 Escherichia coli, 133 Klebsiella pneumonia, 37 Enterobacter cloacae). Of which, according to CLSI CAZ/AVI breakpoint for Enterobacteriaceae (susceptible MIC≤8/4, resistant MIC≥16/4), 27 Klebsiella pneumonia, 41 Escherichia coli, and 31 Enterobacter cloacae are resistant to CAZ/AVI (all resistant isolates are CRE). At present, various resistance mechanisms of CAZ/AVI have been reported, including substitutions of specific amino acid in some beta-lactamases such as KPCs, CTX-M-14 [27,28,29], OXA-2 duplication [30], and loop deletion in AmpC beta-lactamase [31]. In addition, mutations in membrane porin [29, 32, 33], enhanced efflux pump activity [29], and sustained high expression of certain beta lactamases can lead to CAZ/AVI resistance [32]. So in the follow-up study, we will explore the specific resistance mechanisms. Here, our data shows resistant percentages of Klebsiella pneumonia, Escherichia coli, and Enterobacter cloacae are 20.3% (27/133), 70.7% (41/58), and 83.8% (31/37) for CAZ/AVI, respectively, which inconsistent with other researchers [34,35,36]. The follow two causes may be account for the difference. Firstly, our isolates collected according to random selection within group (ESBL negative isolates collected from ESBL negative group, ESBL positive isolates collected from ESBL positive group, CRE isolates collected from CRE group). Second, the last 2 years, new CRE organisms increasing rapidly, such as NDM-1-producing Enterobacteriaceae, which can not be inhibited by AVI [37].
Although CAZ/AVI had a good antibacterial activity against multidrug-resistant Gram-negative bacteria, it was not until 2017 that there was a cut off CAZ/AVI against Enterobacteriaceae in the report of European Committee on Antimicrobial Susceptibility Testing (EUCAST, document. 2017). In 2017, EUCAST report CAZ/AVI inhibition zone diameter cut off and MIC breakpoint, for Kirby-Bauer method (disk content 10 μg /4 μg), susceptible (inhibition zone diameter >13 mm) and resistant (inhibition zone diameter <13 mm), for microdilution broth method (a fixed concentration 4 μg/ml), susceptible (MIC<8/4 μg /ml) and resistant (MIC>8/4 μg /ml). CLSI reported CAZ/AVI inhibition zone diameter cut off and MIC breakpoint in 2018 edition, for Kirby-Bauer method (disk content 30 μg /20 μg), susceptible (inhibition zone diameter ≥ 21 mm) and resistant (inhibition zone diameter ≤ 20 mm), for microdilution broth method (a fixed concentration 4 μg /ml), susceptible (MIC≤8/4 μg /ml) and resistant (MIC≥16/4 μg /ml). Unfortunately, there are still no commercial kits for determining CAZ/AVI antibacterial activity, so we believe that Kirby-Bauer is an economical method for CAZ/AVI. In this study, in order to screen optimal content AVI disk containing ceftazidime (30 μg), inhibition zone diameters of four kinds of ceftazidime (30 μg) disk containing different AVI content (0 μg, 10 μg, 25 μg, 50 μg) against 122 Escherichia coli, 188 Klebsiella pneumonia and 76 Enterobacter cloacae were tested, the CLSI broth microdilution method interpretation was used as the standard to estimate activity.
The result shows that 30 μg/50 μg, 30 μg/25 μg, 30 μg/10 μg CAZ/AVI disk have significant statistical differences and are conducive to determinate antibacterial activity (Fig. 1). Furthermore, disk diffusion breakpoint of CAZ/AVI (30 μg/10 μg) we recommended for Enterobacteriaceae (susceptible ≥20 mm and resistant ≤11 mm) achieves practicable sensitivity and specificity, as shows in Additional file 2: Table S2, for sensitivity (Klebsiella pneumonia:96.9%, Escherichia coli:97.5%, Enterobacter cloacae:95.6%, overall:96.9%) and 100% specificity.
Compare with CLSI breakpoint for CAZ-AVI disk (CAZ-AVI:30 μg/20 μg, susceptible≥21 mm, resistant≤20 mm), the 30 μg/10 μg CAZ/AVI disk inhibition zone diameter is a more simple and effective choice to evaluate CAZ/AVI activity (susceptible ≥20 mm and resistance≤11 mm).
Conclusions
In conclusion, our study indicates that 30 μg/10 μg CAZ/AVI disk is a more feasible choice to evaluate CAZ/AVI activity against Enterobacteriaceae, and disk diffusion breakpoint CAZ/AVI (30 μg/10 μg) we recommended for Enterobacteriaceae (susceptible ≥20 mm and resistant ≤11 mm) has excellent sensitivity and specificity. Those data will provide a rapid and accurate detection of CAZ/AVI activity against the major Enterobacteriaceae and is conducive to provide more effective guidance for treatment of infectious diseases.
Methods
Bacterial strains
A total of 386 Enterobacteriaceae isolates (188 Klebsiella pneumoniae, 122 Escherichia coli, 76 Enterobacter cloacae) were collected according to random selection within group (ESBL negative isolates were random collected from ESBL negative group, ESBL positive isolates were random collected from ESBL positive group, CRE isolates were random collected from CRE group) from two different regions of China (Chengdu and Chongqing), all microorganisms were isolated from patient specimens (such as Urine, blood, sputum and secretion) during treatment, and then those isolates have to be preserved for scientific research. All strains were identified by GN card (bioMerieux, Durham, NC, USA) and ID32 GN (bioMerieux, Durham, NC, USA).
Extended-spectrum β-lactamases experiment
Tests for Extended-Spectrum β-Lactamases was carried out in accordance with Clinical & Laboratory Standards Institute (CLSI, M100, 28th Edition, 2018), in brief, the disk ceftazidime 30 μg (BIO-KONT, Wenzhou, China), ceftazidime-clavulanate 30/10 μg (BIO-KONT, Wenzhou, China), cefotaxime 30 μg (BIO-KONT, Wenzhou, China) and cefotaxime-clavulanate 30/10 μg (BIO-KONT, Wenzhou, China) (Testing necessitates using both cefotaxime and ceftazidime, alone and in combination with clavulanate) was used to test inhibition zone diameter with disk diffusion method. A ≥ 5 mm increase in the inhibition zone diameter for either antimicrobial agent tested in combination with clavulanate vs the inhibition zone diameter of the agent when tested alone is regarded as ESBL positive (ESBL(+)), otherwise ESBL negative (ESBL(−)), K. pneumoniae ATCC® 700603(BIO-KONT, Wenzhou, China) and E. coli ATCC® 25922(BIO-KONT, Wenzhou, China) was used as positive control and negative control, respectively.
CRE experiment
Minimum inhibitory concentration (MIC) of ertapenem, imipenem and meropenem were obtained with broth microdilution, those isolates of which resistant to one or more carbapenems (ertapenem, imipenem and meropenem) using the current MIC breakpoints (CLSI, M100, 28th Edition, 2018) were defined as carbapenem-resistant Enterobacteriaceae (CRE).
CAZ/AVI disks preparation
CAZ (30μg) disk is purchased from Wenzhou Kangtai Biological Company (BIO-KONT, Wenzhou, China) and prepared in regulated good manufacturing practice conditions, AVI was obtained commercially (MedChemExpress. New Jersey. USA) and dissolved in water. CAZ/AVI disks were prepared as follows: 30 μg/0 μg disk: CAZ (30 μg) + 5ul H2O, 30 μg/10 μg disk: CAZ (30 μg) + 5ul AVI (2 mg/ml), 30 μg/25 μg disk: CAZ (30 μg) + 5ul AVI (5 mg/ml), 30 μg/50 μg disk: CAZ (30 μg) + 5ul AVI (10 mg/ml), those disks were dried naturally at room temperature and immediately used in the experiment. At the same time, E. coli ATCC 25922 and 35,218, and Klebsiella pneumoniae ATCC 700603 were used for quality control organisms.
Ceftazidime/avibactam antimicrobial susceptibility test
Ceftazidime/avibactam MIC were tested by broth microdilution according to CLSI guideline (M100, 28th Edition, 2018) using a fixed concentration of avibactam (MedChemExpress. New Jersey. USA) 4 μg/ml. Each isolate was tested in duplicate; a third replicate was necessary if there was disagreement between the first two broth microdilution results. For Kirby-Bauer method, plates were incubated at 35 °C and read after 16–20 h incubation, ceftazidime (30 μg) disk containing different avibactam content (0 μg, 10 μg, 25 μg, 50 μg) were used for disk diffusion test, k diffusion tests were performed in triplicate in parallel with broth microdilution, the mean value of the three inhibition zone diameter is used for statistical analysis. Escherichia coli ATCC 25922 and Escherichia coli ATCC 32518 were used for quality control. Isolates were considered to be susceptible to CAZ/AVI when MICs were ≤ 8/4 mg/L (CLSI, M100, 28th Edition, 2018).
Statistical analysis
T-test was employed to assess the statistical significance of differences intra-group comparisons using GraphPad Prism 5 software (GraphPad Software, Inc., La Jolla, CA, USA). Differences were considered statistically significant at p < 0.01.
Availability of data and materials
All authors decided the datasets used and analyzed in this study are available from the corresponding author upon reasonable request through e-mail. e-mail:yingxu@cmc.edu.cn
Abbreviations
- AVI:
-
avibactam
- CAZ:
-
ceftazidime
- CAZ/AVI:
-
ceftazidime/avibactam
- CLSI:
-
Clinical & Laboratory Standards Institute
- CRE:
-
carbapenem resistant Enterobacteriaceae
- ESBL:
-
Extended spectrum β lactamases
- ESBL(−):
-
Extended spectrum β lactamases negative
- ESBL(+):
-
Extended spectrum β lactamases positive
- EUCAST:
-
European Committee on Antimicrobial Susceptibility Testing
- MIC:
-
Minimum inhibitory concentration
References
van Duin D, Kaye KS, Neuner EA, Bonomo RA. Carbapenem-resistant Enterobacteriaceae: a review of treatment and outcomes. Diagn Microbiol Infect Dis. 2013;75(2):115–20.
Carlet J, Jarlier V, Harbarth S, Voss A, Goossens H, Pittet D. Participants of the 3rd world healthcare-associated infections F: ready for a world without antibiotics? The Pensieres antibiotic resistance call to action. Antimicrob Resist Infect Control. 2012;1(1):11.
Pitout JD. Infections with extended-spectrum beta-lactamase-producing enterobacteriaceae: changing epidemiology and drug treatment choices. Drugs. 2010;70(3):313–33.
Kanj SS, Kanafani ZA. Current concepts in antimicrobial therapy against resistant gram-negative organisms: extended-spectrum beta-lactamase-producing Enterobacteriaceae, carbapenem-resistant Enterobacteriaceae, and multidrug-resistant Pseudomonas aeruginosa. Mayo Clin Proc. 2011;86(3):250–9.
Pfeifer Y, Cullik A, Witte W. Resistance to cephalosporins and carbapenems in gram-negative bacterial pathogens. Int J Med Microbiol. 2010;300(6):371–9.
Nordmann P, Cuzon G, Naas T. The real threat of Klebsiella pneumoniae carbapenemase-producing bacteria. Lancet Infect Dis. 2009;9(4):228–36.
Wenzler E, Deraedt MF, Harrington AT, Danizger LH. Synergistic activity of ceftazidime-avibactam and aztreonam against serine and metallo-beta-lactamase-producing gram-negative pathogens. Diagn Microbiol Infect Dis. 2017;88(4):352–4.
Sader HS, Castanheira M, Flamm RK, Jones RN. Antimicrobial activities of Ceftazidime-Avibactam and comparator agents against gram-negative organisms isolated from patients with urinary tract infections in U.S. medical centers, 2012 to 2014. Antimicrob Agents Chemother. 2016;60(7):4355–60.
van Duin D, Bonomo RA. Ceftazidime/Avibactam and Ceftolozane/Tazobactam: second-generation beta-lactam/beta-lactamase inhibitor combinations. Clin Infect Dis. 2016;63(2):234–41.
Papp-Wallace KM, Bajaksouzian S, Abdelhamed AM, Foster AN, Winkler ML, Gatta JA, Nichols WW, Testa R, Bonomo RA, Jacobs MR. Activities of ceftazidime, ceftaroline, and aztreonam alone and combined with avibactam against isogenic Escherichia coli strains expressing selected single beta-lactamases. Diagn Microbiol Infect Dis. 2015;82(1):65–9.
Del Franco M, Paone L, Novati R, Giacomazzi CG, Bagattini M, Galotto C, Montanera PG, Triassi M, Zarrilli R. Molecular epidemiology of carbapenem resistant Enterobacteriaceae in Valle d'Aosta region, Italy, shows the emergence of KPC-2 producing Klebsiella pneumoniae clonal complex 101 (ST101 and ST1789). BMC Microbiol. 2015;15(1):260.
Guyomard-Rabenirina S, Malespine J, Ducat C, Sadikalay S, Falord M, Harrois D, Richard V, Dozois C, Laboratory working g, Breurec S, et al. Temporal trends and risks factors for antimicrobial resistant Enterobacteriaceae urinary isolates from outpatients in Guadeloupe. BMC Microbiol. 2016;16(1):121.
Bratu S, Landman D, Haag R, Recco R, Eramo A, Alam M, Quale J. Rapid spread of carbapenem-resistant Klebsiella pneumoniae in New York City: a new threat to our antibiotic armamentarium. Arch Intern Med. 2005;165(12):1430–5.
Guh AY, Limbago BM, Kallen AJ. Epidemiology and prevention of carbapenem-resistant Enterobacteriaceae in the United States. Expert Rev Anti-Infect Ther. 2014;12(5):565–80.
Aquino-Andrade A, Merida-Vieyra J, Arias Arzate de la Garza E, Arzate-Barbosa P, De Colsa Ranero A: Carbapenemase-producing Enterobacteriaceae in Mexico: report of seven non-clonal cases in a pediatric hospital. BMC Microbiol 2018, 18(1):38.
Magiorakos AP, Suetens C, Monnet DL, Gagliotti C, Heuer OE, Group EA-NC, participants EA-N. The rise of carbapenem resistance in Europe: just the tip of the iceberg? Antimicrob Resist Infect Control. 2013;2(1):6.
Albiger B, Glasner C, Struelens MJ, Grundmann H, Monnet DL, European survey of Carbapenemase-producing Enterobacteriaceae working g: Carbapenemase-producing Enterobacteriaceae in Europe: assessment by national experts from 38 countries, May 2015. Euro surveillance 2015, 20(45):doi: https://doi.org/10.2807/1560-7917.ES.2015.20.45.30062.
Jean SS, Lee NY, Tang HJ, Lu MC, Ko WC, Hsueh PR. Carbapenem-Resistant Enterobacteriaceae Infections: Taiwan Aspects. Frontiers in Microbiol. 2018;9:2888.
Jean SS, Lee WS, Lam C, Hsu CW, Chen RJ, Hsueh PR. Carbapenemase-producing gram-negative bacteria: current epidemics, antimicrobial susceptibility and treatment options. Future Microbiol. 2015;10(3):407–25.
Navarro-San Francisco C, Mora-Rillo M, Romero-Gomez MP, Moreno-Ramos F, Rico-Nieto A, Ruiz-Carrascoso G, Gomez-Gil R, Arribas-Lopez JR, Mingorance J, Pano-Pardo JR. Bacteraemia due to OXA-48-carbapenemase-producing Enterobacteriaceae: a major clinical challenge. Clin Microbiol Infect. 2013;19(2):E72–9.
Tumbarello M, Viale P, Viscoli C, Trecarichi EM, Tumietto F, Marchese A, Spanu T, Ambretti S, Ginocchio F, Cristini F, et al. Predictors of mortality in bloodstream infections caused by Klebsiella pneumoniae carbapenemase-producing K. pneumoniae: importance of combination therapy. Clin Infect Dis. 2012;55(7):943–50.
Govindaraj Vaithinathan A, Vanitha A. WHO global priority pathogens list on antibiotic resistance: an urgent need for action to integrate one health data. Perspect Public Health. 2018;138(2):87–8.
Fritzenwanker M, Imirzalioglu C, Herold S, Wagenlehner FM, Zimmer KP, Chakraborty T. Treatment options for Carbapenem- resistant gram-negative infections. Deutsches Arzteblatt international. 2018;115(20–21):345–52.
Hidalgo JA, Vinluan CM, Antony N. Ceftazidime/avibactam: a novel cephalosporin/nonbeta-lactam beta-lactamase inhibitor for the treatment of complicated urinary tract infections and complicated intra-abdominal infections. Drug Des Dev Ther. 2016;10:2379–86.
Chahine EB, Sourial M, Ortiz R. Ceftazidime/Avibactam: a new antibiotic for gram-negative infections. Consult Pharm. 2015;30(12):695–705.
Brem J, Cain R, Cahill S, McDonough MA, Clifton IJ, Jimenez-Castellanos JC, Avison MB, Spencer J, Fishwick CW, Schofield CJ. Structural basis of metallo-beta-lactamase, serine-beta-lactamase and penicillin-binding protein inhibition by cyclic boronates. Nat Commun. 2016;7:12406.
Both A, Buttner H, Huang J, Perbandt M, Belmar Campos C, Christner M, Maurer FP, Kluge S, Konig C, Aepfelbacher M, et al. Emergence of ceftazidime/avibactam non-susceptibility in an MDR Klebsiella pneumoniae isolate. J Antimicrob Chemother. 2017;72(9):2483–8.
Giddins MJ, Macesic N, Annavajhala MK, Stump S, Khan S, McConville TH, Mehta M, Gomez-Simmonds A, Uhlemann AC. Successive Emergence of Ceftazidime-Avibactam Resistance through Distinct Genomic Adaptations in blaKPC-2-Harboring Klebsiella pneumoniae Sequence Type 307 Isolates. Antimicrob Agents Chemother. 2018;62(3). https://doi.org/10.1128/AAC.02101-17. Print 2018 Mar.
Nelson K, Hemarajata P, Sun D, Rubio-Aparicio D, Tsivkovski R, Yang S, Sebra R, Kasarskis A, Nguyen H, Hanson BM, et al. Resistance to Ceftazidime-Avibactam Is Due to Transposition of KPC in a Porin-Deficient Strain of Klebsiella pneumoniae with Increased Efflux Activity. Antimicrob Agents Chemother. 2017;61(10). https://doi.org/10.1128/AAC.00989-17. Print 2017 Oct.
Fraile-Ribot PA, Mulet X, Cabot G, Del Barrio-Tofino E, Juan C, Perez JL, Oliver A: In Vivo Emergence of Resistance to Novel Cephalosporin-beta-Lactamase Inhibitor Combinations through the Duplication of Amino Acid D149 from OXA-2 beta-Lactamase (OXA-539) in Sequence Type 235 Pseudomonas aeruginosa. Antimicrob Agents Chemother 2017, 61(9):doi: https://doi.org/10.1128/AAC.01117-17. Print 2017 Sep
Lahiri SD, Walkup GK, Whiteaker JD, Palmer T, McCormack K, Tanudra MA, Nash TJ, Thresher J, Johnstone MR, Hajec L, et al. Selection and molecular characterization of ceftazidime/avibactam-resistant mutants in Pseudomonas aeruginosa strains containing derepressed AmpC. J Antimicrob Chemother. 2015;70(6):1650–8.
Humphries RM, Yang S, Hemarajata P, Ward KW, Hindler JA, Miller SA, Gregson A. First report of Ceftazidime-Avibactam resistance in a KPC-3-expressing Klebsiella pneumoniae isolate. Antimicrob Agents Chemother. 2015;59(10):6605–7.
Shields RK, Nguyen MH, Press EG, Chen L, Kreiswirth BN, Clancy CJ: In Vitro Selection of Meropenem Resistance among Ceftazidime-Avibactam-Resistant, Meropenem-Susceptible Klebsiella pneumoniae Isolates with Variant KPC-3 Carbapenemases. Antimicrob Agents Chemother 2017, 61(5).
Sader HS, Castanheira M, Flamm RK, Mendes RE, Farrell DJ, Jones RN. Ceftazidime/avibactam tested against gram-negative bacteria from intensive care unit (ICU) and non-ICU patients, including those with ventilator-associated pneumonia. Int J Antimicrob Agents. 2015;46(1):53–9.
Sader HS, Castanheira M, Farrell DJ, Flamm RK, Jones RN. Ceftazidime-avibactam activity when tested against ceftazidime-nonsusceptible Citrobacter spp., Enterobacter spp., Serratia marcescens, and Pseudomonas aeruginosa from unites states medical centers (2011-2014). Diagn Microbiol Infect Disease. 2015;83(4):389–94.
Wang X, Zhang F, Zhao C, Wang Z, Nichols WW, Testa R, Li H, Chen H, He W, Wang Q, et al. In vitro activities of ceftazidime-avibactam and aztreonam-avibactam against 372 gram-negative bacilli collected in 2011 and 2012 from 11 teaching hospitals in China. Antimicrob Agents Chemother. 2014;58(3):1774–8.
Zenati K, Touati A, Bakour S, Sahli F, Rolain JM. Characterization of NDM-1- and OXA-23-producing Acinetobacter baumannii isolates from inanimate surfaces in a hospital environment in Algeria. J Hospital Infect. 2016;92(1):19–26.
Acknowledgements
Not applicable.
Funding
This work was supported by the National Natural Science Foundation of China (grant number 81802072), the First Affliated Hospital of Chengdu Medical College programs (grant numbers CYFY2018YB03) and two Chengdu Medical College programs (grant numbers CYZ16–17 and CYTD15–03). The present study was also supported by two grants from Sichuan Province Department of Education Program, China (grant number17TD0012 and grant number 15ZB0249). Those funding bodies provided funds for the purchase of consumption materials for the study and paid scholarships for students. The funding bodies were not involved in study design, data collection, analysis and writing of the manuscript.
Author information
Authors and Affiliations
Contributions
YX and XGY conceived the study, performed data analysis, and wrote the manuscript. DW contributed to data analysis and drafting of the manuscript. QZ and FN designed the study, led collection of clinical isolates, HFD, XLP, YZF and TTB carried out the experimental studies. All authors read and approved the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Microorganisms subject research approval was granted by the Ethics Committee of the First Affiliated Hospital of Chengdu Medical College. All participants gave written consent after the nature of scientific research explained by investigator or designee, in the process of consent, sufficient time is provided for questions and answers.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Additional file 1: Table S1.
The zone diameter range with MIC.
Additional file 2: Table S2.
The sensitivity and specificity of 30 μg/10 μg CAZ/AVI disk.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Yang, X., Wang, D., Zhou, Q. et al. Antimicrobial susceptibility testing of Enterobacteriaceae: determination of disk content and Kirby-Bauer breakpoint for ceftazidime/avibactam. BMC Microbiol 19, 240 (2019). https://doi.org/10.1186/s12866-019-1613-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12866-019-1613-5