Abstract
Although clinically distinguishable, migraine and cluster headache share prominent features such as unilateral pain, common pharmacological triggers such glyceryl trinitrate, histamine, calcitonin gene-related peptide (CGRP) and response to triptans and neuromodulation. Recent data also suggest efficacy of anti CGRP monoclonal antibodies in both migraine and cluster headache. While exact mechanisms behind both disorders remain to be fully understood, the trigeminovascular system represents one possible common pathophysiological pathway and network of both disorders. Here, we review past and current literature shedding light on similarities and differences in phenotype, heritability, pathophysiology, imaging findings and treatment options of migraine and cluster headache. A continued focus on their shared pathophysiological pathways may be important in paving future treatment avenues that could benefit both migraine and cluster headache patients.
Similar content being viewed by others
Background
In the field of cephalalgias, migraine has a prominent role (35,311 publications retrieved for search terms “migraine” in PubMed, accessed on August 15, 2018), with the recent breakthrough in therapeutics, represented by the successful clinical development of calcitonin gene-related peptide (CGRP) antibodies [1]. However, in the last 40 years, the number of papers published yearly for cluster headache (CH) has been constantly increasing (3845 publications retrieved for search terms “cluster headache” in PubMed, accessed on August 15, 2018), and new evidence is accumulating about epidemiology, including gender issues, pathophysiology and imaging. Differences and similarities between the two cephalalgias are presented here with a comparative approach. The clinical continuum that unexpectedly but not infrequently characterizes migraine and CH patients increases the value of such a comparison between the two diseases.
Epidemiology and genetics in migraine and cluster headache
Migraine is a highly prevalent disease, affecting at least 12% of the general population [2], with a lifetime prevalence up to 25% among women [3]. CH is a primary headache disease with an estimated prevalence at 0.5–1.0/1000 of the general population [4]. Both migraine and CH can be present from childhood and their prevalence increases until nearly 40 years of age, after which it gradually decreases [3, 5]. Twin studies demonstrate a heritability around 42% for migraine [6]. Five concordant monozygotic twin pairs with CH have been reported [7], indicating the importance of genetic factors in both disorders. The risk of first-degree relatives of patients with CH to develop CH is between five and fifteen times greater than that of the general population [7]. However, CH does not exhibit a clearly recognizable inheritance pattern. The genetic background of CH has been an unexplored field for years; genetic studies have been performed only recently, in small numbers of patients or as case reports. To date, targeted genes have been investigated, including the calcium voltage-gated channel subunit alpha1 A (CACNA1A) [8], three nitric oxide synthase (NOS) genes [9], the period circadian regulator 3 (PER3) [10] and the hypocretin receptor 2 (HCRTR2) [11], and none showed evidence of involvement in CH. In some families, the mode of inheritance is likely to be autosomal dominant with incomplete penetrance; in other families, it is more likely to be multifactorial or autosomal recessive [12, 13] (see Table 1).
In migraine, first-degree relatives of patients have a 3-fold increase in migraine, compared to the general population [14]. The risk increases in migraine with typical aura, supporting the idea that distinct genetic factors may regulate the inheritance of specific forms of migraine [15]. Rare monogenic migraine subtypes can be caused by precise genetic mutations, as in the case of familial hemiplegic migraine; a rare genetic disorder with dominant autosomal transmission due to mutations of three main genes (CACNA1A, ATP1A2 and the sodium channel 1 A SCN1A) [16]. These genes are not involved in common migraine [17] or in CH [8], in which numerous genes and environmental factors contribute to susceptibility in a manner still unclear. Several studies have failed to identify any association between genetic variants and common forms of migraine indicating that autosomal-dominant inheritance is unlikely, unless the penetrance of the gene is very low. Migraine is currently considered a polygenic disorder: multiple predisposing genes contribute, each with a small effect size, to the underlying risk [16]. New gene alterations have recently been related to CH [18,19,20], and a large meta-analysis has mapped 38 distinct genomic loci expressed in vascular and smooth muscle tissues, associated with migraine [21]. These results should be furthered in larger populations. Although both diseases are characterized by family aggregation, most noticeable in adulthood, CH is a rare disease, with a stronger genetic influence. Accordingly, the mode of inheritance is likely to be different between migraine and CH, and whether some genetic traits are shared between the two disorders is unknown.
Pathophysiology
In the pathophysiology of migraine and CH, both the peripheral nervous system and central nervous system are involved. Three key structures interact and subsequently involve cortical areas as well: the trigeminovascular system, parasympathetic nerve fibers (trigeminal autonomic reflex) and the hypothalamus [22].
Trigeminovascular system and trigemino-cervical reflex
In migraine and CH, pain is likely due to activation of the trigeminovascular system [22]. Nociceptive nerve fibers originate from the trigeminal ganglion (TG) and reach intracranial structures such as the dural, arachnoid and pial blood vessels, cerebral arteries and extracranial structures [22,23,24,25]. From TG the nociceptive signals project to neurons in the trigeminal cervical complex (TCC), including the trigeminal nucleus caudalis (TNC), and the dorsal horn of the upper cervical spinal cord (C1-C2) [24,25,26,27]. These projections from the TCC terminate on neurons of the trigeminal brainstem nuclear complex [28] and transmit all somatosensory information via further projections: to thalamic neurons (via a trigemino-thalamic tract), to hypothalamic nuclei (via a trigemino-hypothalamic tract), to basal ganglia nuclei and to brainstem nuclei including the locus coeruleus (LC) and periaqueductal gray (PAG) [25, 26, 28,29,30]. Subsequently, these structures reach several cortical areas involved in processing aspects of the nociceptive signals [26, 30].
Neuroimaging and neurophysiological investigations
Various neuroimaging studies implicate the brainstem in the pathophysiology of migraine and CH. In migraine, abnormalities are seen in both ascending and descending nociceptive pathways during ictal and inter-ictal phases [31]. Positron emission tomography (PET) imaging studies showed increased dorsal pons activation in migraine patients during the ictal phase [32]. Functional magnetic resonance imaging (fMRI) studies reported increased functional connectivity between the cortical and subcortical regions involved in nociceptive processing and the PAG [33, 34], having connections coming from the thalamus, hypothalamus, and autonomic nervous system [31].
A dysfunction of pain control systems in both headaches and a role of the brainstem in their pathogenesis is also supported by neurophysiological studies. In migraine, loss of habituation, lower cortical pre-activation and abnormal sensitization was seen [35]. In CH, altered pain perception and decreased pain thresholds was found [36].
In migraine some studies reported that the blink reflex (which reflects excitability of interneurons in the brainstem) is delayed and reduced in amplitude [37, 38]. However, other studies did not confirm these conclusions [39, 40]. In cluster headache patients, during active phase, and on the headache side, a pronounced lack of habituation of the brainstem and a general sensitization of pain processing is seen [41]. These results point towards dysfunctional connections between the brainstem and trigeminovascular system, again supporting the trigeminovascular hypothesis [38].
In summary, electrophysiological studies show that the migraine brain presents some interrelated functional characteristics: 1. lack of habituation of evoked responses to repeated stimuli; 2. cortical dysexcitability. The lack of habituation was reported by examining visual evoked potentials (VEP) [41,42,43,44,45,46,47,48,49,50] by using magneto-electroencephalography [51, 52], with somatosensory [45, 46] and auditory [53, 54] evoked cortical potentials pain (laser, LEP) [55] and event-related (contingent negative variation) responses [56, 57] in migraine between attacks [58]. Regarding cortical dysexcitability, conflicting results were presented in various trials, suggesting cortical hypoexcitability [59, 60] as well as hyperexcitability [61, 62]. Recent works suggest that abnormal rhythmic activity between thalamus and cortex induce a low level of cortical preactivation. This might explain the abnormal functional characteristics in migraine mentioned above. Abnormal processing could be due to hypoactivity of some pathways (such as the serotonergic pathway), causing heightened response to repeated stimuli, thus resulting in an excessive energy demand [63]. Changes in energy demand may disrupt cerebral metabolic homeostasis and thus activate the major alarm signalling system of the brain, the trigeminovascular system, ultimately resulting in a migraine attack [63].
Trigeminal autonomic reflex and cranial parasympathetic symptoms
Somatosensory pathways are connected to autonomic pathways through reflex connections from the TNC to the superior salivatory nucleus (SuS). The SuS contains neurons that are part of the cranial parasympathetic autonomic vasodilator pathway [28, 64, 65]. These neurons project to the cranial vasculature, including dura mater, to the nasal and oral mucosa and lacrimal glands mainly through the sphenopalatine ganglion (SPG) [28]. Activation of the cranial SuS-parasympathetic pathway is believed to directly contribute to cranial autonomic symptoms found in cluster headache and up to 50% in migraine patients [29, 66]. Indeed, activation of this pathway induces a dilation of intracranial vessels and a cascade of events that results in plasma protein extravasation, neuropeptide release from dural vascular terminals of post-SPG neurons [28], local dural release of inflammatory mediators with perivascular alteration and activation and sensitization of the trigeminovascular system [23, 27]. The SuS also has a bidirectional connection with the hypothalamus (including the lateral [65, 67], paraventricular, dorsomedial and pre-optic hypothalamic nuclei [65, 68]), as well as with the limbic and cortical areas [65].
Hypothalamus
The hypothalamus is involved in numerous physiological functions including controlling circadian rhythm [22, 69]. Furthermore, it has several connections involved in pain modulation in migraine as well as in cluster headache [36]. The hypothalamus also partakes in autonomic and endocrine regulation [23]. Preclinical data show that specific hypothalamic nuclei, such as the paraventricular and lateral hypothalamus, reach the TNC and SuS neurons through descending projections [22, 65, 67, 68, 70, 71], thus influencing and triggering somatosensory and autonomic neurovascular mechanisms [23]. The premonitory symptoms of headaches are considered the clinical side of an underlying hypothalamic dysregulation. Many neuro-endocrinological data support the hypothesis of hypothalamic–pituitary–adrenal axis failure in these primary headache disorders [72].
Neuroimaging and neurophysiological investigations
fMRI studies report a role of the hypothalamus in pain modulation during the pre-ictal phase of attacks in migraine patients. Particularly, it is hypothesized that the anterior part of the hypothalamus may be involved in migraine chronification, whereas the posterior part may play a role in the acute pain phase [73].
In CH, activation in the hypothalamic grey matter ipsilateral to the side of a headache during attacks is seen with PET [74] and fMRI [75]. Also, altered functional connectivity of the hypothalamus and anterior thalamus were described. A voxel-based morphometry (VBM) study [64] revealed concomitant grey matter volume increase of this hypothalamic region, but other VBM studies did not substantiate these results [76,77,78,79]. Interestingly, a recent work [80] hypothesized that the anterior hypothalamus might contribute to the circadian rhythm of CH attacks [22], whereas the posterior part might generate the restlessness experienced by CH patients during the attack [81].
Alterations of resting state activity [82], were found in the attention network ipsilateral to the pain and in the contralateral cerebellar network. This result coincides with previous repetitive transcranial magnetic stimulation (rTMS) studies showing increased cortical excitability ipsilateral to the pain in CH [82], similar to that in a migraine [83]. Resting state studies showed altered activity of the medial frontal cortex which is part of various resting state networks important in pain perception [75, 84]. This disorganized connectivity could be a consequence of white matter microstructural alteration described in CH [85].
Lastly, cognitive processing studies employing event-related potentials are useful in elucidating cortical activation time courses during cognitive processing [86, 87]. The hypothalamic dysfunction might also explain the habituation deficit of the brainstem and the general sensitization of pain processing detected in patients with CH [88]. Neurophysiological studies of sensory evoked potentials show various abnormalities but not as homogenously as displayed in migraine [89,90,91]. The intensity dependence of auditory evoked potentials is also increased in CH patients, during and outside active phase, possibly suggesting decreased serotoninergic activity in the hypothalamic pathways [92].
Other brain structures
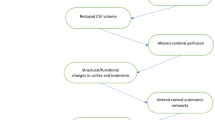
In addition to the above-mentioned studies involving brainstem and hypothalamus, patients with primary headaches experience dynamic structural [93] and functional [75] changes in cortical-subcortical areas involved in nociception.
In migraine, fMRI and resting-state fMRI studies show marked abnormalities both ictally and interictally in areas involved in nociceptive processing and networks involved in mediating cognitive, attentional, somatosensory and emotional components of pain [33, 52, 94,95,96,97], respectively. These networks may influence multisensory integration and pain experience in migraine patients. Structural MRI studies also show a decrease in grey matter in various regions of the brain such as frontal, parietal and temporal cortex (Table 2). However, neuroimaging data on the association of white matter hyperintensities and migraine has been conflicting. Some studies show a higher occurrence of subcortical, deep, and cerebellar ischemic hyperintensities in migraineurs [98], whereas other studies fail to confirm such findings [99].
In CH, a decrease of the grey matter in several regions was shown using structural MRI [78]. Structural alterations in the striatum [93, 100] and atrophy of the thalamus and the caudate nucleus has also been reported. Importantly, in addition to a decrease also an increase in the right cuneus was observed [78]. Recent resting-state fMRI studies found abnormal functional connectivity in the sensorimotor and primary visual networks during the pain-free period, as well as between the hypothalamus and pain network areas in active phase [84, 87, 95] (Table 2). Diffusion-tensor imaging studies investigating white matter microstructural changes offer contradictory findings [36, 78, 101]. Some report the absence of white matter abnormalities [78]. Others report widespread white matter microstructural changes, particularly in the pain networks such as the frontal lobe, parietal lobe, temporal lobe and thalamus [36, 85].
Clinical picture
Phenotypes
Migraine and CH are diagnosed according to the International Classification of Headache Disorders (ICHD-3), which are evidence-based primarily on patient populations [102]. Although the clinical presentation of migraine and CH is usually different, these primary headaches often share some similarities in the headache phenotype, such as unilateral and severe pain and some associated symptoms including aura [103, 104] (Table 3). Moreover, coexistence of these two primary headaches simultaneously has been reported [105].
CH attacks are usually associated with multiple prominent ipsilateral cranial autonomic symptom, such as conjunctival injection, lacrimation, rhinorrhea, forehead sweating, miosis and/or ptosis [22, 106]. These symptoms have also been described in migraineurs, but patients usually report only one symptom (forehead sweating the most frequent) and in contrast to CH, they are less frequent, bilateral and mild [66].
Interestingly, different cohorts have revealed that CH patients without comorbid migraine frequently experience accompanying ‘migrainous associated symptoms’, such as photophobia, phonophobia, nausea or vomiting [104, 107]. Furthermore, CH attacks are associated with specific chronobiological features, mainly circadian (most frequently nocturnal) and circannual (most frequently spring or autumn) rhythms [22]. In contrast, migraine attacks are most frequently reported to occur during the day and no clear seasonal rhythm has been stablished yet [108].
When migraine attacks occur on 15 or more days/month for more than three months it is considered chronic [102]. Each year 2.5–3% of patients with episodic migraine transform to chronic migraine (CM), fortunately these patients frequently revert back to episodic migraine [109, 110].
CH attacks occurring for one year or longer without remission or with remission periods lasting less than three months (10–15%) are classify as chronic [102]. CCH may be unremitting from onset (de novo), or evolve from the episodic type and in some patients a change from chronic to episodic may occur [111]. Furthermore, a recent consensus from the European Headache Federation defined refractory CCH as a situation that fulfills ICHD-3 for CCH with at least three severe attacks per week despite at least three consecutive trials of adequate preventive treatments [112].
Triggers
Migraine and CH patients report a remarkable number of common triggers – both naturally occurring events such as stress, sleep, alcohol intake and weather changes [106, 107, 113], but also pharmacological triggers [22, 114]. It has been suggested that these triggers are common trigeminal system activators [105, 109].
Identification and avoidance of attack triggers plays an important role in management of patients with migraine and CH. Attack triggers may also provide clues to their underlying pathophysiology [115]. While naturally occurring attack triggers are useful in management of individual patients they may be of limited use in experimental provocation studies. Thus, in a study of self-reported triggers of migraine with aura only 17% of patients developed an attack following exposure to their natural attack trigger [116]. For a comprehensive review on specific natural attack triggers of primary headaches see Pellegrino et al. 2017 [115].
The earliest pharmacological provocation studies in migraine and CH patients explored histamine [117,118,119] and found that histamine infusion, which causes endogenous nitric oxide (NO) formation, induces attacks in both migraine and CH. In a double-blind, randomized pretreatment study in 20 migraine patients without aura (MwoA) [117], a 20 min intravenous histamine infusion was pretreated with mepyramine (0.5 μg/kg/min for 10 min) or placebo infusion (n = 10, each). In the placebo pretreated group 7 of 10 MwoA patients reported migraine-like attack following histamine infusion compared to 0 of 10 in the mepyramine group. In the placebo pretreated group the average time to peak headache was 5 h. In CH, nine patients received subcutaneous injection of histamine (0.01 mg/kg body weight) [120]. All nine CH patients developed CH-like attacks after a median time of 45 min. The study was neither blinded nor placebo controlled.
Glyceryl trinitrate (GTN), a prodrug of NO, was given intravenously (0.5 μg/kg/min for 20 min) in a double-blind, placebo controlled, cross over study on 12 MO patients [121]. At a median time of 5.5 h after GTN infusion 8 of 10 patients fulfilled criteria for a migraine attack compared to only one after placebo. In CH, several non-placebo controlled provocation studies found that GTN induces CH in episodic active phase in 33–100% of patients [122,123,124,125] and in CCH in 20–78% of patients [125, 126]. In remission phase episodic CH patients GTN induced no attacks [122,123,124]. Mean time to onset of attacks was 12–72 min after infusion start [120, 122, 124]. NO, among other things, increases intracellular cyclic guanosine monophosphate (cGMP) [127]. Sildenafil, a phosphodiesterase 5 inhibitor, which also increases intracellular cGMP, also induced migraine attacks in 10 of 12 MO patients compared to 2 of 12 after placebo [128]. In CH, cases of sildenafil (prescribed for erectile dysfunction) triggering CH attacks in active phase [129, 130] and even triggering an active phase itself [131] have been reported. In a randomized, double-blind, placebo controlled cross-over study in 12 MwoA patients, vasoactive signaling molecule CGRP was infused intravenously (2.0 μg/min for 20 min) [132]. In the paper, authors stated that three of nine MO patients developed delayed migraine attacks as strictly defined by criteria from the International Headache Society on CGRP compared to zero of nine on placebo. When revisiting these results and applying newer, modified criteria for pharmacologically induced migraine-like attacks, CGRP induced delayed migraine-like attacks in six of nine MO patients compared to one in nine after placebo [133]. In a recent study, 32 CH patients (9 episodic active phase, 9 episodic remission phase, and 14 chronic) received intravenous infusion of CGRP (1.5 μg/min for 20 min) or placebo in a randomized, double-blind, placebo controlled cross-over study [134]. CGRP induced cluster-like attacks in 89% of episodic active phase patients compared to 11% after placebo, and in 50% of chronic patients compared to 0% after placebo. In episodic remission phase CH patients neither CGRP nor placebo induced any attacks. Median time to onset of attacks was 20 min in CCH and 30 min in episodic active phase CH. This was the first placebo controlled provocation study in CH. Authors conclude that these findings point to the possibility of efficacy of CGRP antagonism, already known to prevent and abort migraine [135,136,137,138], in CH as well. Such antibodies against CGRP are currently under investigation in CH [22]. Recently the efficacy in reduction of weekly attacks in episodic but not CCH was announced [139].
Thus, although migraine and CH have several pharmacological triggers in common, the time to onset of attacks seems to vary predictably between the two diseases with CH generally being triggered faster than the average induced migraine attack [117, 120, 132, 140, 141]. In migraine, delayed attacks are thought to arise from the pharmacological trigger playing a role relatively early in spontaneous migraine attack initiation [114]. Thus, the short time to attack in CH might reflect a shorter cascade of events in CH attack initiation relative to migraine.
Migraine and CH are linked pathophysiologically by common neuronal structures, however, they are (usually) influenced differently by lifestyle, environmental, hormonal and genetic factors [107]. This shared pathophysiology is supported by common environmental and pharmacological attack triggers and similar efficacy with some treatments (see next section). Unfortunately, research about the pathophysiological interactions between diseases is scarce and these questions remain to be elucidated.
Gender aspects
Migraine and CH show distinct and inverse gender-related characteristics. Migraine is two to three times more common in women than in men, estimates varying from 13% to 17% for females and 7.6% to 10% for males [142]. On the contrary, CH is a male-dominated disorder with the ratio of men to women estimated to range from 3:1 to 7:1 [143]. Puberty is a turning point for the predominance of gender in both primary headaches, which in childhood show a similar distribution by gender [144, 145]. According to the onset of the disease, gender differences are more evident in the third decade of life for both migraine and CH, and women with CH show a further peak of incidence between the ages of fifty and sixty years [143]. In older people, the gender-related aspects vanish in both disorders.
Women experience migraine or CH differently than men. Women report more severe and longer attacks [146]. Moreover, women with migraine are more likely to report nausea, vomiting, photophobia, phonophobia and aura associated with headache [147]. Men and women with CH have similar clinical phenotypes [148], with no apparent differences in pain intensity, quality and location. Women with CH report more nausea and vomiting than men, but it is unclear whether this is caused by a generally higher proportion of concomitant migraine [149]. Additionally, women with CH appear to respond more poorly to some abortive and preventative treatments [150]. The reasons for the opposite gender characteristics in migraine and CH are not fully understood. The underlying causes are likely to be multifactorial, involving both biological and psychosocial factors. Among biological factors, previous studies have focused on fluctuations in sex hormones and the exploration of genetic factors, without obtaining a definitive response [151].
Treatment
Migraine and CH therapy includes the acute therapy to abort the single attack, and preventive therapy to reduce attack frequency, duration and severity and the use of acute headache medications.
Acute therapy
As in migraine, CH attacks respond well to acute therapy with triptans [152,153,154]. Nevertheless, differently from migraine, the oral route of administration is not usually recommended in CH, because of the delayed effect compared to subcutaneous or intranasal administration. On the other hand, acetaminophen and non-steroidal anti-inflammatory drugs are only used in the acute therapy of migraine and not in CH [155]. Shared pathophysiological mechanisms as reviewed in previous sections could explain the efficacy of triptans in both diseases.
Another acute approach for the treatment of CH attacks is inhalation of 100% oxygen through a face mask (with a flow of 12–15 l/min). Interestingly, a recent randomized placebo-controlled clinical trial on 22 patients reported that high-flow oxygen was significantly more effective than air in the acute treatment of migraine attacks [156], and it has been suggested that this treatment could have greater responses in migraine patients with cranial autonomic symptoms [157] or migraine-cluster and cluster-migraine variants (these rare phenotypes are not included in the ICHD-3). An inhibition of activated trigeminal nociceptive afferents or the autonomic pathway could be one of the mechanisms explaining its efficacy in both migraine and CH [158].
Lastly, in patients affected by CH, when oxygen and triptans are ineffective, intranasal lidocaine (sprayed in the ipsilateral nostril) should be considered [125]. Clinical trials provided conflicting data about its efficacy in migraine [159,160,161].
Taken together, the previous suggests that, although with different preferable route of administration (for triptans) and response rate (for oxygen inhalation) migraine and CH share responsiveness to some acute strategies (see Table 4).
Preventive therapy
Different drug categories are effective in the prophylactic treatment of patients affected by episodic or CCH, even though, unlike in migraine, few randomized clinical trials have been conducted [162]. Similarities and differences in migraine and CH preventive therapies are summarized in Table 5.
High-dose verapamil is the most frequently used in CH preventive therapy [163]. Interestingly, few studies suggested the efficacy of verapamil in migraine prophylaxis [164, 165]. Lithium carbonate is mainly used as a prophylactic drug in CCH to reduce the attack frequency in patients [166, 167]. To date, no randomized clinical trials have studied the efficacy of lithium in migraine prophylaxis. Small open trials reported conflicting results in migraine [168, 169]. A short term effective therapy for CH is represented by prednisone [77, 170] which can be used for short-duration episodes or to induce a rapid remission (usually within 3–10 days). Evidences about the use of steroids in the preventive therapy of migraine do not allow precise conclusions. Nevertheless a recent review showed that steroids demonstrated a good efficacy in reducing the recurrence of migraine in patients visiting the emergency department for acute attacks [171]. Blockade of the greater occipital nerve (GON) ipsilateral to the pain, with injection of corticosteroids and local anesthetic is effective in CH [172] and was also shown to be effective in treatment of CM [173].
In migraine, the efficacy of sodium valproate and topiramate has been documented in RCTs [174, 175]. In CH, even though open uncontrolled studies indicated a good efficacy, RCTs did not show any clinical efficacy of sodium valproate and topiramate [176,177,178,179,180].
Open trials showed clinical efficacy of local injection of onabotulinumtoxin A into the sphenopalatine ganglion (SPG) both in CH [181] and in refractory CM therapy [181]. The Phase III REsearch Evaluating Migraine Prophylaxis Therapy 1 and 2 (PREEMPT 1 and 2) have shown the efficacy of Onabotulinumtoxin A in reduction of headache days in CM, using a specific injection protocol [182, 183]. The PREEMPT study protocol was also used in a 28 week, open-label trial, with refractory CCH [184]. A more than 50% reduction in headache minutes was reached in 58.8%, whereas 29.4% experienced a 30–50% of improvement. Mean frequency of headache days dropped from 28 to 12 days at week 24 (p = .0001). Randomized controlled trials are needed to confirm these encouraging results.
Randomized clinical trials have indicated that melatonin may be effective for the preventive treatment of CH, with a daily dose of 10 mg [185] and migraine, with a dose of 3 mg [186].
Anti CGRP monoclonal antibodies (mAbs) are effective in migraine prophylaxis [135,136,137,138] and the anti CGRP receptor mAbs erenumab is now approved by Food and Drug Administration (FDA) [187]. Ongoing trials (NCT02964338, NCT02797951, NCT02397473, NCT02438826) are investigating the efficacy of anti CGRP mAbs in CH. Recently, an Eli Lilly press release announced that a phase 3 study (NCT02797951) showed that galcanezumab reduced weekly attacks in episodic but not CCH patients [188].
The efficacy of anti CGRP monoclonal antibodies and greater occipital nerve (GON) blockade in both migraine and CH indicates that the activation of the trigeminovascular system (with consequent release of CGRP) and the TCC is a key mechanism involved in the pathogenesis of both migraine and CH. Furthermore, the good response to oral corticosteroids as a transitional treatment may indicate that they may reduce the neurogenic inflammation induced by the activation of the trigeminovascular system in both diseases. The efficacy of melatonin in the prophylactic therapy for both migraine and CH points towards a pathogenetic role for the hypothalamus and the circadian rhythm regulation system in both migraine and CH. The pharmacological effect of verapamil is probably due to the interactions with muscarinic, serotoninergic and dopaminergic receptors, the inhibition of presynaptic adrenergic receptors (with a consequent increase in noradrenaline release) and the modulation of pain pathways. Its efficacy in both migraine and CH could be due to the modulation of brainstem circuitries, the rebalancing of autonomic system and the restoration of the pain control system [189].
In conclusion, even though the first line strategies for migraine and CH treatment seem to be quite different, most of the drugs used for CH prophylaxis also demonstrated a certain degree of efficacy in migraine prophylaxis, showing that migraine and CH, even with their clinical differences may share some of their basic pathophysiological mechanisms.
Neuromodulation
Invasive neuromodulatory procedures comprise stimulation of the central nervous system, hypothalamic deep brain stimulation (hDBS) and of the peripheral nerves (occipital nerve stimulation, ONS; SPG). Non-invasive variants comprise vagus nerve stimulation (VNS), supraorbital nerve stimulation (SNS), rTMS and transcranial direct current stimulation (tDCS).
The rationale for the use of hDBS is an increased blood flow in the posterior hypothalamus during cluster [74] and migraine attacks [190], which was interpreted as neuronal activation of that brain area. hDBS has been shown to be highly effective in CH, with significant reduction of attack frequency and with the ability to change disease course [22, 191,192,193]. Although the treatment effects seem clinically equal, the side effects of the more invasive hDBS treatment are to be considered [194]. So far, there is no evidence to support the use of hDBS in CM.
The basis for the use of ONS in headaches came from animal studies showing the convergence of cervical, somatic and dural afferents on second order nociceptors in the trigeminocervical complex [195, 196]. More or less all these structures are involved in the pathophysiology of CM and CH. For ONS, to date, 3 RCTs have been performed in CM [197,198,199], and their outcome is overall disappointing. For CH multiple isolated reports, case series, small cohort studies and observational studies suggested a 50% improvement in headache frequency or intensity with ONS [200, 201].
The SPG is a large extracranial parasympathetic ganglion located in the pterygopalatine fossa. Post-ganglionic parasympathetic fibers from the SPG innervate facial structures such as the salivary and lacrimal glands, the nasopharyngeal mucosa and the cerebral and meningeal blood vessels [202]. Mainly all these structures are involved in the pathophysiology of CH and partially also in CM. SPG electrical stimulation via an implantable device was proven for effective in a multicentre randomised, double-blind and sham-controlled trial in refractory CCH [203]. Full stimulation of the SPG versus sham stimulation resulted in a significant pain relief (67%) and a significant reduction in attack frequency (34%) [203]. Only anecdotal cases have been reported for migraine treatment with SPG, usually reserved for cases of refractory migraine [204]. SPG has been targeted also with blockade via bupivacaine, which showed, in CM, a sustained reduction of headache frequency in a double-blind, parallel-arm, placebo-controlled, randomized pilot study [205].
VNS has been shown to be effective in both migraine and CH. Indeed, in small open-label single-arm studies, VNS had good migraine abortive effect, with 43 to 65% of patients obtaining pain relief [206, 207]. The recent multicenter, double-blind, randomized, sham-controlled PRESTO trial confirmed VNS effective as abortive treatment for migraine attacks, with consistent therapeutic benefit compared to sham stimulation [208]. In the EVENT trial, a double-blind sham-controlled study on migraine prevention, though not reaching the primary outcome, VNS led to a slight reduction in migraine frequency [209].
CH patients can also benefit from VNS. In an open-label, prospective, randomized study, a significant reduction in weekly attack frequency was observed among patients with CCH receiving VNS plus standard of care compared to standard of care alone [210, 211]. Moreover, VNS has been shown to be cost-effective, providing economic benefits as an adjunct treatment to standard of care in CCH [212].
rTMS has effect as prophylactic treatment in migraine with aura. In a sham controlled randomized trial, single pulse rTMS has been shown to increase in freedom from pain after 2 h when applied early in the treatment of migraine with aura, with substantial benefit for up to 48 h after treatment [213] Although cortical excitability has been implicated in CH [82], to date few data exist on rTMS in CH.
In migraine prevention, SNS has been extensively studied and shown to provide a significant reduction of migraine days compared to sham stimulation [214, 215]. On the contrary, SNS in CH has been poorly investigated, and only isolated reports of possible positive neuromodulation among CH are available [216].
Overall, few data still exist on neuromodulation strategies in headache disorders. Nevertheless, data from randomised controlled trials seem to suggest safety and effectiveness in both migraine and CH (see Table 6), supporting the concept that these two diseases, despite their differences, might share pathophysiological mechanisms. The common denominator might be the hyperexcitability of brain network, progressive changes in nociceptive thresholds and subsequent central sensitization. For CCH, SPG [217, 218] or ONS [197, 219], given the risk/benefit profile of the intervention, might be considered before hDBS. In migraine VNS might be considered as an abortive effective treatment, also able to spare symptomatic drugs. For patients with CM the use of ONS, as well as the application of the non-invasive VNS, tDCS, rTMS, cannot be recommended so far, given the poor amount of controlled data.
Conclusions
Migraine and CH show remarkable similarities with common triggers [22, 114], efficacy of triptans [220, 221], anti CGRP monoclonal antibodies [135,136,137,138, 188] and neuromodulation [222]. These observations raise an important question on possible shared pathophysiological mechanisms. The central denominator in both diseases may be the trigeminovascular pathway, alteration in hypothalamic activity and functional changes in hypothalamic–brainstem connectivity. A key signalling molecule, CGRP, is involved in migraine and CH [223, 224]. The importance of the pituitary adenylate-cyclase activating peptide (PACAP) is well established in migraine [140] and an ongoing phase 2 study is testing the efficacy of a PAC1 receptor antibody for migraine prevention [225]. Future studies will show whether migraine and CH shares the involvement of PACAP signalling in pathophysiology.
Abbreviations
- CACNA1A:
-
calcium voltage-gated channel subunit alpha1 A
- CCH:
-
chronic cluster headache
- cGMP:
-
cyclic guanosine monophosphate
- cGMP:
-
cyclic guanosine monophosphate
- CGRP:
-
calcitonin gene-related peptide
- CH:
-
cluster headache
- CM:
-
chronic migraine
- FDA:
-
Food and Drug Administration
- fMRI:
-
functional magnetic resonance imaging
- GON:
-
greater occipital nerve
- GTN:
-
glyceryl trinitrate
- HCRTR2:
-
hypocretin receptor 2
- hDBS:
-
hypothalamic deep brain stimulation
- ICHD 3:
-
International Classification of Headache Disorders 3rd edition
- LC:
-
locus coeruleus
- MwoA:
-
migraine without aura
- NO:
-
nitric oxide
- NOS:
-
nitric oxide synthase
- ONS:
-
occipital nerve stimulation
- PAC1:
-
pituitary adenylate cyclase receptor 1
- PACAP:
-
pituitary adenylate-cyclase activating peptide
- PAG:
-
periequiductal grey
- PER3:
-
period circadian regulator 3
- PET:
-
positron emission tomography
- PREEMPT:
-
Phase III REsearch Evaluating Migraine Prophylaxis Therapy
- rTMS:
-
repetitive transcranial magnetic stimulation
- rTMS:
-
repetitive transcranial magnetic stimulation
- SCN1A:
-
sodium channel 1 A
- SNS:
-
supraorbital nerve stimulation
- SPG:
-
sphenopalatine ganglion
- SuS:
-
superior salivatory nucleus
- TCC:
-
trigeminal cervical complex
- tDCS:
-
transcranial direct current stimulation
- TG:
-
trigeminal ganglion
- TNC:
-
Trigeminal nucleus caudalis
- VEP:
-
visual evoked potentials
- VNS:
-
vagus nerve stimulation
References
Schuster NM, Rapoport AM (2016) New strategies for the treatment and prevention of primary headache disorders. Nat Rev Neurol 12:635–650
Lipton RB, Bigal ME, Diamond M et al (2007) Migraine prevalence, disease burden, and the need for preventive therapy. Neurology 68:343–349
Rasmussen B (2001) Epidemiology of headache. Cephalalgia 21:774–777
Fischera M, Marziniak M, Gralow I et al (2008) The incidence and prevalence of cluster headache: a meta-analysis of population-based studies. Cephalalgia 28:614–618
Bahra A, May A, Goadsby PJ (2002) Cluster headache: a prospective clinical study with diagnostic implications. Neurology 58:354–361
Mulder EJ, Van Baal C, Gaist D et al (2003) Genetic and environmental influences on migraine: a twin study across six countries. Twin Res 6:422–431
Russell MB (2004) Epidemiology and genetics of cluster headache. Lancet Neurol 3:279–283
Haan J, Van Vliet JA, Kors EE et al (2001) No involvement of the calcium channel gene (CACNA1A) in a family with cluster headache. Cephalalgia 21:959–962
Sjöstrand C, Modin H, Masterman T et al (2002) Analysis of nitric oxide synthase genes in cluster headache. Cephalalgia 22:758–764
Ofte HK, Tronvik E, Alstadhaug KB (2016) Lack of association between cluster headache and PER3 clock gene polymorphism. J Headache Pain 17:18
Weller CM, Wilbrink LA, Houwing-Duistermaat JJ et al (2015) Cluster headache and the hypocretin receptor 2 reconsidered: a genetic association study and meta-analysis. Cephalalgia 35:741–747
Russell MB, Andersson PG, Thomsen LL et al (1995) Cluster headache is an autosomal dominantly inherited disorder in some families: a complex segregation analysis. J Med Genet 32:954–956
De Simone R, Fiorillo C, Bonuso S et al (2003) A cluster headache family with possible autosomal recessive inheritance. Neurology 61:578–579
Russell MB, Hilden J, Sorensen SA et al (1993) Familial occurrence of migraine without aura and migraine with aura. Neurology 43:1369–1373
Russell MB, Ulrich V, Gervil M et al (2002) Migraine without Aura and Migraine with Aura are distinct disorders. A population-based twin survey. Headache J Head Face Pain 42:332–336
Sutherland HG, Griffiths LR (2017) Genetics of migraine: insights into the molecular basis of migraine disorders. Headache 57:537–569
Kirchmann M, Thomsen LL, Olesen J (2006) The CACNA1A and ATP1A2 genes are not involved in dominantly inherited migraine with aura. Am J Med Genet - Neuropsychiatr Genet 141(B):250–256
Costa M, Squassina A, Piras IS et al (2015) Preliminary transcriptome analysis in Lymphoblasts from cluster headache and bipolar disorder patients implicates dysregulation of circadian and serotonergic genes. J Mol Neurosci 56:688–695
Bacchelli E, Cainazzo MM, Cameli C et al (2016) A genome-wide analysis in cluster headache points to neprilysin and PACAP receptor gene variants. J Headache Pain 17:114
Fourier C, Ran C, Zinnegger M et al (2018) A genetic CLOCK variant associated with cluster headache causing increased mRNA levels. Cephalalgia 38:496–502
Gormey P, Antilla V, Winsvold B et al (2016) Meta-analysis of 375,000 individuals identifies 38 susceptibility loci for migraine. Nat Genet 48:856–866
Hoffmann J, May A (2018) Diagnosis, pathophysiology, and management of cluster headache. Lancet Neurol 17:75–83
Hoffmann J, Baca SM, Akerman S. Neurovascular mechanisms of migraine and cluster headache. J Cereb Blood Flow Metab 2017; 271678X17733655
Puledda F, Messina R, Goadsby PJ (2017) An update on migraine: current understanding and future directions. J Neurol 264:2031–2039
Goadsby PJ, Holland PR, Martins-Oliveira M et al (2017) Pathophysiology of migraine: a disorder of sensory processing. Physiol Rev 97:553–622
Dodick DW (2018) A phase-by-phase review of migraine pathophysiology. Headache 58(Suppl 1):4–16
Burstein R, Noseda R, Borsook D (2015) Migraine: multiple processes, complex pathophysiology. J Neurosci 35:6619–6629
Vila-Pueyo M, Hoffmann J, Romero-Reyes M et al (2018) Brain structure and function related to headache: brainstem structure and function in headache. Cephalalgia 333102418784698
Akerman S, Holland PR, Goadsby PJ (2011) Diencephalic and brainstem mechanisms in migraine. Nat Rev Neurosci 12:570–584
Zhang X, Levy D, Kainz V et al (2011) Activation of central trigeminovascular neurons by cortical spreading depression. Ann Neurol 69:855–865
Boran HE, Bolay H (2013) Pathophysiology of migraine. Noro Psikiyatr Ars 50:S1–S7
Afridi SK, Matharu MS, Lee L et al (2005) A PET study exploring the laterality of brainstem activation in migraine using glyceryl trinitrate. Brain 128:932–939
Mainero C, Boshyan J, Hadjikhani N (2011) Altered functional magnetic resonance imaging resting-state connectivity in periaqueductal gray networks in migraine. Ann Neurol 70:838–845
Schwedt TJ, Schlaggar BL, Mar S et al (2013) Atypical resting-state functional connectivity of affective pain regions in chronic migraine. Headache 53:737–751
Abanoz Y, Abanoz Y, Gunduz A et al (2016) Trigeminal somatosensorial evoked potentials suggest increased excitability during interictal period in patients with long disease duration in migraine. Neurosci Lett 612:62–65
May A, Schwedt TJ, Magis D et al (2018) Cluster headache. Nat Rev Dis Prim 4:18006
Avramidis TG, Podikoglou DG, Anastasopoulos IE et al (1998) Blink reflex in migraine and tension-type headache. Headache 38:691–696
Unal Z, Domac FM, Boylu E et al (2016) Blink reflex in migraine headache. North Clin Istanbul 3:1–8
Aktekin B, Yaltkaya K, Ozkaynak S et al (2001) Recovery cycle of the blink reflex and exteroceptive suppression of temporalis muscle activity in migraine and tension-type headache. Headache 41:142–149
Galeotti F, Truini A, Cruccu G (2006) Neurophysiological assessment of craniofacial pain. J Headache Pain 7:61–69
Coppola G, Di Lorenzo C, Schoenen J et al (2013) Habituation and sensitization in primary headaches. J Headache Pain 14:65
Schoenen J, Wang W, Albert A et al (1995) Potentiation instead of habituation characterizes visual evoked potentials in migraine patients between attacks. Eur J Neurol 2:115–122
Wang W, Wang GP, Ding XL et al (1999) Personality and response to repeated visual stimulation in migraine and tension-type headaches. Cephalalgia 19:718
Bohotin V, Fumal A, Vandenheede M et al (2002) Effects of repetitive transcranial magnetic stimulation on visual evoked potentials in migraine. Brain 125:912–922
Ozkul Y, Bozlar S (2002) Effects of fluoxetine on habituation of pattern reversal visually evoked potentials in migraine prophylaxis. Headache 42:582–587
Coppola G, Curra A, Serrao M et al (2010) Lack of cold pressor test-induced effect on visual-evoked potentials in migraine. J Headache Pain 11:115–121
Coppola G, Curra A, Sava SL et al (2010) Changes in visual-evoked potential habituation induced by hyperventilation in migraine. J Headache Pain 11:497–503
Coppola G, Curra A, Di Lorenzo C et al (2010) Abnormal cortical responses to somatosensory stimulation in medication-overuse headache. BMC Neurol 10:126
Fumal A, Coppola G, Bohotin V et al (2006) Induction of long-lasting changes of visual cortex excitability by five daily sessions of repetitive transcranial magnetic stimulation (rTMS) in healthy volunteers and migraine patients. Cephalalgia 26:143–149
Schoenen J, Clemente L Di, Vandenheede M, et al. Hypothalamic stimulation in chronic cluster headache : a pilot study of efficacy and mode of action 2005; 940–947
Chen W-T, Wang S-J, Fuh J-L et al (2009) Peri-ictal normalization of visual cortex excitability in migraine: an MEG study. Cephalalgia 29:1202–1211
Chen W-T, Wang S-J, Fuh J-L et al (2011) Persistent ictal-like visual cortical excitability in chronic migraine. Pain 152:254–258
Wang W, Timsit-Berthier M, Schoenen J (1996) Intensity dependence of auditory evoked potentials is pronounced in migraine: an indication of cortical potentiation and low serotonergic neurotransmission? Neurology 46:1404–1409
Ambrosini A, Rossi P, De Pasqua V et al (2003) Lack of habituation causes high intensity dependence of auditory evoked cortical potentials in migraine. Brain 126:2009–2015
de Tommaso M, Libro G, Guido M et al (2005) Habituation of single CO2 laser-evoked responses during interictal phase of migraine. J Headache Pain 6:195–198
Maertens de Noordhout A, Timsit-Berthier M, Timsit M et al (1986) Contingent negative variation in headache. Ann Neurol 19:78–80
Kropp P, Gerber WD (1995) Contingent negative variation during migraine attack and interval: evidence for normalization of slow cortical potentials during the attack. Cephalalgia 15:123–129
Sand T, Zhitniy N, White LR et al (2008) Visual evoked potential latency, amplitude and habituation in migraine: a longitudinal study. Clin Neurophysiol 119:1020–1027
Bohotin V, Fumal A, Vandenheede M et al (2003) Excitability of visual V1-V2 and motor cortices to single transcranial magnetic stimuli in migraine: a reappraisal using a figure-of-eight coil. Cephalalgia 23:264–270
Brighina F, Piazza A, Daniele O et al (2002) Modulation of visual cortical excitability in migraine with aura: effects of 1 Hz repetitive transcranial magnetic stimulation. Exp Brain Res 145:177–181
Aurora SK, Cao Y, Bowyer SM et al (1999) The occipital cortex is hyperexcitable in migraine: experimental evidence. Headache 39:469–476
Young WB, Oshinsky ML, Shechter AL et al (2004) Consecutive transcranial magnetic stimulation: phosphene thresholds in migraineurs and controls. Headache 44:131–135
Ambrosini A (2018) Neurophysiology of migraine. Neurol Sci Off J Ital Neurol Soc Ital Soc Clin Neurophysiol 39:59–60
May A, Ashburner J, Büchel C et al (1999) Correlation between structural and functional changes in brain in an idiopathic headache syndrome. Nat Med 5:836–838
Spencer SE, Sawyer WB, Wada H et al (1990) CNS projections to the pterygopalatine parasympathetic preganglionic neurons in the rat: a retrograde transneuronal viral cell body labeling study. Brain Res 534:149–169
Lai TH, Fuh JL, Wang SJ (2009) Cranial autonomic symptoms in migraine: characteristics and comparison with cluster headache. J Neurol Neurosurg Psychiatry 80:1116–1119
Hosoya Y, Matsushita M, Sugiura Y (1983) A direct hypothalamic projection to the superior salivatory nucleus neurons in the rat. A study using anterograde autoradiographic and retrograde HRP methods. Brain Res 266:329–333
Robert C, Bourgeais L, Arreto C-D et al (2013) Paraventricular hypothalamic regulation of trigeminovascular mechanisms involved in headaches. J Neurosci 33:8827–8840
Leone M, Lucini V, D’Amico D et al (1995) Twenty-four-hour melatonin and cortisol plasma levels in relation to timing of cluster headache. Cephalalgia 15:224–229
Hosoya Y, Sugiura Y, Ito R et al (1990) Descending projections from the hypothalamic paraventricular nucleus to the A5 area, including the superior salivatory nucleus, in the rat. Exp Brain Res 82:513–518
Goadsby PJ, May A (1999) PET demonstration of hypothalamic activation in cluster headache. Neurology 52:1522
Holle D, Obermann M (2011) Cluster headache and the hypothalamus: causal relationship or epiphenomenon? Expert Rev Neurother 11:1255–1263
Schulte LH, Allers A, May A (2017) Hypothalamus as a mediator of chronic migraine. Neurology 88:2011–2016
May A, Bahra A, Büchel C et al (1998) Hypothalamic activation in cluster headache attacks. Lancet 352:275–278
Farago P, Szabo N, Toth E et al (2017) Ipsilateral alteration of resting state activity suggests that cortical dysfunction contributes to the pathogenesis of cluster headache. Brain Topogr 30:281–289
Matharu M, May A (2008) Functional and structural neuroimaging in trigeminal autonomic cephalalgias. Curr Pain Headache Rep 12:132–137
Naegel S, Holle D, Obermann M. Structural imaging in cluster headache. Curr Pain Headache Rep; 18. Epub ahead of print 2014. DOI: https://doi.org/10.1007/s11916-014-0415-6
Absinta M, Rocca MA, Colombo B et al (2012) Selective decreased grey matter volume of the pain-matrix network in cluster headache. Cephalalgia 32:109–115
Yang FC, Chou KH, Fuh JL et al (2013) Altered gray matter volume in the frontal pain modulation network in patients with cluster headache. Pain 154:801–807
Arkink EB, Schmitz N, Schoonman GG et al (2016) The anterior hypothalamus in cluster headache. Cephalalgia 033310241666055:0
Messina R, Filippi M, Goadsby PJ (2018) Recent advances in headache neuroimaging. Curr Opin Neurol 31:379–385
Cosentino G, Brighina F, Brancato S et al (2015) Transcranial magnetic stimulation reveals cortical hyperexcitability in episodic cluster headache. J Pain 16:53–59
Chadaide Z, Arlt S, Antal A et al (2007) Transcranial direct current stimulation reveals inhibitory deficiency in migraine. Cephalalgia 27:833–839
Rocca MA, Valsasina P, Absinta M et al (2010) Central nervous system dysregulation extends beyond the pain-matrix network in cluster headache. Cephalalgia 30:1383–1391
Szabó N, Kincses ZT, Párdutz A et al (2013) White matter disintegration in cluster headache. J Headache Pain 14:1–6
Wang R, Dong Z, Chen X et al (2014) Cognitive processing of cluster headache patients: evidence from event-related potentials. J Headache Pain 15:66
Qiu E, Wang Y, Ma L et al (2013) Abnormal brain functional connectivity of the hypothalamus in cluster headaches. PLoS One e57896:8
Perrotta A, Serrao M, Sandrini G et al (2008) Reduced habituation of trigeminal reflexes in patients with episodic cluster headache during cluster period. Cephalalgia 28:950–959
Holle D, Gaul C, Zillessen S et al (2012) Lateralized central facilitation of trigeminal nociception in cluster headache. Neurology 78:985–992
Holle D, Zillessen S, Gaul C et al (2012) Habituation of the nociceptive blink reflex in episodic and chronic cluster headache. Cephalalgia 32:998–1004
Casale MS, Baratto M, Cervera C et al (2008) Auditory evoked potential abnormalities in cluster headache. Neuroreport 19:1633–1636
Afra J, Ertsey C, Bozsik G et al (2005) Cluster headache patients show marked intensity dependence of cortical auditory evoked potentials during and outside the bout. Cephalalgia 25:36–40
Kiraly A, Szabo N, Pardutz A et al (2018) Macro- and microstructural alterations of the subcortical structures in episodic cluster headache. Cephalalgia 38:662–673
Chong CD, Dumkrieger GM, Schwedt TJ (2017) Structural co-variance patterns in migraine: a cross-sectional study exploring the role of the Hippocampus. Headache 57:1522–1531
Yang F-C, Chou K-H, Kuo C-Y et al (2017) The pathophysiology of episodic cluster headache: insights from recent neuroimaging research. Cephalalgia 0:033310241771693
Xue T, Yuan K, Zhao L et al (2012) Intrinsic brain network abnormalities in migraines without aura revealed in resting-state fMRI. PLoS One 7:e52927
Xue T, Yuan K, Cheng P et al (2013) Alterations of regional spontaneous neuronal activity and corresponding brain circuit changes during resting state in migraine without aura. NMR Biomed 26:1051–1058
Cheng C-Y, Cheng H-M, Chen S-P et al (2018) White matter hyperintensities in migraine: clinical significance and central pulsatile hemodynamic correlates. Cephalalgia 38:1225–1236
Zhang J, Wu Y-L, Su J et al (2017) Assessment of gray and white matter structural alterations in migraineurs without aura. J Headache Pain 18:74
Schmidt-Wilcke T, Leinisch E, Ganssbauer S et al (2006) Affective components and intensity of pain correlate with structural differences in gray matter in chronic back pain patients. Pain 125:89–97
Teepker M, Menzler K, Belke M et al (2012) Diffusion tensor imaging in episodic cluster headache. Headache 52:274–282
Headache Classification Committee of the International Headache Society (IHS) The International Classification of Headache Disorders, 3rd edition. Cephalalgia 2018; 38: 1–211
Lipton RB, Stewart WF, Diamond S et al (2001) Prevalence and burden of migraine in the United States: data from the American migraine study II. Headache 41:646–657
Schürks M, Kurth T, De Jesus J et al (2006) Cluster headache: clinical presentation, lifestyle features, and medical treatment. Headache J Head Face Pain 46:1246–1254
D’Amico D, Centonze V, Grazzi L et al (1997) Coexistence of migraine and cluster headache: report of 10 cases and possible pathogenetic implications. Headache J Head Face Pain 37:21–25
Barloese M, Lund N, Petersen A et al (2015) Sleep and chronobiology in cluster headache. Cephalalgia 35:969–978
Rozen TD, Fishman RS (2012) Cluster headache in the United States of America: demographics, clinical characteristics, triggers, suicidality, and personal burden. Headache J Head Face Pain 52:99–113
van Oosterhout W, van Someren E, Schoonman GG et al (2018) Chronotypes and circadian timing in migraine. Cephalalgia 38:617–625
Schwedt TJ (2014) Chronic migraine. BMJ 348:g1416
Su M, Yu S (2018) Chronic migraine: a process of dysmodulation and sensitization. Mol Pain 14:174480691876769
Favier I, Haan J, Ferrari MD (2005) Chronic cluster headache: a review. J Headache Pain 6:3–9
Mitsikostas DD, Edvinsson L, Jensen RH et al (2014) Refractory chronic cluster headache: a consensus statement on clinical definition from the European headache federation. J Headache Pain 15:79
Kelman L (2007) The triggers or precipitants of the acute migraine attack. Cephalalgia 27:394–402
Schytz HW, Schoonman GG, Ashina M (2010) What have we learnt from triggering migraine? Curr Opin Neurol 23:259–265
Pellegrino ABW, Davis-Martin RE, Houle TT, et al. Perceived triggers of primary headache disorders: a meta-analysis. Cephalalgia 2017; 0: 033310241772753
Hougaard A, Amin F, Hauge AW et al (2013) Provocation of migraine with aura using natural trigger factors. Neurology 80:428–431
Lassen LH, Thomsen LL, Olesen J (1995) Histamine induces migraine via the H1-receptor. Support for the NO hypothesis of migraine. Neuroreport 6:1475–1479
Krabbe AA, Olesen J (1980) Headache provocation by continuous intravenous infusion of histamine. Clinical results and receptor mechanisms. Pain 8:253–259
Horton BT (1956) Histaminic cephalgia: differential diagnosis and treatment. Proc Staff Meet Mayo Clin 31:325–333
Bogucki a (1990) Studies on nitroglycerin and histamine provoked cluster headache attacks. Cephalalgia 10:71–75
Thomsen LL, Kruuse C, Iversen HK et al (1994) A nitric oxide donor (nitroglycerin) triggers genuine migraine attacks. Eur J Neurol 1:73–80
Ekbom K (1968) Nitrolglycerin as a provocative agent in cluster headache. Arch Neurol 19:487–493
Fanciullacci M, Alessandri M, Figini M et al (1995) Increase in plasma calcitonin gene-related peptide from the extracerebral circulation during nitroglycerin-induced cluster headache attack. Pain 60:119–123
Fanciullacci M, Alessandri M, Sicuteri R et al (1997) Responsiveness of the trigeminovascular system to nitroglycerine in cluster headache patients. Brain 120:283–288
Costa A, Pucci E, Antonaci F et al (2000) The effect of intranasal cocaine and lidocaine on nitroglycerin-induced attacks in cluster headache. Cephalalgia 20:85–91
Hannerz J (1995) Jogestrand T. Chronic Cluster Headache : Provocation With Carbon Dioxide Breathing and Nitroglycerin:10–12
Garthwaite J, Boulton CL (1995) Nitric oxide signaling in the central nervous system. Annu Rev Physiol 57:683–706
Kruuse C, Thomsen LL, Birk S et al (2003) Migraine can be induced by sildenafil without changes in middle cerebral artery diameter. Brain 126:241–247
Figuerola MDL, Bruera O, Lestón J et al (2006) Cluster headache attack due to sildenafil intake. Cephalalgia 26:617–619
Evans RW. Sildenafil can trigger cluster headache. Headache 2006; 46: 165–167
Lin GY, Lee JT, Peng GS et al (2014) Sildenafil can induce the onset of a cluster headache bout. J Can Urol Assoc 8:378–380
Lassen L, Haderslev P, Jacobsen V et al (2002) CGRP may play a causative role in migraine. Cephalalgia 22:54–61
Ashina H, Schytz HW, Ashina M. CGRP in human models of primary headaches. Cephalalgia 2016; 0: 1–8
Vollesen LH, Snoer A, Beske RP, Guo S, Hoffmann J, Jensen RHAM Infusion of calcitonin gene-related peptide provokes cluster headache attacks. JAMA Neurol
Dodick DW, Goadsby PJ, Spierings ELH et al (2014) Safety and efficacy of LY2951742, a monoclonal antibody to calcitonin gene-related peptide, for the prevention of migraine: a phase 2, randomised, double-blind, placebo-controlled study. Lancet Neurol 13:885–892
Dodick DW, Goadsby PJ, Silberstein SD et al (2014) Safety and efficacy of ALD403, an antibody to calcitonin gene-related peptide, for the prevention of frequent episodic migraine: a randomised, double-blind, placebo-controlled, exploratory phase 2 trial. Lancet Neurol 13:1100–1107
Sun H, Dodick DW, Silberstein S et al (2016) Safety and efficacy of AMG 334 for prevention of episodic migraine: a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Neurol 15:382–390
Bigal ME, Edvinsson L, Rapoport AM et al (2015) Safety, tolerability, and efficacy of TEV-48125 for preventive treatment of chronic migraine: a multicentre, randomised, double-blind, placebo-controlled, phase 2b study. Lancet Neurol 14:1091–1100
Eli Lilly and Company. A Study of LY2951742 in Participants With Episodic Cluster Headache, https://clinicaltrials.gov/ct2/show/NCT02397473?term=eli+lilly+cluster&rank=1 (accessed 1 March 2018)
Schytz HW (2010) Investigation of carbachol and PACAP38 in a human model of migraine. Dan Med Bull 57:B4223
Christiansen I, Daugaard D, Thomsen LL et al (2000) Glyceryl trinitrate induced headache in migraineurs - relation to attack frequency. Eur J Neurol 7:405–411
Stewart WF, Simon D, Schechter A et al (1995) Population Variation in Migraine a Meta-Analysis Prevalence. J Clin Epidemiol 48:269–280
Ekbom K, Svensson DA, Träff H et al (2002) Age at onset and sex ratio in cluster headache: observations over three decades. Cephalalgia 22:94–100
Mortimer MJ, Kay J, Jaron A (2008) Epidemiology of headache and childhood migraine in an URBAN general practice using ad hoc, VAHLQUIST and IHS criteria. Dev Med Child Neurol 34:1095–1101
Taga A, Manzoni GC, Russo M et al (2018) Childhood-onset cluster headache: observations from a personal case-series and review of the literature. Headache J Head Face Pain 58:443–454
Vetvik KG, MacGregor EA (2017) Sex differences in the epidemiology, clinical features, and pathophysiology of migraine. The Lancet Neurology 16:76–87
Lipton RB, Diamond S, Reed M et al (2001) Migraine diagnosis and treatment: results from the American migraine study II. Headache 41:638–645
Lund N, Barloese M, Petersen A et al (2017) Chronobiology differs between men and women with cluster headache, clinical phenotype does not. Neurology 88:1069–1076
Rozen TD, Niknam RM, Shechter AL et al (2001) Cluster headache in women: clinical characteristics and comparison with cluster headache in men. J Neurol Neurosurg Psychiatry 70:613–617
Rozen TD, Niknam R, Shechter AL, Young WB, Silberstein S Gender differences in clinical characteristics and treatment response in cluster headache patients. Cephalalgia 19:323
Peterlin BL, Gupta S, Ward TN et al (2011) Sex matters: evaluating sex and gender in migraine and headache research. Headache 51:839–842
Xu H, Han W, Wang J et al (2016) Network meta-analysis of migraine disorder treatment by NSAIDs and triptans. J Headache Pain 17:113
Cittadini E, May A, Straube A et al (2006) Effectiveness of intranasal zolmitriptan in acute cluster headache: a randomized, placebo-controlled, double-blind crossover study. Arch Neurol 63:1537–1542
Rapoport AM, Mathew NT, Silberstein SD et al (2007) Zolmitriptan nasal spray in the acute treatment of cluster headache: a double-blind study. Neurology 69:821–826
Lampl C, Voelker M, Steiner TJ (2012) Aspirin is first-line treatment for migraine and episodic tension-type headache regardless of headache intensity. Headache 52:48–56
Singhal AB, Maas MB, Goldstein JN et al (2017) High-flow oxygen therapy for treatment of acute migraine: a randomized crossover trial. Cephalalgia 37:730–736
Jürgens TP, Schulte LH, May A (2013) Oxygen treatment is effective in migraine with autonomic symptoms. Cephalalgia 33:65–67
Akerman S, Holland PR, Lasalandra MP et al (2009) Oxygen inhibits neuronal activation in the Trigeminocervical complex after stimulation of trigeminal autonomic reflex, but not during direct Dural activation of trigeminal afferents. Headache J Head Face Pain 49:1131–1143
Maizels M, Geiger AM (1999) Intranasal lidocaine for migraine: a randomized trial and open-label follow-up. Headache 39:543–551
Avcu N, Dogan NO, Pekdemir M et al (2017) Intranasal lidocaine in acute treatment of migraine: a randomized controlled trial. Ann Emerg Med 69:743–751
Blanda M, Rench T, Gerson LW et al (2001) Intranasal lidocaine for the treatment of migraine headache: a randomized, controlled trial. Acad Emerg Med 8:337–342
Leone M, Giustiniani A, Cecchini AP (2017) Cluster headache: present and future therapy. Neurol Sci Off J Ital Neurol Soc Ital Soc Clin Neurophysiol 38:45–50
Leone M, D’Amico D, Frediani F et al (2000) Verapamil in the prophylaxis of episodic cluster headache: a double-blind study versus placebo. Neurology 54:1382–1385
Solomon GD, Steel JG, Spaccavento LJ (1983) Verapamil prophylaxis of migraine. A double-blind, placebo-controlled study. JAMA 250:2500–2502
Paterna S, Martino SG (1990) Campisi D, et al. [evaluation of the effects of verapamil, flunarizine, diltiazem, nimodipine and placebo in the prevention of hemicrania. A double-blind randomized cross-over study]. Clin Ter 134:119–125
Bussone G, Leone M, Peccarisi C et al (1990) Double blind comparison of lithium and verapamil in cluster headache prophylaxis. Headache 30:411–417
Steiner TJ, Hering R, Couturier EG et al (1997) Double-blind placebo-controlled trial of lithium in episodic cluster headache. Cephalalgia 17:673–675
Peatfield RC, Rose FC (1981) Exacerbation of migraine by treatment with lithium. Headache 21:140–142
Medina JL, Diamond S (1981) Cyclical migraine. Arch Neurol 38:343–344
Couch JRJ, Ziegler DK (1978) Prednisone therapy for cluster headache. Headache 18:219–221
Orr SL, Friedman BW, Christie S et al (2016) Management of Adults with Acute Migraine in the emergency department: the American headache society evidence assessment of parenteral pharmacotherapies. Headache 56:911–940
Ambrosini A, Vandenheede M, Rossi P et al (2005) Suboccipital injection with a mixture of rapid- and long-acting steroids in cluster headache: a double-blind placebo-controlled study. Pain 118:92–96
Inan LE (2016) Greater occipital nerve blockade for the treatment of chronic migraine. Cephalalgia 36:1095
Bostani A, Rajabi A, Moradian N et al (2013) The effects of cinnarizine versus sodium valproate in migraine prophylaxis. Int J Neurosci 123:487–493
Afshari D, Rafizadeh S, Rezaei M (2012) A comparative study of the effects of low-dose topiramate versus sodium valproate in migraine prophylaxis. Int J Neurosci 122:60–68
Leone M, Dodick D, Rigamonti A et al (2003) Topiramate in cluster headache prophylaxis: an open trial. Cephalalgia : an international journal of headache 23:1001–1002
Wheeler SD, Carrazana EJ (1999) Topiramate-treated cluster headache. Neurology 53:234–236
Lainez MJA, Pascual J, Pascual AM et al (2003) Topiramate in the prophylactic treatment of cluster headache. Headache 43:784–789
Hering R, Kuritzky A (1989) Sodium valproate in the treatment of cluster headache: an open clinical trial. Cephalalgia 9:195–198
El Amrani M, Massiou H, Bousser MG. A negative trial of sodium valproate in cluster headache: methodological issues. Cephalalgia 2002; 22: 205–208
Bratbak DF, Nordgard S, Stovner LJ et al (2017) Pilot study of sphenopalatine injection of onabotulinumtoxinA for the treatment of intractable chronic migraine. Cephalalgia 37:356–364
Diener HC, Dodick DW, Aurora SK et al (2010) OnabotulinumtoxinA for treatment of chronic migraine: results from the double-blind, randomized, placebo-controlled phase of the PREEMPT 2 trial. Cephalalgia 30:804–814
Aurora SK, Dodick DW, Turkel CC et al (2010) OnabotulinumtoxinA for treatment of chronic migraine: results from the double-blind, randomized, placebo-controlled phase of the PREEMPT 1 trial. Cephalalgia 30:793–803
Lampl C, Rudolph M, Brautigam E (2018) OnabotulinumtoxinA in the treatment of refractory chronic cluster headache. J Headache Pain 19:45
Leone M, D’Amico D, Moschiano F et al (1996) Melatonin versus placebo in the prophylaxis of cluster headache: a double-blind pilot study with parallel groups. Cephalalgia 16:494–496
Peres MFP, Zukerman E, da Cunha Tanuri F et al (2004) Melatonin, 3 mg, is effective for migraine prevention. Neurology 63:757
Novartis. Novartis and Amgen announce FDA approval of Aimovig(TM) (erenumab), a novel treatment developed specifically for migraine prevention, https://www.novartis.com/news/media-releases/novartis-and-amgen-announce-fda-approval-aimovigtm-erenumab-novel-treatment-developed-specifically-migraine-prevention (2018, accessed 4 June 2018)
Lilly. Lilly’s Galcanezumab Meets Primary Endpoint in Phase 3 Study Evaluating Galcanezumab for the Prevention of Episodic Cluster Headache, https://investor.lilly.com/news-releases/news-release-details/lillys-galcanezumab-meets-primary-endpoint-phase-3-study (accessed 17 May 2018)
Costa A, Antonaci F, Ramusino MC et al (2015) The neuropharmacology of cluster headache and other trigeminal autonomic Cephalalgias. Curr Neuropharmacol 13:304–323
Denuelle M, Fabre N, Payoux P et al (2007) Hypothalamic activation in spontaneous migraine attacks. Headache 47:1418–1426
Leone M, Franzini A, Bussone G (2001) Stereotactic stimulation of posterior hypothalamic gray matter in a patient with intractable cluster headache. N Engl J Med 345:1428–1429
Leone M, Cecchini AP, Franzini A et al (2008) Lessons from 8 years’ experience of hypothalamic stimulation in cluster headache. Cephalalgia 28:789–797
Leone M, Franzini A, Broggi G et al (2004) Long-term follow-up of bilateral hypothalamic stimulation for intractable cluster headache. Brain 127:2259–2264
Cortelli P, Guaraldi P, Leone M et al (2007) Effect of deep brain stimulation of the posterior hypothalamic area on the cardiovascular system in chronic cluster headache patients. Eur J Neurol 14:1008–1015
Bartsch T, Goadsby PJ (2003) Increased responses in trigeminocervical nociceptive neurons to cervical input after stimulation of the dura mater. Brain 126:1801–1813
Bartsch T, Goadsby PJ (2002) Stimulation of the greater occipital nerve induces increased central excitability of dural afferent input. Brain 125:1496–1509
Lipton R, Goadsby J (2009) P, Cady R, et al. In: PRISM study: occipital nerve stimulation for treatment-refractory migraine
Saper JR, Dodick DW, Silberstein SD et al (2011) Occipital nerve stimulation for the treatment of intractable chronic migraine headache: ONSTIM feasibility study. Cephalalgia 31:271–285
Dodick DW, Silberstein SD, Reed KL et al (2015) Safety and efficacy of peripheral nerve stimulation of the occipital nerves for the management of chronic migraine: long-term results from a randomized, multicenter, double-blinded, controlled study. Cephalalgia 35:344–358
Magis D, Schoenen J (2012) Advances and challenges in neurostimulation for headaches. Lancet Neurol 11:708–719
Fontaine D, Blond S, Lucas C et al (2016) Occipital nerve stimulation improves the quality of life in medically-intractable chronic cluster headache: results of an observational prospective study. Cephalalgia 0:033310241667320
Ruskell GL (2003) Orbital passage of pterygopalatine ganglion efferents to paranasal sinuses and nasal mucosa in man. Cells Tissues Organs 175:223–228
Schoenen J, Jensen RH, Lanteri-Minet M et al (2013) Stimulation of the sphenopalatine ganglion (SPG) for cluster headache treatment. Pathway CH-1: a randomized, sham-controlled study. Cephalalgia 33:816–830
Tepper SJ, Rezai A, Narouze S et al (2009) Acute treatment of intractable migraine with sphenopalatine ganglion electrical stimulation. Headache 49:983–989
Cady RK, Saper J, Dexter K et al (2015) Long-term efficacy of a double-blind, placebo-controlled, randomized study for repetitive sphenopalatine blockade with bupivacaine vs. saline with the Tx360 device for treatment of chronic migraine. Headache 55:529–542
Goadsby PJ, Grosberg BM, Mauskop A et al (2014) Effect of noninvasive vagus nerve stimulation on acute migraine: an open-label pilot study. Cephalalgia 34:986–993
Barbanti P, Grazzi L, Egeo G et al (2015) Non-invasive vagus nerve stimulation for acute treatment of high-frequency and chronic migraine: an open-label study. J Headache Pain 16:61
Tassorelli C, Grazzi L, de Tommaso M et al (2018) Noninvasive vagus nerve stimulation as acute therapy for migraine. Neurology 0. https://doi.org/10.1212/WNL.0000000000005857
Silberstein SD, Calhoun AH, Lipton RB et al (2016) Chronic migraine headache prevention with noninvasive vagus nerve stimulation. Neurology 87:529–538
Gaul C, Diener H-C, Silver N et al (2016) Non-invasive vagus nerve stimulation for PREVention and acute treatment of chronic cluster headache (PREVA): a randomised controlled study. Cephalalgia 36:534–546
Gaul C, Magis D, Liebler E, et al. Effects of non-invasive vagus nerve stimulation on attack frequency over time and expanded response rates in patients with chronic cluster headache: a post hoc analysis of the randomised, controlled PREVA study. J Headache Pain; 18. Epub ahead of print 2017. DOI: https://doi.org/10.1186/s10194-017-0731-4
Morris J, Straube A, Diener H-C et al (2016) Cost-effectiveness analysis of non-invasive vagus nerve stimulation for the treatment of chronic cluster headache. J Headache Pain 17:43
Lipton RB, Dodick DW, Silberstein SD et al (2010) Single-pulse transcranial magnetic stimulation for acute treatment of migraine with aura: a randomised, double-blind, parallel-group, sham-controlled trial. Lancet Neurol 9:373–380
Magis D, Sava S, d’Elia TS et al (2013) Safety and patients’ satisfaction of transcutaneous supraorbital neurostimulation (tSNS) with the Cefaly(R) device in headache treatment: a survey of 2,313 headache sufferers in the general population. J Headache Pain 14:95
Schoenen J, Vandersmissen B, Jeangette S et al (2013) Migraine prevention with a supraorbital transcutaneous stimulator: a randomized controlled trial. Neurology 80:697–704
Haane DYP, Koehler PJ (2014) Nociception specific supraorbital nerve stimulation may prevent cluster headache attacks: serendipity in a blink reflex study. Cephalalgia 34:920–926
Blumenfeld A, Ashkenazi A, Napchan U et al (2013) Expert consensus recommendations for the performance of peripheral nerve blocks for headaches--a narrative review. Headache 53:437–446
Ansarinia M, Rezai A, Tepper SJ et al (2010) Electrical stimulation of sphenopalatine ganglion for acute treatment of cluster headaches. Headache 50:1164–1174
Reed KL, Black SB, Banta CJ 2nd et al (2010) Combined occipital and supraorbital neurostimulation for the treatment of chronic migraine headaches: initial experience. Cephalalgia 30:260–271
Ferrari MD, Roon KI, Lipton RB et al (2001) Oral triptans (serotonin 5-HT(1B/1D) agonists) in acute migraine treatment: a meta-analysis of 53 trials. Lancet (London, England) 358:1668–1675
Ekbom K, Monstad I, Prusinski A et al (1993) Subcutaneous sumatriptan in the acute treatment of cluster headache: a dose comparison study. The Sumatriptan cluster headache study group. Acta Neurol Scand 88:63–69
Schwedt TJ, Vargas B (2015) Neurostimulation for treatment of migraine and cluster headache. Pain Med 16:1827–1834
Edvinsson L (2017) The Trigeminovascular pathway: role of CGRP and CGRP receptors in migraine. Headache J Head Face Pain 57:47–55
Khan S, Olesen A, Ashina M (2017) CGRP, a target for preventive therapy in migraine and cluster headache: systematic review of clinical data. Cephalalgia 0 033310241774129
Patrick M. Behind Amgen’s Plans to Penetrate the Migraine Segment, http://marketrealist.com/2016/05/amgen-plans-penetrate-migraine-segment-multiple-investigational-drugs/ (2016, accessed 27 February 2017)
Rocca MA, Ceccarelli A, Falini A et al (2006) Brain gray matter changes in migraine patients with T2-visible lesions: a 3-T MRI study. Stroke 37:1765–1770
Schmitz N, Arkink EB, Mulder M et al (2008) Frontal lobe structure and executive function in migraine patients. Neurosci Lett 440:92–96
Kim JH, Suh SI, Seol HY et al (2008) Regional grey matter changes in patients with migraine: a voxel-based morphometry study. Cephalalgia 28:598–604
Jin C, Yuan K, Zhao L et al (2013) Structural and functional abnormalities in migraine patients without aura. NMR Biomed 26:58–64
Valfre W, Rainero I, Bergui M et al (2008) Voxel-based morphometry reveals gray matter abnormalities in migraine. Headache 48:109–117
DaSilva AFM, Granziera C, Nouchine Hadjikhani JS (2007) Thickening in the somatosensory cortex of patients with migraine. Neurology 69:1990–1995
Seifert CL, Magon S, Staehle K et al (2012) A case-control study on cortical thickness in episodic cluster headache. Headache 52:1362–1368
Tessitore A, Russo A, Esposito F et al (2011) Interictal cortical reorganization in episodic migraine without aura: an event-related fMRI study during parametric trigeminal nociceptive stimulation. Neurol Sci Off J Ital Neurol Soc Ital Soc Clin Neurophysiol 32(Suppl 1):S165–S167
Cao Y, Aurora SK, Nagesh V et al (2002) Functional MRI-BOLD of brainstem structures during visually triggered migraine. Neurology 59:72–78
Jia Z, Yu S (2017) Grey matter alterations in migraine: a systematic review and meta-analysis. NeuroImage Clin 14:130–140
Yuan K, Zhao L, Cheng P et al (2013) Altered structure and resting-state functional connectivity of the basal ganglia in migraine patients without aura. J Pain 14:836–844
Coppola G, Petolicchio B, Di Renzo A et al (2017) Cerebral gray matter volume in patients with chronic migraine: correlations with clinical features. J Headache Pain 18:115
Wang Y, Zhang X, Guan Q et al (2015) Altered regional homogeneity of spontaneous brain activity in idiopathic trigeminal neuralgia. Neuropsychiatr Dis Treat 11:2659–2666
Goadsby PJ, Lipton RB, Ferrari MD (2002) Migraine--current understanding and treatment. N Engl J Med 346:257–270
Cohen AS, Burns B, Goadsby PJ (2009) High-flow oxygen for treatment of cluster headache: a randomized trial. JAMA 302:2451–2457
Robbins L (1995) Intranasal lidocaine for cluster headache. Headache 35:83–84
Fonzari M, Santoloci D, Farinini D (1994) The use of verapamil in the treatment of hemicrania without aura. Clin Ter 144:329–332
Peres MFP, Stiles MA, Siow HC et al (2002) Greater occipital nerve blockade for cluster headache. Cephalalgia 22:520–522
Acknowledgements
This work was supported by the School of Advanced Studies of the European Headache Federation (EHF-SAS).
Funding
We thank the Lundbeck Foundation (R155–2014-171), Research Foundation of the Capital Region of Copenhagen, Danish Council for Independent Research, Medical Sciences and Novo Nordisk Foundation (NNF11OC101433).
Availability of data and materials
All papers included in this review can be found online.
Author information
Authors and Affiliations
Consortia
Contributions
All Authors equally contributed to the review. LV, SB, FC, ALR, FM, LP, MR are Junior Fellows of EHF-SAS. MA and CL are Senior Fellows of EHF-SAS. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
Prof Messoud Ashina is a consultant or scientific advisor for Allergan, Amgen, Alder, Eli Lilly, Novartis and Teva, primary investigator for Amgen 20,120,178 (Phase 2), 20,120,295 (Phase 2), 20,130,255 (OLE), 20,120,297 (Phase 3) and GM-11 gamma- Core-R trials, and reports grants from Lundbeck Foundation (R155–2014-171), Research Foundation of the Capital Region of Copenhagen, Danish Council for Independent Research, Medical Sciences and Novo Nordisk Foundation (NNF11OC101433). Prof Christian Lampl is a consultant or scientific advisor for Novartis and Teva. Other authors have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Vollesen, A.L., Benemei, S., Cortese, F. et al. Migraine and cluster headache – the common link. J Headache Pain 19, 89 (2018). https://doi.org/10.1186/s10194-018-0909-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s10194-018-0909-4