Abstract
A hallmark of biological invasion is the rapid reproduction of invasive species. However, we still do not have an adequate understanding of how fluctuations in environmental factors affect the reproduction of invasive species, particularly in estuarine habitats. In this study, we evaluated the reproduction of the Gulf toadfish Opsanus beta and its relationships with temperature and rainfall in the Paranaguá estuary, a subtropical region in southern Brazil. This species was likely introduced into the Brazilian ecosystems through the discharge of ballast water. Since invasion, which was first reported in port areas, the species has rapidly spread along the Brazilian coast line. We collected specimens monthly from October 2011 to September 2012 and from October 2019 to September 2020, to give us insight into the seasonal and temporal changes in the reproduction of this species. We found that O. beta population uses the estuary throughout its life cycle, including during spawning. Females and males spawned throughout almost the entire sampling period and spawning was specified as partially. Peak reproductive activity occurred in periods of lower temperature and was also related to rain. The length of first maturation in females (10cm) was considered early, indicating reproductive activity in smaller individuals. Our results demonstrate that the reproductive strategy used by the invasive species supports its ability to successfully colonize new environments.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Biological invasions are one of the main threats to the world’s biodiversity [1]. In the aquatic environment, invasive species can affect biodiversity [2, 3] due to the absence of predators, the ability to adapt to the new location, and interfere with the economy, by negatively influencing the fishing and aquaculture activities and increasing health risks [3, 4]. Therefore, we need to better understand the biological processes that support the success of invasive species in colonizing new habitats in order to design effective conservation strategies to protect native species and for better management of ecosystem functions [5, 6].
Reproduction is one of the main biological processes that can ensure the establishment and maintenance of exotic species in an unfamiliar environment. In fish, reproduction is linked to environmental factors [7], and requires a variation in some specific environmental factors for the initiation (environmental triggers) and development of the gonads [8]. Moreover, for a given species to achieve reproductive success, reproduction must occur under favorable environmental conditions for the development of larvae and alevins. Thus, invasive fish species have to rapidly adapt their reproductive signals and timing to the environmental fluctuations of the new environment in order to successfully colonize the new habitat.
An emerging threat to tropical and subtropical estuarine ecosystems in Brazil is the invasion of Opsanus beta (Goode and Bean, 1880). Opsanus beta has its natural distribution in the western Central Atlantic, and from the Gulf of Mexico to Palm Beach, Florida [9,10,11]. The species was reported on the southeastern and southern coast of Brazil, in the port areas of Santos (state of São Paulo, southeastern Brazil) in 2005 [12], Paranaguá (state of Paraná, southern Brazil) in 2007 [13], Guanabara Bay (state of Rio de Janeiro, southeastern Brazil) in 2017 [14], in Sepetiba Bay (state of Rio de Janeiro, southeastern Brazil) in 2018 [15] and in the non-port area of Guaratuba Bay (state of Paraná, southern Brazil) in 2020 [16]. Given that 95% of foreign trade in Brazil is carried out through maritime transport, there is a high probability that the introduction of O. beta occurred through the release of ballast water [17].
In Brazil, there is detailed information on the distribution of this invasive species, however, information on the impacts caused by this species is limited [18]. Nevertheless, from the first report in 2005 of this species in Brazil, there is evidence of geographical expansion, usually occurring in shipping port related regions, while more recent observations in a non-port area reflect the ability of the invasive species to disperse. This temporal expansion suggests that O. beta has successfully invaded the new habitat and is expanding and potentially threating the homeostasis of estuarine and non-estuarine habitats in the Brazilian coast. A main factor underpinning this invasion is likely the ability of O. beta to reproduce successfully across seasons, although evidence of this reproductive process remains allusive.
To address this we characterized the reproductive patterns of O. beta in subtropical estuaries to better understand its impact on the functioning of the estuarine ecosystem, which is a nursery for marine biodiversity [19]. Furthermore, in the Paranaguá Estuarine Complex (where this study was carried out), different species of interest for fishing use estuaries in one or more phases of their life cycle and, therefore, any impact of invasive species can influence fishing. We evaluated O. beta reproductive patterns across two time series to ensure that our analyses were robust and captured the true reproductive dynamics of this species. This is because after analyzing the first sampling period, we found that some parameters, such as the length of first maturation and the period of recruitment, were biased by the small number of young individuals (1.4% of the total, all males). By sampling a second time, it was possible to verify and validate our observations at a second point in time, corroborating the variation in reproductive tactics over 7 years. Across our experiments, we found that O. beta reproduction patterns were associated with the seasonal variation of environmental factors across two time series.
The invasion of O. beta into the estuarine ecosystem likely results in negative impacts to estuarine functioning, such as loss of biodiversity due to overlapping niches, increased inter- and intraspecific competition, as well as a decline in fishing yields, which can directly affect communities local and leading to socioeconomic impacts in the region. Our results show the reproductive patterns of an important estuary invasive species, which can spread due to human activities and colonize new environments.
2 Material and methods
2.1 Study area
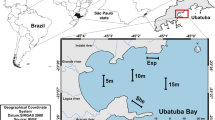
The study was carried out in the Paranaguá Estuarine Complex, comprised of the bays in Paranaguá and Antonina (east–west axis), Guaraqueçaba, Pinheiros and Laranjeiras bay (north–south axis) [20], located on the coast of the state of Paraná, in southern Brazil (Fig. 1). The climate in the region is mesothermal humid subtropical, according to the Köppen classification system, which includes hot summers and without a well-defined dry season. The average temperature in summer is above 22 ℃ while in winter the average is below 18 ℃ [21]. The rainy season corresponds to spring and summer months (from October to March), while the dry season occurs during the autumn and winter (from April to September) [20], with an annual average of 2500 mm of rain [22]. The rainiest period (summer) can accumulate precipitation levels between 900 and 1000 mm. While the driest period (winter) can accumulate precipitation levels between 300 and 400 mm [23].
Map of the estuarine complex in Paranaguá, on the northern shore of the State of Paraná, Brazil, locating the sampling sites. The ccircle with a dotted line in red refers to the collections during the years 2011/2012 and the circle with a solid line in blue refers to the collections during the years 2019/2020. The Location of the Port of Paranaguá refers to the yellow star
Samples were collected monthly during two periods. The first period was from October 2011 to September 2012 and the second from October 2019 to September 2020. Specimens were captured with longlines in the first series and manually at low tide in the second time series. It is important to note that the locations sampled in each collection period are close together (less than 10 km) and under the same environmental conditions [23]. There are no differences between salinity, pH, dissolved oxygen, temperature and precipitation. Reproductive analyzes included maturation curve, frequency of gonadal development (monthly and seasonal), total and somatic condition factors, and length at first maturation.
2.2 Data collection
Specimens were obtained through catches made by local fishermen. The collections were conducted monthly, over a period of 1 year, and in each period the fishing gear used was different. The specimens sampled from October 2011 to September 2012 were obtained using longlines on a rocky bottom, and the specimens gathered from October 2019 to September 2020 were obtained manually, at low tide. The use of different equipment allowed for an evaluation of the entire population being studied.
After collection, specimens were cooled on ice and transported to the laboratory for analysis. License for collection and transport nº 14683-4, issued by the Chico Mendes Institute for Biodiversity Conservation (ICMBio)—Biodiversity Authorization and Information System (SISBIO). In the laboratory, the specimens were identified according to the descriptions by Collette [11] and Caires et al. [13]. Each specimen had morphometric data of total length (cm) and total weight (g) recorded. Subsequently, gonads were removed, weighed (g) and identified macroscopically for sex and gonadal development. Some gonads were destined for the routine histological process. The gonads were fixed in ALFAC for 18 h, embedded in paraffin and stained with Hematoxylin–Eosin (HE), and processed according to Fávaro and Oliveira [24].
Tissues were analyzed with histology under a light microscope to correct the macroscopic analysis of sex and determine the stages of gonadal development, when necessary. Analyzes of gonadal development were carried out based on studies by Vazzoler [25], Brown-Peterson et al. [26], on the ovarian maturity scale proposed by Lowerre-Barbieri et al. [27]and in the testicular maturity scale proposed by Gomes and Araújo [28]. Abiotic precipitation and temperature data for the sampled periods were obtained from the Paraná Meteorological System (SIMEPAR).
2.3 Data analysis
2.3.1 Maturation curve and frequency of gonadal development stages
The data obtained were analyzed in the same way for both time series. The reproductive period of the species was initially characterized by the maturation curve, for separate sexes, and later corroborated by other reproductive analyses. To create the maturation curve, the individual Gonadal-Somatic Index (GSI) was determined, calculated using the formula GSI = (Wg/Wt) × 100; Wg representing the weight of the gonad, and Wt referring to the total weight of the fish. From the individual GSI, the average monthly GSI was calculated to create the gonadal maturation curve. We used a linear model with GSI as the response variable and the linear and quadratic effect of month, seasonality and sex (and their interactions) as explanatory variables. GSI values were log-transformed to improve model fit. P values were obtained with ANOVA function, with a significance threshold of 0.05. The frequency of gonadal development, throughout the monthly distribution for both sexes, stages and the classification of spawning type were done using histological analysis of the gonads.
2.3.2 Energy allocation
To verify the allocation of energy in the reproductive process, the weight-length ratio was initially determined using the expression Wt = aLt b (least squares method). The value of “a” that corresponds to the linear coefficient and the value of “b” that corresponds to the angular coefficient [29]. Subsequently, the monthly means of the total (K) and somatic (K’) condition factors were calculated separately for each gender, using the following formulas: K = Wt/Lt b and K' = Wc/Lt b, where Wt = total weight, Lt = total length, Wc = body weight (Wc = Wt-Wg, where Wg = gonadal weight), b = angular coefficient obtained from the weight-length ratio. The result of this analysis was obtained according to the spacing of the K and K' curves, the greater the distance between the curves, the greater the energy consumed in order to reach maturation and development of the gonads. For this reason, immature individuals were excluded from the analysis, to avoid data masking.
2.3.3 Length of the first maturation
The length at first sexual maturation was determined using the curve that relates to the relative frequency of adult individuals to the midpoint of the length classes (established using the Sturges Postulate), adjusted by the equation: Fr = 1–(e –aLmb) where: Fr is the relative frequency of adult individuals, “ e” is the base of the Napierian logarithm. “A” and “b” are the linear and angular coefficients estimated by the method of least squares [29] and applied in the linear relation obtained by transforming the variables involved. “Lm” is represented as the midpoint of the total length classes. Due to the low frequency and abundance of young specimens obtained from October 2011 to September 2012, it was not possible to determine the length at first maturation for either sex. Thus, this analysis was performed only for specimens obtained in the 2019–2020 time series, for both sexes.
To evaluate the variation in temperature and precipitation and their possible relationships with the reproductive process, in the two-time series, we tested the differences of these environmental factors between seasons, using the Kruskal–Wallis test with a significance limit of 0.05. Seasons were considered as: Spring (October to December), Summer (January to March), Autumn (April to June) and Winter (July to September).
3 Results
Based on the morphological data obtained in both sampling periods and across months, our results indicate that the population is composed of both young and adult individuals which use the area of the Paranaguá estuarine complex during all months of the year. In the period from 2011–2012, 440 individuals were analyzed, 147 females (33.5%) and 293 males (66.5%). Total lengths ranged from 6.5 cm to 28.5 cm for females, and 6.6 cm to 32 cm for males. In the 2019–2020 time series, 365 individuals were analyzed, 162 females (44.4%) and 203 males (55.6%). There was a range in total length from 6.2 cm to 29 cm in the female population, and from 5.2 cm to 28.8 cm for the males. The abundance of young individuals (immature gonads) was reduced in the period from 2011–2012 (1.4% of the total) and abundant in the years from 2019–2020 (47% of the total).
Microscopic analysis of the gonads showed five stages of ovarian development: Immature (A), in development (B), capable of spawning (C), partially spawned (PS) and spawned (S). In addition, five stages of testicular development were categorized: Immature (A), developing (B), able to sperm (C), partially emptied (PE) and emptied (E). The observation of female and male gonads with a partial release of gametes, the characterization of the partially spawned and partially emptied stages for females and males, respectively, allowed for characterizing partial spawning for O. beta (Fig. 2).
Histological section of O. beta gonads, captured in the Paranaguá estuarine complex. A Ovary capable of spawning with hyalinized follicles (*); B Partially spawned ovary with post-ovulatory follicles (POF); C Testis able to sperm with seminiferous tubules filled almost exclusively with spermatozoa (white arrow); D Partially emptied testis, seminiferous tubules with decreased sperm count and presence of empty spaces (black arrows)
There were statistically significant interactions between sex, time period, and the linear or quadratic effect of month (Sex:Year:month: F1,732 = 10.533; p = 0.001; Sex:Year:month2: F1,732 = 7.224; p = 0.007) (Supplements 1). This suggests that the linear and quadratic fluctuations of IGS varied between sexes and between sampling time periods. This was evident from the sexual maturation curves, which showed a trend of synchronized gonadal development between the sexes but which was highly variable (Fig. 3). The highest IGS values were observed in the periods from June to November, corresponding to the occurrence of a greater number of individuals with gonads capable of spawning/spermiation (C) and partially spawned (PS) and partially emptied (PE) for the two analyzed periods (Fig. 4).
Distribution of monthly percentage frequency of the gonadal development stages of O. beta captured in the Paranaguá estuary, subtropical region of Brazil. A- females, B- males collected from the 2011/2012 time series, C- females and D- males from the 2019/2020 time series. Stages: A—immature; B—under development; C—able to spawn/spermiate, PS Partially spawned, PE Partially emptied, S spawned and E emptied
We found that the abundance of young specimens in our monthly and seasonal analyses of gonadal development stages (Figs. 4 and 5), obtained in the period 2019/2020. We also found that individuals capable of reproducing occur in all seasons, although more predominantly in winter and spring (Fig. 5). The data showed that in Autumn, the abundance of individuals with gonads capable of reproducing was greater in the period 2019/2020 than in the first time series of 2011–2012. This can potentially indicate that reproductive activity, despite occurring in autumn in both series analyzed, was more intense in the period of 2019/2020, further into the invasion process. The recruitment peak occurred in spring and summer, coinciding with the most intense end of the reproductive period (Fig. 5c, d).
Seasonal percentage frequency distribution of gonadal development stages of O. beta captured in the Paranaguá estuary, subtropical region of Brazil. A- females, B- males collected from the 2011/2012 time series, C- females and D- males from the 2019/2020 time series. Stages: A—immature; B—under development; C—able to spawn/spermiate; PS partially spawned, PE partially emptied, S spawned and E emptied
The period of greater energy investment in the reproductive process was evidenced by the greater distances between the total condition (K) and somatic (K’) factor curves (Fig. 6). More energy consumption was detected in females, in the two periods sampled, from June to January, coinciding with the highest frequencies and abundances of ovaries in the spawning stage. O. beta males allocated a greater consumption of energy during the development of the testes, throughout the year.
The results obtained from the analyzes of the gonadal maturation curve, frequency of gonadal development stages (monthly and seasonal) and distribution of the monthly K and K’ values allowed characterizing the occurrence of the reproductive period from late autumn to spring in both analyzed periods.
In 2011–2012, the majority of our specimens were adults. Young specimens of both sexes were sampled infrequently. They corresponded to 1.4% of the total specimens obtained, not allowing the determination of length at first sexual maturation. Thus, the determination of the length at the first maturation (L50) for females and males of O. beta (Fig. 7) was carried out with specimens obtained in the period from 2019–2020, where the frequency and abundance of young individuals were satisfactory, corresponding to 47% of the total obtained in the period. The L50 determined for females was 10.0 cm and the L 100 was 17.4 cm. For males, the L50 corresponded to 14.35 cm and the L 100 was 18.0 cm.
There was a small monthly and seasonal variation in temperature between the time periods. However, precipitation data showed more notable montly and seasonal variations. In the 9 months of the 2019/2020 period, precipitation values were lower when compared to the 2011/2012 period, corresponding to an 18.5% reduction in total annual precipitation, between the two periods analyzed. Specifically, in autumn 2020, precipitation was 59.6% lower than in autumn 2012, a period in which a significant difference was detected using the Kruskal–Wallis test (p = 0.049) (Fig. 8, Supplements 2 and 3).This matches the earlier onset and peak of reproductive activity inferred from the gonadal analyses (see above), suggesting that O. beta reproductive activity is sensitive to environmental fluctuations with respect to rainfall.
4 Discussion
Here, we conducted a survey of the population of an invasive species of subtropical estuaries to characterize their reproductive biology in early and later stages of biological invasion. We sampled monthly, in two time periods 7 years apart, and with different capturing techniques to gain distinct length structure for the same population of O. beta. As a result of this, smaller individuals were captured in greater numbers in the 2019–2020 time period, allowing to achieve results that had not been obtained with the collections conducted from the first collection in 2011–2012. The use of specimens obtained from different extraction techniques does not make the reproductive analyzes unfeasible and provides a positive aspect pertaining to the analyzes by including a portion of the population that would not be normally analyzed if only one fishing extraction technique was used. Various authors have employed different fish extraction techniques to overcome the selectivity of fishing gear and cover all length ranges of the population. Sometimes, different fishing gears have been used at different times and in different locations [30,31,32,33]. The 2019–2020 sampling was more extensive, and allowed us to estimate parameters such as length of maturation, which gives us a more granular understanding of the reproductive dynamics of the population.
Our histological analysis of gonadal development agrees with the maturity scales already established for other species in studies carried out in a subtropical estuary in southern Brazil [34,35,36,37], which were based on, and adapted from studies carried out by Vazzoler [25] and by Brown-Peterson et al. [26]. The characterization of a batch spawning for O. beta, in the analyzed estuary, was made possible through microscopic observations of the partially depleted ovaries and testes. The same type of spawning was observed for the species in the natural distribution area [9, 38, 39] and recorded for the congener species O. tau [40,41,42]. This suggests that our findings are in broad agreement with the findings of other species in the literature, corroborating the validity of our findings.
The maturation curve of females and males showed a graphic difference between the analyzed periods, which showed a drop in GSI values in the winter months of 2020, due to the greater number of young individuals obtained. For this reason, it was necessary to complement our analysis of the maturation curve to determine the reproductive period of a species. The reproductive period was established by the higher frequencies of specimens with gonads capable of spawning/emptying (stage C) and partially exhausted gonads (stages PS/PE). Comparing time series, we found that the reproductive period occurred in the winter and spring months in 2011–2012. However, in 2019–2020 the reproductive period came earlier, starting in late autumn with a high frequency of individuals capable of spawning/emptying, and extending throughout the winter and part of the spring. This was in agreement with the significant reduction in rainfall for the autumn in 2019–2020 compared with 2011–2012, indicating that reproductive activity may be dependent on raifall in this species. Despite the earlier reproductive period from 2019–2020, the duration of the reproductive period was the same during the 2011–2012 time period. This result demonstrates that the population of the analyzed invasive species is well adapted to the new environment, and able to adjust the reproductive cycle with the abiotic characteristics. This characteristic is necessary for an invasive population to stabilize in invaded environments [43, 44].
The small variation observed in the reproductive period, between the analyzed time series, can be explained by the variation in precipitation, which showed a significant difference between the autumn seasons of the two-time series. Rainfall and changes in abiotic factors can change the pattern of marine or fresh water entering the estuarine environment, also interfering with flow. The reduction in precipitation in the 2019/2020 period probably influenced other dependent environmental variables, such as salinity and pH balance. When comparing the two-time series, the precipitation in autumn of 2020 was reduced by approximately 60% compared to autumn in 2012. It is also important to note that autumn is the period of the year where an environmental trigger is likely to occur, which promotes the end of gonadal development and the beginning of the reproductive period.
The O. beta population analyzed had a long reproductive period, similar to that recorded by Barimo et al. [39], but contradicting to the data recorded by Breder [38] and Franco-Lopez et al. [9]. They reported a restricted reproductive period in the winter, in an area of natural distribution of the referred species. Otolith analysis from O. beta from two Brazilian estuaries [45] found that the Brazilian coastal populations are connected and probably isolated from the native Mexican population. In our analyses of K', it was possible to observe that the highest energy consumption destined for the reproductive process coincides with the period with the highest values of average monthly GSI and with the reproductive periods in each analyzed time series. This reflects the highest frequencies of individuals with gonads able to reproduce and/or that have been partially depleted. A similar result was described by Franco-Lopez et al. [9] for the same species in its place of origin.
Determining the length of the first sexual maturation is a biological parameter widely used in fish reproductive studies, as it allows for the creation of laws to standardize the sustainable exploitation of the resource (commercial species. In addition, it ensures the reproduction of the species, and maintains stock. However, this same parameter is important for monitoring exotic species, predicting reproductive potential, and possible damage to the environment and native species.
Based on the L50/Lmaximum ratio, the values obtained were 0.34 for females and 0.50 for males of O. beta. Considering that the value of the L50/Lmaximum ratio is between 0.40 and 0.90 for varied species [46], females of O. beta, in the Paranaguá estuary, were characterized with earlier maturity. This evidence, regarding the first maturation of females, can be considered a temporary reproductive tactic used to increase reproductive success in the invaded area. Gertzen et al. [47] described the reproductive characteristics and modes of reproduction of three invasive species of Gobiidae in native and invaded regions, highlighting interspecific differences. In the same study, it was shown that females of one of the species matured at much smaller sizes in the invaded area in the Lower Rhine (Germany) compared to other regions.
The multifaceted analysis of reproductive parameters presented here, such as the gonadal maturation curve, frequency distribution of the gonadal development stages, the determination of K and K', allowed for the characterization of the reproductive period of the invasive species O. beta in the estuary. Our data showed that the population of Opsanus beta uses the Paranaguá estuary, a subtropical region in the south of Brazil, throughout its life cycle. The use of this particular estuary as a reproduction area and spawning site was confirmed by the presence of ovaries with hyalinized follicles and post-ovulatory follicles, according to Vazzoler [25] and Dias et al. [48].
Reproduction strategies play an important role in biological invasion processes and help predict future population development [47]. The reproductive tactics used by the species include a high frequency of the gonadal development in the capable reproduction stage during most of the analyzed period. In addition, a prolonged reproductive period in the months of lower temperature (most estuarine species reproduce in periods of higher temperatures—spring and summer), partial spawning, and early maturation for females, make it possible to achieve reproductive success and remain in the invaded environment. Moreover, our results contributes to our understanding of how invasive species can adapt to novel environmental clines, for example, due to climate change, enabling a better understanding of the responses of invasive species across different scenarios.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
McGeoch MA, Butchart SHM, Spear D, Marais E, Kleynhans EJ, Symes A, Chanson J, Hoffmann M. Global indicators of biological invasion:species numbers, biodiversity impact andpolicy responses. Div Distrib. 2010;16:95–108. https://doi.org/10.1111/j.1472-4642.2009.00633.x.
Jeschke JM, Bacher S, Blackburn TM, Dick JT, Essl F, Evans T, Gaertner M, Hulme PE, Ku¨hn I, Mrugała A, Pergl J, Pyˇsek P, Rabitsch W, Ricciardi A, Richardson DM, Sendek A, Vila` M, Winter M, Kumschick S. Defining the impact of non-native species. Conserv Biol. 2014;28:1188–94. https://doi.org/10.1111/cobi.12299.
Ricciardi A, Blackburn TM, Carlton JT, Dick JTA, Hulme PE, Iacarella JC, Jeschke JM, Liebhold AM, Lockwood JL, MacIsaac HJ, Pysˇek P, Richardson DM, Ruiz GM, Simberloff D, Sutherland WJ, Wardle DA, Aldridge DC. Invasion science: a horizon scan of emerging challenges and opportunities. Trends Ecol Evol. 2017;32(6):464–74. https://doi.org/10.1016/j.tree.2017.03.007.
Lima Junior DP, Magalhães ALB, Pelicice FM, Vitule JRS, Azevedo-Santos VM, Orsi ML, Simberloff D, Agostinho AA. Aquaculture expansion in Brazilian freshwaters against the aichi biodiversity targets. Ambio. 2018;47(4):427–40. https://doi.org/10.1007/s13280-017-1001-z.
Walsh JR, Carpenter SR, Vander Zanden MJ. Invasive species triggers a massive loss of ecosystem services through a trophic cascade. Proc Natl Acad Sci. 2016;113(15):4081–5.
Kiruba-Sankar R, Raj JP, Saravanan K, Kumar KL, Angel JRJ, Velmurugan A, Roy SD. Invasive species in freshwater ecosystems–threats to ecosystem services. In: Biodiversity and climate change adaptation in tropical islands. Cambridge: Academic Press; 2018. p. 257–96.
Godinho AL, Lamas IR, Godinho HP. Reproductive ecology of Brazilian freshwater fishes. Environ Biol Fish. 2010;87:143–62. https://doi.org/10.1007/s10641-009-9574-4.
Ribeiro CS, Moreira RG. Environmental factors and fish reproduction. Revista da Biologia—Universidade de São Paulo USP. 2012;8(1):58–61.
Franco-López J, Alma GSG, Arenas LGA, Sánchez CB, Escorcia HB, Pérez JAM, Rodríguez EP, Legorreta JLV. Ecología y reproducción de Opsanus beta (Actinopterygii: Batrachoididae) en la Laguna de Alvarado, Veracruz México. Rev Biol Trop. 2017;65(4):1381–96. https://doi.org/10.15517/rbt.v65i4.27239.
Thorson RF, Fine ML. Acoustic competition in the gulf toadfish Opsanus beta: acoustic tagging. Acoust Soc Am. 2002;111(5):2302–7. https://doi.org/10.1121/1.1466865.
Collette BB. Batrachoididae. In: Carpenter KE (ed). The Living marine resources of the Western Central Atlantic. Vol 2: Bony Fishes part 1 (Acipenseridae to Grammatidae). FAO Species Identification Guide for Fishery Purposes and American Society of Ichthyologists and Herpetologists Special Publication, 2002; 5:1026–1042
Tomás ARG, Tutui SDS, Fagundes L, Souza MR. Opsanus beta: an invasive fish species in the Santos estuary. Brazil Bol Inst Pesca. 2012;38(4):349–55.
Caires RA, Pichler HA, Spach HL, Ignácio JM. Opsanus brasiliensis Rotundo, Spinelli & Zavalla-Camin, 2005 (Teleostei: Batrachoidiformes: Batrachoididae), a junior synonym of Opsanus beta (Goode & Bean, 1880), with notes on its occurrence in the Brazilian coast. Biota Neotrop. 2007;7(2):136–9. https://doi.org/10.1590/S1676-06032007000200015.
Cordeiro BD, Bertoncini AA, Abrunhosa FE, Corona LS, Araújo FG, Santos LN. First report of the nonnative gulf toadfish Opsanus beta (Goode & Bean, 1880) on the coast of Rio de Janeiro—Brazil. BioInvasions Rec. 2020;9:279–86. https://doi.org/10.3391/bir.2020.9.2.13.
Andrade-Tubino MF, Salgado FLK, Uehara W, Utsunomia R, Araújo FG. Opsanus beta (Goode & Bean, 1880) (Acanthopterygii: Batrachoididae), a nonindigenous toadfish in Sepetiba Bay, south-eastern Brazil. J Mar Biol Assoc UK. 2021. https://doi.org/10.1017/S0025315421000011.
Carvalho BM, Ferreira Junior AL, Fávaro LF, Artoni RF, Vitule J. Human-facilitated dispersal of the Gulf toadfish Opsanus beta (Goode & Bean, 1880) in the Guaratuba Bay, south-eastern Brazil. J Fish Biol. 2020;97(3):686–90. https://doi.org/10.1111/jfb.14421.
Agência Nacional de Vigilância Sanitária (ANVISA). Estudo exploratório para identificação e caracterização de espécies patogênicas em água de lastro em portos selecionados no Brasil. Relatório Técnico, Agência Nacional de Vigilância Sanitária, Ministério da Saúde, Brasília, DF. 2002
Bueno ML, Magalhães ALB, Andrade-Neto FR, Alves CBM, Rosa DM, Junqueira NT, Pessali TC, Pompeu PS, Zenni RD. Alien fish fauna of southeastern Brazil: species status, introduction pathways, distribution and impacts. Biol Invasions. 2021;23:3021–34. https://doi.org/10.1007/s10530-021-02564-x.
Martinho F, Cabral HN, Azeiteiro UM, Pardal MA. Estuarine nurseries for marine fish: connecting recruitment variability with sustainable fisheries management. Manag Environ Qual Int J. 2012;23(4):414–33.
Lana PC, Marone E, Lopes RM, Machado EC. The subtropical estuarine complex of Paranaguá Bay, Brazil. In: Seeliger U, Kjerfre B, editors. Coastal Marine Ecosystems of Latin American. Ecological Studies, vol. 144. Berlin, Heidelberg: Springer; 2001. https://doi.org/10.1007/978-3-662-04482-7_11.
Maack R. Geografia física do Estado do Paraná. 4ª ed. UEPG, 2017.
Bigarella JJ. A serra do mar e a porção oriental do Estado do Paraná Contribuição à geografia, geologia e ecologia regional. Curitiba: Secretaria do Estado do Planejamento—Associação de Defesa e Educação Ambiental (ADEA). 1978. p. 248
Nitsche PR, Caramori PH, Ricce WS, Pinto LFD. Atlas climático do estado do Paraná. Palotina: Instituto Agronômico do Paraná (IAPAR); 2019.
Fávaro LF, Oliveira EC. Ecologia reprodutiva em peixes, p. 321–339. In: Ribeiro CAO,Reis Filho HS,.Grotzner SR. (Org.) Técnicas e Métodos para a Utilização Prática de Microscopia. 1ed.São Paulo: SANTOS, 2012. pp. 422
Vazzoler AEAM. Biologia da reprodução de peixes teleósteos: teoria e prática. Maringá: EDUEM/Nupélia. 1996. pp. 169
Brown-Peterson NJ, Wyanski DM, Saborido-Rey F, Macewicz BJ, Lowerre-Barbieri SK. A standardized terminology for describing reproductive development in fishes Mar. Coast Fish. 2011;3:52–70. https://doi.org/10.1080/19425120.2011.555724.
Lowerre-Barbieri SK, Brown-Peterson NJ, Murua H, Tomkiewicz J, Wyanski DM, Saborido-Rey F. Emerging issues and methodological advances in fisheries reproductive biology. Mar Coast Fish. 2011;3(1):32–51. https://doi.org/10.1080/19425120.2011.555725.
Gomes ID, Araújo FG. Reproductive biology of two marine catfishes (Siluriformes, Ariidae) in the Sepetiba Bay. Brazil Rev biol trop. 2004;52(1):143–56.
Le Cren ED. The length-weight relationship and seasonal cycle in gonad weight and condition in the perch (Perca fluviatilis). J Anim Eco. 1951;20(2):201–19. https://doi.org/10.2307/1540.
Martin LK, Cailliet GM. Aspects of the reproduction of the Bat Ray, Myliobatis californica, in Central California. Copeia. 1988;3:754–62. https://doi.org/10.2307/1445398.
Silva CELS, Feitosa CV, Fernandes CAF. Fishing gear selectivity on sub-adults and spawning stock of the Tarpon Megalops atlanticus (Actinopterygii: Megalopidae) in Northeast Brazil. Reg Stud Mar Sci. 2021;44: 101727. https://doi.org/10.1016/j.rsma.2021.101727.
Freire JL, Marques CB, Silva BB. Estrutura populacional e biologia reprodutiva do camarão-da-amazônia Macrobrachium amazonicum (Heller 1862 (Decapoda:Palaemonidae) em um estuário da região nordeste do Pará. Brasil J Aquat Sci Technol. 2012;16(2):65–76.
Gray CA, Barnes LM. Reproduction and growth of dusky flathead (Platycephalus fuscus) in NSW estuaries. NSW department of primary industries—fisheries final report series, 2008; No. 101 ISSN 1449–9967
Fávaro LF, Lopes SCG, Spach HL. Reprodução do peixe-rei, Atherinella brasiliensis (Quoy & Gaimard) (Atheriniformes, Atherinidae), em uma planície de maré adjacente à gamboa do Baguaçu, Baía de Paranaguá, Paraná. Brasil Rev Bras Zool. 2003;20(3):501–6. https://doi.org/10.1590/S0101-81752003000300022.
Oliveira EC, Fávaro LF. Reproduction of the flatfish Achirus lineatus (Pleuronectiformes, Achiridae) in Paranaguá Bay-PR, a subtropical region in Brazil. Zoologia (Curitiba). 2010;27(4):523–32. https://doi.org/10.1590/S1984-46702010000400004.
Oliveira EC, Favaro LF. Reproductive biology of the flatfish Etropus crossotus (Pleuronectiformes: Paralichthyidae) in the Paranaguá Estuarine Complex, Paraná State, subtropical region of Brazil. Neotrop Ichthyol. 2011;9(4):795–805. https://doi.org/10.1590/S1679-62252011005000043.
Possamai B, Fávaro LF. Using mariculture as a breeding site: reproduction of Hypleurochilus fissicornis (Actinopterygii: Blenniidae). Sci Mar. 2015;79(3):335–43. https://doi.org/10.3989/scimar.04176.19B.
Breder CM. On the reproduction of Opsanus beta Goode and Bean. Zoologica. 1941;26:229–32.
Barimo JF, Serafy JE, Frezza PE, Walsh PJ. Habitat use, urea production and spawning in the gulf toadfish Opsanus beta. Mar Biol. 2007;150:497–508. https://doi.org/10.1007/s00227-006-0356-7.
Gray GA, Winn HE. Reproductive ecology and sound production of scarecrow toadfish Opsanus tau. Ecology. 1961;42(2):274–82. https://doi.org/10.2307/1932079.
Schwartz FJ. Movements of the oyster toadfish (Pisces: Batrachoididae) about Solomons Maryland. Ches Sci. 1974;15:155–9. https://doi.org/10.2307/1351033.
Wilson CA, Dean JM, Radtke R. Age, growth rate and feeding habits of the oyster toadfish, Opsanus tau (Linnaeus) in South Carolina. J Exp Mar Biol Ecol. 1982;62:251–9. https://doi.org/10.1016/0022-0981(82)90205-2.
Moyle PB, Marchetti MP. Predicting Invasion Success: Freshwater Fishes in California as a Model. Bioscience. 2006;56(6):515–24. https://doi.org/10.1641/0006-3568(2006)56[515:PISFFI]2.0.CO;2.
Blackburn TM, Pyšek P, Bacher S, Carlton JT, Duncan RP, Jarošík V, Richardson D. A proposed unified framework for biological invasions. Trends Ecol Evol. 2011;26(7):333–9. https://doi.org/10.1016/j.tree.2011.03.023.
Carvalho B, Pérez J, Aguilar-Perera A, Noh Quiñones V, Tomás A, Vitule J, Volpedo A. Inferring ecological connectivity between Opsanus beta (Goode & Bean, 1880) populations from the southern Gulf of Mexico and southwestern Atlantic Coast. J Mar Biol Assoc. 2022;102(8):597–603. https://doi.org/10.1017/S0025315422000935.
Beverton RJH, Holt SJ. A review of the lifespans and mortality rates of fish in nature, and their relation to growth and other physiological characteristics. In: Wolstenholme GEW, Oconnor M (eds). Ciba Foundation Colloquia on Ageing 1959; 5:142–180
Gertzen S, Fidler A, Kreische F, Kwabek L, Schwamborn V, Borcherding J. Reproductive strategies of three invasive Gobiidae co-occurring in the Lower Rhine (Germany). Limnologica. 2016;56:39–48. https://doi.org/10.1017/S0025315422000935.
Dias JF, Peres-Rio E, Chaves PTC, Rossi-Wongtschowski CLDB. Análise macroscópica dos ovários de teleósteos: problemas de classificação e recomendações de procedimentos. Rev Bras Biol. 1998;58(1):55–69.
Funding
This work was supported by the ‘Vice-chancellor’s funds for support of research activities’ of the Federal University of Paraná (PRPPG 04/2018 e PRPPG 02/2020). TSA and JKN were financed by and acknowledge the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) (Grant 02).
Author information
Authors and Affiliations
Contributions
All authors (TSA, JKN, AAE, JM, LFF) contributed to the conception and design of the study, preparation of the material, collection and analysis of data. Specifically, JKN was responsible for obtaining the fish and histology in the first stage of the study. TSA was responsible for obtaining the fish and histology in the second stage. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
44274_2024_34_MOESM1_ESM.docx
Supplementary file1 (Supplement 1 Complete model output for the analysis of GSI. We included sex, year of collection, the linear and quadratic effects of month and their two- and three-way interactions. Bold: p < 0.05. Supplement 2 Monthly distribution of Average Temperature (TºC) and Total Rainfall (mm) in the study area of the Paranaguá estuary, a subtropical region in southern Brazil referring to the 2011/2012 and 2019/2020 sample series. The values in bold represent the months with the lowest total precipitation values in the 2019/2020 period, when compared to the 2011/2012 period. Supplement 3 Seasonal distribution of the Average Temperature (TºC) and Total Rainfall (mm) in the study area of the Paranaguá estuary, subtropical region in southern Brazil referring to the 2011/2012 and 2019/2020 sample series. The values in bold represent the stations with the lowest total precipitation values in the 2019/2020 period, when compared to the 2011/2012 period.)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
de Assis, T.S., Nagata, J.K., Evans, A.A. et al. Influence of environmental factors on reproduction of the invasive Gulf toadfish Opsanus beta (Teleostei: Batrachoididae) in a subtropical estuary. Discov Environ 2, 7 (2024). https://doi.org/10.1007/s44274-024-00034-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s44274-024-00034-4