Abstract
In the marine environment, control of invasive species’ population levels, that is, keeping them at an abundance level which is below a density-dependent adverse effect, may be the most attainable goal for the management of introduced bryozoans. An improved understanding of reproductive strategies and life history traits is key in order to understand the spreading potential. The assessment of the magnitude and temporal dynamics of propagule pressure from the reproducing population important for the success of control actions and needs is to be determined prior to any field intervention. The reproductive cycle of three fouling bryozoans (Bugula neritina, Tricellaria inopinata and Virididentula dentata) in the waters of the Azores Archipelago was assessed. The study revealed that although the release of larvae can occur throughout the entire year, its intensity and developmental and the attachment success of the ancestrula are not even throughout the year and that each species’ reproductive development needs to be determine independently. In the light of these findings, it is possible to determine the best time to apply field actions aimed at controlling invasive population’s density levels, optimizing the always scarce financial resources for marine management.
Similar content being viewed by others
Background
Coastal ecosystems around the world are increasingly being exposed to a pool of potential non-indigenous species (NIS) translocated through a variety of vectors [17, 62, 71]. As a consequence, the risk that populations of potential invaders establish themselves in recipient environments is rapidly increasing [35]. Marine invasive species may create and modify habitats, prey upon or outcompete native species, and act as either disease agents or vectors, or both. Ultimately, invading species are agents of homogenization of the species composition of separated communities and can destabilize, at least temporarily, ecosystem structure [42].
The impact of NIS introduction is viewed as negative, namely due to decreases in the economic benefits accrued from activities based on marine environments and resources such as fisheries, aquaculture, tourism and marine infrastructure [8]. For example, the ecological and economic damage caused by the sea lamprey (Petromyzon marinus) has cost millions of dollars in losses to commercial Great Lake fisheries and millions of dollars in control programmes [58]. Additionally, people’s welfare may decrease from the reduced quality of their environments and natural surroundings. For example, the increased frequency of toxic red tides, which threaten both public health and marine fisheries, has been partly attributed to the worldwide transfer of dinoflagellates and their cysts in ships’ ballast tanks [71]. Finally, from a conservation viewpoint, the pristine biodiversity of invaded ecosystems is threatened by invaders: in the Azorean Archipelago, biological invasions are seen as a potential factor affecting Marine Protection Areas [1]. Other studies (e.g. [8] contend that, in economic and social terms, the impacts of an invader may be positive, improving aesthetic values, developing new economic activities (e.g. creating commercial and recreational fisheries and aquaculture opportunities—[20, 71] and creating employment for the exploitation of natural resources produced by NIS.
The invasiveness and impact of a NIS population are not simply a function of its presence, but also of its biological features, the site-specific environmental conditions and the recipient ecosystem structure [67]. In the marine environment, the control or eradication of invasive species is technically and financially difficult [54]. Prevention, reducing the risk of introductions, is considered the most effective management strategy [6, 8, 60, 92]. However, with the continued movement of craft, people and goods, even with a stricter regulation of vectors, some incursions are inevitable [53].
If a marine NIS invader is detected shortly after arrival, its eradication may be possible [23], but until the present time, only a few cases have been successful (e.g. the alga Caulerpa taxifolia in California [4]; the green-lipped mussel Perna canaliculus [83] and the dreissenid mussel Mytilopsis sallei [7], both in Australia). Failures to eradicate introduced species are far more common (although not so well or often documented) (e.g. the ascidian Didemnum vexillum or the Asian kelp Undaria pinnatifida, both in New Zealand [21]; the northern Pacific seastar Asterias amurensis [87] and the Pacific oyster Crassostrea gigas, both in Tasmania [59]; the alga Caulerpa webbiana in the Faial Island of the Azorean Archipelago (Cardigos et al. [15]).
Control of population levels, that is, to keep the species at an abundance level which is below a density-dependent adverse effect, or to contain or reduce the spread of target organisms, may be a more attainable goal than eradication for the management of non-indigenous species [31, 43, 60, 79, 92]. There are three main methods to keep an introduced species at low levels: (1) physical and mechanical control, often highly effective but labour-intensive [80]; (2) chemical control, sometimes effective but often controversial [80] and (3) biological control, considered arguable because many introduced species’ enemies never become established [88], non-target impacts occasionally occur [22] or biological control agents may spread to distant areas where they are unwanted [80].
Physical and mechanical controls seem to be the less controversial methods and those with less side effects, but the effectiveness of control, at a particular point in time, is dependent on species features such as the reproductive cycle, which need to be determined prior to any field intervention.
As a remote oceanic archipelago, the nine islands of the Azores are particularly vulnerable to marine introductions, especially due to a limited biotic resistance to introduced invaders and the characteristic high availability of empty niches, as described by Micael et al. [56]. In the last decade, several marine invertebrates have been reported as being introduced recently into the archipelago [14]: Amathia verticillata (Delle Chiaje, 1822) (Gymnolaemata) [3]; Perforatus perforatus (Bruguière, 1789) (Thecostraca) [84]; Schizoporella errata (Waters, 1878) (Gymnolaemata) [57]; Phorcus sauciatus (Koch, 1845) (Gastropoda) [5]. It has been shown, moreover, that the non-indigenous marine algal flora of these remote islands is double the number known at a global scale (6 vs. 3 % non-indigenous macroalgae) [56].
Similarly Souto et al. [81], following Berning [10], observed that the recent increase in bryozoan biodiversity in the waters off Madeira may be linked to two factors. Firstly, the evolution of taxonomic studies, helped by modern investigation methods, has revealed distinct species, several being endemic to Madeira. Secondly, the monitoring of marinas and harbours for non-indigenous species has detected several introduced bryozoans [13, 89].
Insular marine ecosystems are characterized by environmental specificity achieved subsequent to millions of years of physical isolation and which comprises, at least in part, habitats and species that should be preserved due to their uniqueness [56].
The focus of the present study was to provide insights into the reproductive strategies of three bryozoan species (Bugula neritina, Tricellaria inopinata and Virididentula dentata), introduced into the Azores and showing an invasive potential to determine the best time of the year to apply field management action aimed at reducing their population numbers.
Methods
The three target species
Diverse species of Bryozoa are achieving distributions that far exceed their inherent dispersal potential [36]. A species trait related to the ability to foul and the generalized use of different types of substrata to settle upon, together with their abundance and their natural tolerance to a broad range of prevailing temperatures, salinities and pollution parameters characteristic of harbours and marinas have been identified as fundamental features explaining the recent increases in the geographic ranges of bryozoans [16, 19, 77, 85].
Records of coastal bryozoans from the Azores date from the early 1900s [11, 12, 37]. In 1975, Ctenostoma and Cheilostome obtained from the Azores were reported upon by d’Hondt [24], and Harmelin [32] wrote a thesis on Sur quelques Cribrimorphes (Bryozoa, Cheilostomata) de l’Atlantique Oriental including several taxa from Azorean coastal waters. In the last 15 years, some field reports and scientific papers have been adding bryozoan records to the Azorean marine fauna [3, 30, 57, 74, 76, 82], Micael et al. in prep.).
In the summer of 2013, a systematic survey was carried out in Ponta Delgada Harbour with the aim of studying non-indigenous species in the marina there and which is mainly used by recreational vessels (Micael et al. in prep.). During this preliminary study, three cheilostome erect bryozoan species with potential invasive behaviours were identified, mainly due to their abundance in the marina (Joana Micael, personal observation):
-
1.
Bugula neritina Linnaeus, 1758
Bugula neritina is one of the most common fouling organisms worldwide and was considered a cosmopolitan species. Recent molecular evidence suggests that B. neritina consists of at least three genetically distinct types of colonies along the coasts of the USA [41, 46]. One of the colony types (Type S) can be distinguished through two mitochondrial (cytochrome c oxidase subunit I [COI] and large ribosomal RNA subunit [16S]) and two nuclear genes (dynein light chain roadblock type-2 protein [DYN] and a voltage-dependent anion-selective channel protein [VDAC]) [27]. Type S colonies have an invasive behaviour, are globally distributed, including genetic confirmation of colonies from Galicia—in the north-eastern Atlantic [27], and are considered to have undergone widespread introduction as a fouling organism [74]. The status of this species cannot in general be defined with certainty, pending confirmation from the above ongoing research: some authors consider it cryptogenic, while the National Exotic Marine and Estuarine Species Information System (NEMESIS http://invasions.si.edu/nemesis/browseDB/SpeciesSummary.jsp?TSN=-95) considers it as NIS in the Azores.
In the Azores, B. neritina has only recently (2001) been recorded from several islands of the Archipelago [30, 74, 76, 82]. No attempt has been made to investigate the type of colonies collected.
-
2.
Tricellaria inopinata d’Hondt and Occhipinti-Ambrogi, 1985
Although not previously reported upon in the literature, the cheilostome T. inopinata, whose origin is considered to be the Pacific, has become invasive along Mediterranean and Atlantic coastlines (d’Hondt and Occhipinti-Ambrogi 1985; Marchini et al. [47]), and is now well established in several marinas of the Azorean Archipelago. Large colonies bearing ovicells with embryos confirm that the species has become acclimated to and reproducing in the Azores (J Micael pers. observation).
As pointed out by Marchini et al. [47], T. inopinata was first recorded, in 1982, from the Venice Lagoon in the Adriatic Sea (d’Hondt and Occhipinti-Ambrogi 1985), and although new to science, it has been considered introduced from the area where congeneric taxa are native. In the Venice Lagoon, it showed an invasive behaviour, with the subsequent decline of native bryozoan species. In the Atlantic, the first record dates from 1998, that is, T. inopinata in Poole Harbour, on the central southern coast of England [26], and probably representing the initial stage of a range expansion of the species along Atlantic coasts, even reaching high latitudes in northern Scotland and Norway [19, 44, 64].
-
3.
Virididentula dentata (Lamouroux, 1816)—formerly Bugula dentata Lamouroux, 1816 [28].
At present, Virididentula dentata encompasses a complex of species characterized by intraspecific morphological variation [45, 73] and divergent lineages based on COI sequences [28, 45] reported from several localities around the Australia–New Guinea continent, Brazil, Cape Verde, Celebes Sea, Hawaii, Japan, Madeira, Mediterranean Sea (Cadiz Bay) and South Africa [12, 40, 45, 65, 73]. As discussed above for B. neritina, a consensus has not yet been reached on establishing the status of this species: Cardigos et al. [14] consider B. dentata as cryptogenic.
In the Azores, the species has been recorded from several islands of the Archipelago, since 2001 [30, 76, 82].
Field sampling
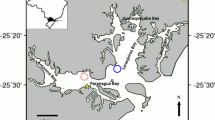
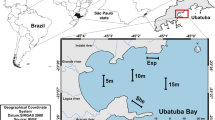
The Azores Archipelago is located in the North Atlantic between latitudes 36 55′ and 39 43′N and longitudes 24 46′ and 31 16′W, approximately 1600 km from mainland Portugal (Fig. 1). At monthly intervals from March 2014 to February 2015, colonies (N = 15–20) of Bugula neritina, Tricellaria inopinata and Virididentula dentata were collected randomly (snorkelling at the depth of 0–2 m) from the marina of Ponta Delgada (that was enlarged in 2008), located on the south coast of São Miguel Island (37 44′N; 25 39′W) (Fig. 1).
It has been argued that the size of clonal organisms positively correlates with reproductive output [33, 34, 38, 78]. As such, and following Keough [38], who determined that mean size of B. neritina colonies at first reproduction achieves five bifurcations, our sampling target colonies presented at least five bifurcations (corresponding to a minimum of 15 mm in height) and were separated from each other by a distance of more than 5 m.
Samples were transported to the laboratory in solid, inert, plastic bags filled with local sea water and placed within a thermic box. Upon laboratory arrival, samples were cleaned of associated fauna and flora, and living specimens were inspected for embryos under a light microscope (amplification 10×—Olympus CX41). After screening, specimens were preserved in plastic jars with 10 % neutral formalin.
Ambient sea water temperature was recorded monthly (three measures per month) from 0.5 m below the low-water mark, using a multi-parameter water quality meter probe (Horiba u-50). Measurements were taken in the same site as biological samples. Day length (photoperiod) data for São Miguel were obtained from Beck [9].
Reproductive cycle
According to Fernández Pulpeiro et al. [29] and Marshall et al. [49], the reproductive development state of each bryozoan colony was assessed recording the presence of ovicells (brood structures) with embryos, the number of ancestrulae and the number of young colonies attached to the adult colony, observed under a light microscope.
Statistical analyses
Statistical differences between the percentages of colonies with ovicells with embryos and with ancestrulae and young colonies (up to a dozen zooids) throughout the year were examined for each species, using one-way analysis of variance (ANOVA). The post hoc Tukey’s HSD was applied to determine in which months the percentages of ovicells with embryos or ancestrulae (plus young colonies) were significantly different among each other.
Spearman’s rank correlation analysis was used to establish any relationship between the number of ovicells with embryos and ancestrulae plus young colonies and between each of these variables and the environmental variables (temperature, photoperiod).
Results
Bugula neritina
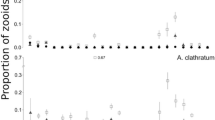
The number of ovicells with embryos did not differ significantly throughout the year [ANOVA, F(11, 228) = 1.317, p = 0.216]. Nevertheless, in August, 100 % of the colonies presented ovicells with embryos (Fig. 2a). Fewer colonies with ovicells and embryos were identified in May (70 % of the colonies, Fig. 2a).
The number of ancestrulae plus young colonies of B. neritina was significantly different throughout the year [ANOVA, F(11, 228) = 6.521, p = 0.001]. Fewer recruits were identified in April and December (Fig. 2b and Additional file 1).
A negative correlation (albeit without statistical significance) was noted between the number of ovicells with embryos and temperature and day length (r s = −0.367, p = 0.313, r s = −0.319, p = 0.241, respectively). The correlation between ancestrulae plus young colonies and temperature was also negative, but not statistically significant (r s = −0.053, p = 0.871), while the correlation between ancestrulae plus young colonies and day length was positive, but also without statistical significance (r s = 0.133, p = 0.681).
Tricellaria inopinata
The number of ovicells with embryos differed significantly throughout the year [ANOVA, F(11, 206) = 6.396, p = 0.001], being especially higher during the summer months in contrast to autumn (Fig. 2 and Additional file 1). Fewer colonies with ovicells and embryos (18–20 % of the colonies, Fig. 2a) were identified in October and September.
The number of ancestrulae plus young colonies of T. inopinata was significantly different throughout the year [ANOVA, F(11, 206) = 2.904, p = 0.001]. Fewer ancestrulae plus young colonies (Fig. 2b and Additional file 1) were identified in October.
The correlation between the number of ovicells with embryos or ancestrulae plus young colonies and temperature, although positive, was not significant (r s = 0.468, p = 0.125; r s = 0.134, p = 0.677, respectively). Nevertheless, there was a significant positive linear relationship between the number of ovicells with embryos and day length (r s = 0.870, p = 0.001), and the number of ancestrulae and young colonies and day length (r s = 0.631, p = 0.028).
Virididentula dentata
The number of ovicells with embryos was significantly different throughout the year [ANOVA, F(11, 213) = 2.339, p = 0.01], being higher in May and September (Fig. 2a and Additional file 2). Fewer colonies with ovicells and embryos (5 % of the colonies, Fig. 2a) were identified in December.
The number of ancestrulae plus young colonies of V. dentata in July was almost three times more than in other months; ANOVA, F(11, 212) = 6.307, p = 0.001) (Fig. 2b and Additional file 1).
Although a positive correlation between the number of ovicells with embryos and temperature and day length was detected, it was not statistically significant (r s = 0.264, p = 0.408, r s = 0.506, p = 0.093, respectively). Similar results were obtained for ancestrulae plus young colonies and temperature and day length r s = 0.153, p = 0.636, r s = 0.428, p = 0.165, respectively).
The three target species in this study produced ovicells with embryos throughout the year, with a higher frequency at the end of the spring and during summer (Fig. 2a). Recruitment of ancestrulae was also observed throughout the year, with each species producing different recruitment peaks along the course of the year (Fig. 2b). Correlation between the number of ovicells with embryos and the number of ancestrulae (plus young colonies) was only obtained for Tricellaria inopinata (r s = 0.796, p = 0.002).
Temperature and photoperiod data are shown in Additional file 2.
Discussion
Our observations on the reproduction and recruitment of the Azorean bryozoans described and discussed herein confirm the present and previous records of abundant colonies in different locations within the Archipelago, making it possible to conclude that the three species, after their recent introduction are well established locally. Moreover, the potential of the three studied bryozoan species to further colonize more coastal stretches of the Azores is suggested by the year-round presence of colonies at all stages of development.
Species traits that control geographic range and the spreading phase following introduction include larval mode, environmental tolerance and the ability to float or raft [85]. Currently, over large distances, human-mediated transport may be far more important for dispersal than natural methods [16], but at a local scale, their success in establishing large populations and further colonizing new habitats can be evaluated in the medium and longer term through the study of their biological traits, among their reproductive strategies.
The populations of the three species considered in the present study are characterized by a constant presence of adults capable of continuous reproduction. Embryos were observed every month, indicating that the larvae are released throughout the year and, thus, settle and produce new colonies continuously. Nevertheless, seasonality in the reproductive cycle is worth to be considered, in order to optimize control strategies.
Bryozoa typically have short-lived non-feeding larvae with limited dispersal capabilities that will usually initiate metamorphosis within a few hours of release [86, 91]. Due to anthropogenic dispersal, however, many species can be found in subtropical and temperate waters worldwide [68, 74].
A substantial body of experimental work on the reproductive biology of Bugula neritina has been accumulated [48, 50, 90]. According to Keough and Chernoff [39] and Keough [38], B. neritina larvae have a short pelagic phase (0.5–2 h), suggesting limited natural dispersal. These wide-ranging research results encompass different aspects of larval, post-settlement and colonial performance. Bugula neritina embryos grow about 500 times larger, favoured by a placenta-like mechanism [90], while the increase factor in other Bryozoa [25] varies between 7 and 30; in general, the larger the egg, the lesser the nutrient input during the embryonic stage.
Mawatari [51, 52] published two papers dealing with the cheilostomes Bugula neritina and Tricellaria occidentalis (probably T. inopinata but it may be T. catalinensis), respectively. In Bugula, zooids were described as simultaneous hermaphrodites performing self-fertilization. Mawatari studied embryogenesis, larval structure, larval release and locomotion, as well as larval attachment and metamorphosis. He also presented data on the B. neritina life cycle throughout the year, including peaks of reproduction and larval settlement and the rate of colony growth and maturation. In Tricellaria, zooids were said to be non-simultaneous hermaphrodites. Mawatari [52] also briefly described oogenesis in T. occidentalis, and it is clear from his text and illustrations that oocytes develop in pairs in this species.
Observations on the larvae of T. inopinata have shown that in still waters, the larvae swim actively for a few hours and tend to attach onto aquaria glass walls where settlement occurs within a few minutes [63].
Although some differences have been recorded in the timing of preferential reproductive activity by bryozoans in Ponta Delgada Harbour, the continuous recruitment of larvae and the lack of die back in the winter months indicate a potential advantage of the three invasive species over native ones (for example, Bugula flabellata and Crisia denticulata) that are known from the literature to disappear in winter and then re-establish themselves—creating a yearly cycle, settling only during a short period [19, 63, 72]. In Eel Pond (Woods Hole, Massachusetts), observations on T. inopinata showed no sign of colony regrowth until May and no new colonies until June [36]. Differences in the timing of settlement could provide T. inopinata with sufficient time to recruit to available substrata and begin growing, preventing other species from forming dense aggregations where they had done so previously.
The amount of embryos was not, however, constant throughout the year during the observations made within Ponta Delgada, especially for Tricellaria inopinata and Virididentula dentata, these being less during the autumn months. The successful attachment of the larva in the area near the parent colonies was, moreover, also not equal throughout the year, especially for V. dentata for which July seems to be particular favourable for larval settlement. This information can allow managers to schedule the best time of the year to apply field management actions aimed at lowering population numbers of invading bryozoan species.
It has been reported that Bugula neritina and other erect bryozoans serve as habitats for other smaller NIS such as caprellids and isopods [66, 69, 70]. The dynamics of each studied population was not found to be related to variations in temperature or photoperiod characteristic of the geographic area. Instead, it may be related to the availability of food, especially for Virididentula dentata, since the highest percentage of ancestrulae plus young colonies of this species anticipates the months when the summer plankton community in the Azores reaches its maximum [18]. These factors could also suggest that V. dentata may in fact be a native species in the Azores, adapted to the regional abiotic/biotic characteristics [55] or simply that it is not so well adapted to the point of displaying an invasive behaviour, being more dependent on food availability than the other two studied species.
By the time of this study, no serious impacts on other species, such as, overgrowing, or serious modifications of the benthic community have been observed. Indeed the availability of reference community data in the specific marina under study are scarce, so we can rely only on the cursory experience of previous visits to the site. Nevertheless, caution is needed in relation to the dynamics of this biological community, as these Bryozoa may compete with native species for habitat and food, especially B. neritina and T. inopinata [2]. Bugula neritina may, moreover, affect the mariculture of bivalves, by coating reproductive structures or the valves of the growing cultures [75]. A similar behaviour can be expected from T. inopinata as it has been reported to overgrow several other species of arborescent bryozoans and various other organisms, including mussels, sponges, ascidians and barnacles in the Venice Lagoon [61], with the observation of a subsequent decline of the native bryozoan species [47]. Ecological information on V. dentata is scarce, and the status of the species as invasive is not yet established.
The observations on the reproductive status of the three species suggest that the propagule pressure from the established populations is potentially increasing and promote further spread to other localities in the islands of the Archipelago of Azores. The observed differences in periods of successful reproduction and recruitment in the three species examined herein may offer opportunities to focus control and mitigation measures in some periods. More generally, the knowledge of each species’ reproductive development is important for management planning to control the population numbers of invasive species. The next research step should consist of the determination of the best removal or control strategies and the choice of intervention areas.
References
Abecassis RC, Afonso P, Colaço A, Longnecker N, Clifton J, Schmidt L, Santos RS. Marine conservation in the Azores: evaluating marine protected area development in a remote island context. Front Mar Sci. 2015;2:104. doi:10.3389/fmars.2015.00104.
Aguilar LR, Ugaz LT, Garay JC. Variación estacional de Bugula neritina (Bryozoa, Cheilostomata) en las estructuras de cultivo suspendido de Argopecten purpuratus en bahía Samanco (Ancash, Perú). Revista científica de la Sociedad Española de Acuicultura. 2014;40:1–10.
Amat JN, Tempera F. Zoobotryon verticillatum Delle Chiaje, 1822 (Bryozoa), a new occurrence in the archipelago of the Azores (North-eastern Atlantic). Mar Pollut Bull. 2009;58:761–4.
Anderson LWJ. California’s reaction to Caulerpa taxifolia: a model for invasive species. Biol Invasions. 2005;7:1003–16.
Ávila SP, Madeira P, Rebelo AC, Melo C, Hipólito A, Pombo J, Botelho AZ, Cordeiro R. Phorcus sauciatus (Koch, 1845) (Gastropoda: Trochidae) in Santa Maria, Azores archipelago: the onset of a biological invasion. J Molluscan Stud. 2015. doi:10.1093/mollus/eyv012.
Bax N, Carlton JT, Mathews-Amos A, Haedrich RL, Howarth FG, Purcell JE, Rieser A, Gray A. The control of biological invasions in the world’s oceans. Conserv Biol. 2001;15:1234–46.
Bax N, Hayes K, Marshall A, Parry D, Thresher R. Man-made marinas as sheltered islands for alien marine organisms: establishment and eradication of an alien invasive marine species. In: Veitch CR, Clout MN, editors. Turning the tide: the eradication of invasive species. Gland, Cambridge: IUCN SSC Invasive Species Specialist Group. IUCN [World Conservation Union]; 2002. p. 26–39.
Bax N, Williamson A, Aguero M, Gonzalez E, Geeves W. Marine invasive species: a threat to global biodiversity. Mar Policy. 2003;27:313–23.
Beck SD. Insect photoperiodism. New York: Academic; 1968.
Berning B. Taxonomic notes on some Cheilostomata (Bryozoa) from Madeira. Zootaxa. 2012;3236:36–54.
Calvet L. Diagnoses de quelque espéces nouvelles de Bryozoaires Cyclostomes provenant des campagnes scientifiques accomplies par S. A. S. le Prince de Monaco, à bord de la Princesse-Alice (1889–1910). Bulletin de l’Institut Océanographicque du Monaco. 1911;8:1–9.
Calvet L. Bryozoaires provenant des campagnes scientifiques du Prince Albert I de Monaco. Monaco: Resultats des Campagnes Scientifiques; 1931.
Canning-Clode J, Fofonoff P, McCann L, Carlton JT, Ruiz G. Marine invasions on a subtropical island: fouling studies and new records in a recent marina on Madeira Island (Eastern Atlantic Ocean). Aquat Invasions. 2013;8:261–70.
Cardigos F, Tempera F, Ávila S, et al. Non-indigenous marine species of the Azores. Helgol Mar Res. 2006;60:160–9.
Cardigos F, Monteiro J, Fontes J, Parretti P, Serrão Santos R. Fighting Invasions in the Marine Realm, a Case Study with Caulerpa webbiana in the Azores. In: Canning Çlode J, editor. Biological invasions in changing ecosystems (Ch. 12). Berlin: Walter de Gruyter GmbH & Co KG; 2015. p. 279–300.
Carlton JT. Patterns of transoceanic marine biological invasions in the Pacific Ocean. Bull Mar Sci. 1987;41:452–65.
Carlton JT. Man’s role in changing the face of the ocean: biological invasions and implications for conservation of near-shore environments. Conserv Biol. 1989;3:265–73.
Colebrook JM. Continuous plankton records: relationships between species of phytoplankton and zooplankton in the seasonal cycle. Mar Biol. 1984;83:313–23.
Cook EJ, Stehlíková J, Beveridge CM, Burrows MT, De Blauwe H, Faasse M. Distribution of the invasive bryozoan Tricellaria inopinata in Scotland and a review of its European distribution. Aquat Invasions. 2013;8:281–8.
Courtney WR Jr, Williams JD. Dispersal of exotic species from aquaculture sources, with emphasis on freshwater fishes. In: Rosenfield A, Mann R, editors. Dispersal of living organisms into aquatic ecosystems. College Park: University of Maryland; 1992. p. 49–82.
Coutts ADM, Forrest BM. Development and application of tools for incursion response: lessons learned from the management of the fouling pest Didemnum vexillum. J Exp Mar Biol Ecol. 2007;342:154–62.
Cowie R. Invertebrate invasions on Pacific islands and the replacement of unique native faunas: a synthesis of the land and freshwater snails. Biol Invasions. 2002;3:119–36.
Culver CS, Kuris AM. The apparent eradication of a locally established introduced marine pest. Biol Invasions. 2000;2:245–53.
d’Hondt J-L. Bryozoaires cténostomes et cheilostomes (Cribrimorphes et Escharellidae exceptés) provenant des dragages de la campagne océanographique Biacores du “Jean-Charcot”. Bull Mus Natl Hist Nat. 1975;209:553–600.
Dyrynda PEJ, King PE. Gametogenesis in placental and non- placental ovicellate cheilostome Bryozoa. J Zool. 1983;200:471–92.
Dyrynda PJ, Fairall VR, Occhipinti-Ambrogi A, d’Hondt J-L. The distribution, origins and taxonomy of Tricellaria inopinata d’Hondt and Occhipinti Ambrogi, 1985, an invasive bryozoan new to the Atlantic. J Nat Hist. 2000;34:1993–2006.
Fehlauer-Ale KH, Mackie JA, Lim-Fong GE, Ale E, Pie MR, Waeschenbach A. Cryptic species in the cosmopolitan Bugula neritina complex (Bryozoa, Cheilostomata). Zool Scr. 2014;43:193–205.
Fehlauer-Ale KH, Winston JE, Tilbrook KJ, Nascimento KB, Vieira LM. Identifying monophyletic groups within Bugula sensu lato (Bryozoa, Buguloidea). Zool Scr. 2015. doi:10.1111/zsc.12103.
Fernández Pulpeiro E, Cesar-Aldariz J, Reverter Gil O. Sobre la presencia de Tricellaria inopinata d'Hondt and Occhipinti Ambrogi, 1985 (Bryozoa, Cheilostomatida) en el litoral gallego (N.O. España). Nova Acta Cientifica Compostelana. Bioloxia. 2001;11:207–13.
Ferraz RR, Santos V, Vizinho S, Guerreiro V, Cardigos F, Frade P, Tempera F, Santos RS. Caracterização Ecológica e Sócio-económica do Sítio de Importância Comunitária Costa Nordeste e Ponta do Topo (PTJOR0014) e Medidas de Gestão Propostas. Arquivos do DOP: Série Estudos no. 20/2004, Horta; 2004.
Forrest BM, Hopkins GA. Population control to mitigate the spread of marine pests: insights from management of the Asian kelp Undaria pinnatifida and colonial ascidian Didemnum vexillum. Manag Biol Invasion. 2013;4:317–26.
Harmelin JG. Sur Quelques Cribrimorphes (Bryozoa, Cheilostomata) de l’Atlantique Oriental. Tethys. 1978;8:173–92.
Harvell CD, Grosberg RK. The timing of sexual maturity in clonal organisms. Ecology. 1988;69:1855–64.
Hayward PJ. Preliminary observations on settlement and growth in populations of Akyonidium hirsutum (Fleming). In: Larwood GP, Rosen BR, editors. Living and fossil Bryozoa. New York: Academic; 1973. p. 107–13.
Hopkins GA, Forrest BM, Jiang W, Gardner JPA. Successful eradication of a non-indigenous marine bivalve from a subtidal soft-sediment environment. J Appl Ecol. 2011;48:424–31.
Johnson CH, Winston JE, Woollacott RM. Western Atlantic introduction and persistence of the marine bryozoan Tricellaria inopinata. Aquat Invasions. 2012;7:295–303.
Jullien J, Calvet L. Bryozoaires Provenant des Campagnes de l’Hirondelle (1886–1888). Résultats des Campagnes Scientifiques Accomplies sur son Yacht par Albert 1er Prince Souverain de Monaco. 1903;23:1–188.
Keough MJ. Dispersal of the bryozoan Bugula neritina and effects of adults on newly metamorphosed juveniles. Mar Ecol Prog Ser. 1989;57:163–71.
Keough MJ, Chernoff H. Dispersal and population variation in the bryozoan Bugula neritina. Ecology. 1987;68:199–210.
Lim G. Bugula (Bryozoa) and their bacterial symbionts: a study in symbiosis, molecular phylogenetics and secondary metabolism. Ph.D. Dissertation, University of California; 2004.
Linneman J, Paulus D, Lim-Fong G, Lopanik NB. Latitudinal Variation of a Defensive Symbiosis in the Bugula neritina (Bryozoa) sibling species complex. PLoS One. 2014;9(10):e108783. doi:10.1371/journal.pone.0108783.
Locke A, Hanson JM. Rapid response to nonindigenous species. 3. A proposed framework. Aquat Invasions. 2009;4:259–73.
Locke A, Hanson JM, MacNair NG, Smith AH. Rapid response to nonindigenous species. 2. Case studies of invasive tunicates in Prince Edward Island. Aquat Invasions. 2009;4:249–58.
Lodola A, Savini D, Occhipinti-Ambrogi A. First record of Tricellaria inopinata (Bryozoa: Candidae) in the harbours of La Spezia and Olbia, Western Mediterranean Sea (Italy). Mar Biodivers Rec. 2012;5:e41. doi:10.1017/S1755267212000309.
Mackie JA, Keough MJ, Norman JA, Christidis L. Mitochondrial evidence of geographical isolation within Bugula dentata Lamouroux. In: Wyse Jackson PN, Buttler CJ, Spencer JME, editors. Bryozoan studies 2001, proceedings of 12th international bryozoology association conference, Balkema, Lisse, Netherlands, 2002. p. 199–206.
Mackie J, Keough M, Christidis L. Invasion patterns inferred from cytochrome oxidase I sequences in three bryozoans, Bugula neritina, Watersiporas ubtorquata, and Watersipora arcuata. Mar Biol. 2006;149:285–95.
Marchini A, Cunha MR, Occhipinti-Ambrogi A. First observations on bryozoans and entoprocts in the Ria de Aveiro (NW Portugal) including the first record of the Pacific invasive cheilostome Tricellaria inopinata. Mar Ecol. 2007;28:154–60.
Marshall DJ, Keough MJ. Offspring size plasticity in response to intraspecific competition: and adaptive maternal effect across life-history stages. Am Nat. 2008;171:225–37.
Marshall DJ, Bolton TF, Keough MJ. Offspring size affects the post-metamorphic performance of a colonial marine invertebrate. Ecology. 2003;84:3131–7.
Marshall DJ, Allen RM, Crean AJ. The ecological and evolutionary importance of maternal effects in the sea. Oceanogr Mar Biol. 2008;46:203–50.
Mawatari S. On the natural history of a common fouling bryozoan, Bugula neritina (Linnaeus). Misc Rep Res Inst Nat Resour. 1951;20:47–54.
Mawatari S. On Tricellaria occidentalis (Trask), one of the fouling bryozoans in Japan. Misc Rep Res Inst Nat Resour. 1951;22:9–16.
McEnnulty FR, Bax NJ, Schaffelke B, Campbell ML. A review of rapid response options for the control of ABWMAC listed introduced marine pest species and related taxa in Australian waters, CRIMP Technical Report Number 23. Hobart: CSIRO Marine Research; 2001.
Meyerson LA, Reaser JK. Biosecurity: moving toward a comprehensive approach. Bioscience. 2002;52:593–600.
Micael J, Rodrigues AS, Barreto MC, Alves MJ, Jones MB, Costa AC. Allocation of nutrients during the reproductive cycle of Ophidiaster ophidianus (Echinodermata: Asteroidea). Invertebr Reprod Dev. 2011;55:205–16.
Micael J, Parente M, Costa AC. Tracking macroalgae introductions in North Atlantic oceanic islands. Helgol Mar Res. 2014;68:209–19.
Micael J, Marina J, Costa AC, Occhipinti-Ambrogi A. The non-indigenous Schizoporella errata (Bryozoa: Cheilostomatida) introduced into the Azores Archipelago. Mar Biodivers Rec. 2014. doi:10.1017/S1755267214001298.
Mills EL, Leach JH, Carlton JT, Secor CL. Exotic species in the Great Lakes: a history of biotic crises and anthropogenic introductions. J Great Lakes Res. 1993;19:1–57.
Mitchell I, Jones A, Crawford C. Distribution of feral Pacific oysters and environmental conditions. Hobart: Marine Research Laboratories-Tasmanian Aquaculture and Fisheries Institute, University of Tasmania; 2000.
Myers JH, Simberloff D, Kuris A, Carey J. Eradication revisited: dealing with exotic species. Trends Ecol Evol. 2000;15:316–20.
Occhipinti-Ambrogi A. The spread of Tricellaria inopinata into the lagoon of Venice: an ecological hypothesis. Bulletin Societé Sciences Naturels Ouest France Mémoires Hors Série. 1991;1:299–308.
Occhipinti-Ambrogi A. Global change and marine communities: alien species and climate change. Mar Pollut Bull. 2007;55:342–52.
Occhipinti-Ambrogi A, d’Hondt J-L. The invasion ecology of Tricellaria inopinata in the lagoon of Venice: morphological notes on larva and ancestrula. In: Hayward PJ, Ryland JS, Taylor PD, editors. Biology and palaeobiology of bryozoans. Fredensberg: Olsen & Olsen; 1994. p. 139–44.
Porter JS, Spencer Jones ME, Kuklinski P, Rouse S. First records of marine invasive non-native Bryozoa in Norwegian coastal waters from Bergen to Trondheim. Bioinvasions Rec. 2015;4:157–69.
Ramalho LV, Muricy G, Taylor PD. Taxonomy and distribution of Bugula (Bryozoa: Cheilostomata: Anasca) in Rio de Janeiro state, Brazil. In: Moyano HI, Cancino JM, Wyse-Jackson PN, editors. Bryozoan studies 2005. Leiden: AA Balkema Publishers; 2005. p. 231–43.
Ramalhosa P, Canning-Clode J. The invasive caprellid Caprella scaura Templeton, 1836 (Crustacea: Amphipoda: Caprellidae) arrives to Madeira Island, Portugal. Bioinvasions Rec. 2015;4:97–102.
Rew LJ, Lehnhoff EA, Maxwell BD. Non-indigenous species management using a population prioritization framework. Can J Plant Sci. 2007;87:1029–36.
Rodgers PJ, Woollacott RM. Systematics, variation, and developmental instability: analysis of spine patterns in ancestrulae of a common bryozoan. J Nat Hist. 2006;40:1351–68.
Ros M, Guerra-García JM, González-Macías M, Saavedra Á, López-Fe CM. Influence of fouling communities on the establishment success of alien caprellids (Crustacea: Amphipoda) in Southern Spain. Mar Biol Res. 2013;9:261–73.
Ros M, Guerra-García J, Navarro-Barranco C, Cabezas MP, Vázquez-Luis M. The spreading of the non-native caprellid (Crustacea: Amphipoda) Caprella scaura Templeton, 1836 into southern Europe and northern Africa: a complicated taxonomic history. Mediterr Mar Sci. 2014;15:145–55.
Ruiz GM, Carlton JT, Hines AH. Global invasions of marine and estuarine habitats by non-indigenous species: mechanisms, extent, and consequences. Am Zool. 1997;37:621–32.
Ryland JS. The British species of Bugula (Polyzoa). Proc Zool Soc Lond. 1960;134:65–105.
Ryland JS. Bryozoa in the Great Barrier Reef Province. Proc Int Coral Reef Symp. 1974;2:341–8.
Ryland JS, Bishop JDD, de Blauwe H, Nagar AE, Minchin D, Wood CA, Yunnie ALE. Alien species of Bugula (Bryozoa) along the Atlantic coasts of Europe. Aquat Invasions. 2011;6:17–31.
Sá FS. O efeito dos organismos incrustantes e sua fauna associada, no crescimento do mexilhão Perna perna (Linnaeus, 1758) em estruturas de cultivo. Dissertation, Universidade Federal do Espírito Santo; 2004.
Santos V, Cardigos F, Guerreiro V, Diez C, Vizinho S, Ferraz RR, Tempera F, Frade P, Santos RS. Caracterização Ecológica e Sócio-Económica do Sítio de Importância Comunitária Costa das Quatro Ribeiras (PTTER0018) e Medidas de Gestão Propostas. Arquivos Internos do DOP: Série Estudos no. 13/2004, Horta; 2004.
Scholz J, Nakajima K, Nishikawa T, Kaselowsky J, Mawatari FS. First discovery of Bugula stolonifera Ryland, 1960 (Phylum Bryozoa) in Japanese waters, as an alien species to the Port of Nagoya. Bull Nagoya Univ Mus. 2003;19:9–19.
Sebens KP. Competition for space: growth rate, reproductive output, and escape in size. Am Nat. 1982;120:189–97.
Simberloff D. Today Tititiri Matangi, tomorrow the world! Are we aiming too low in invasives control? In: Veitch CR, Clout MN, editors. Turning the tide: the eradication of invasive species. Gland, Cambridge: IUCN SSC Invasive Species Specialist Group, IUCN; 2002. p. 4–12.
Simberloff D, Parker IM, Windle PN. Introduced species policy, management, and future research needs. Front Ecol Environ. 2005;3:12–20.
Souto J, Kaufmann MJ, Canning-Clode J. New species and new records of bryozoans from shallow waters of Madeira Island. Zootaxa. 2015;3925:581–93.
Tempera F, Afonso P, Morato T, Prieto R, Silva M, Cruz A, Gonçalves J, Santos RS. Comunidades Biológicas dos Sítios de Interesse Comunitário do Canal Faial-Pico. Arquivos do DOP. Série de Relatório Internos, 5. Departamento de Oceanografia e Pescas da Universidade dos Açores, Horta; 2001.
Thresher RE, Kuris AM. Options for managing invasive marine species. Biol Invasions. 2004;6:295–300.
Torres P, Costa AC, Dionísio MA. New alien barnacles in the Azores and some remarks on the invasive potential of Balanidae. Helgol Mar Res. 2012;66:513–22.
Watts PC, Thorpe JP, Taylor PD. Natural and anthropogenic dispersal mechanisms in the marine environment: a study using cheilostome Bryozoa. Philos Trans R Soc Lond B. 1998;353:453–64.
Wendt DE, Woollacott RM. Ontogenies of phototactic behavior and metamorphic competence in larvae of three species of Bugula (Bryozoa). Invertebr Biol. 1999;118:75–84.
Whitehead J. Derwent Estuary introduced marine and intertidal species: review of distribution, issues, recent actions and management options. Tasmania: Derwent Estuary Program; 2008.
Williamson M. Biological invasions. London: Chapman and Hall; 1996.
Wirtz P, Canning-Clode J. The invasive bryozoan Zoobotryon verticillatum has arrived at Madeira Island. Aquat Invasions. 2009;4:669–70.
Woollacott RM, Zimmer RL. A simplified placenta-like system for the transport of extraembryonic nutrients during embryogenesis of Bugula neritina (Bryozoa). J Morphol. 1975;147:355–78.
Woollacott RM, Pechenik JA, Imbalzano KM. Effects of duration of larval swimming period on early colony development in Bugula stolonifera (Bryozoa: Cheilostomata). Mar Biol. 1989;102:57–63.
Wotton DM, Hewitt CL. Marine biosecurity post-border management: developing incursion response systems for New Zealand. N Z J Mar Freshw Res. 2004;38:553–9.
Authors' contributions
JM contributed to all stages of the work; NJ contributed to the acquisition, analysis and interpretation of data; CN contributed to the acquisition, analysis and interpretation of data; AO contributed to conception, design and revising the work critically for important intellectual content; AC contributed to conception, design and revising the work critically for important intellectual content. All authors’ read and approved the final manuscript.
Acknowledgements
The authors are thankful to Nuno Barata from the Portos dos Açores for allowing the development of the work within the Ponta Delgada Marina. This project was funded by the Direcção Regional de Ciência e Tecnologia (DRCT)—‘Açores: Stopover for Marine Alien Species?’—ASMAS—M2.1.2/I/032/2011.
Competing interests
The authors declare that they have no competing interests.
Author information
Authors and Affiliations
Corresponding author
Additional files
10152_2016_458_MOESM1_ESM.doc
Additional file 1. Results of the Tukey HSD post-hoc test to discriminate significant variations in the presence of ovicells with embryos and ancestrulae plus young colonies of the Bryozoa species among months. Significance levels are marked with * P < 0.05; ** P < 0.01; and *** P < 0.001. APR April, AUG August, FEB February, DEC December, MAR March, MAY May, JAN January, JUL July, JUN June, NOV November, OCT October, SEP September.
10152_2016_458_MOESM2_ESM.doc
Additional file 2. Monthly variation in day length and seawater temperature at São Miguel. Seawater temperature data recorded from 0.5 m below the low water mark using a multi-parameter water quality meter probe (Horiba u-50). Day length at São Miguel was obtained from Beck [9].
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Micael, J., Jardim, N., Núñez, C. et al. Some Bryozoa species recently introduced into the Azores: reproductive strategies as a proxy for further spread. Helgol Mar Res 70, 7 (2016). https://doi.org/10.1186/s10152-016-0458-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s10152-016-0458-7