Abstract
Background
Several association studies have shown that -844 G/A and HindIII C/G PAI-1 polymorphisms are related with increase of PAI-1 levels, obesity, insulin resistance, glucose intolerance, hypertension and dyslipidemia, which are components of metabolic syndrome. The aim of this study was to analyze the allele and genotype frequencies of these polymorphisms in PAI-1 gene and its association with metabolic syndrome and its components in a sample of Mexican mestizo children.
Methods
This study included 100 children with an age range between 6-11 years divided in two groups: a) 48 children diagnosed with metabolic syndrome and b) 52 children metabolically healthy without any clinical and biochemical alteration. Metabolic syndrome was defined as the presence of three or more of the following criteria: fasting glucose levels ≥ 100 mg/dL, triglycerides ≥ 150 mg/dL, HDL-cholesterol < 40 mg/dL, obesity BMI ≥ 95th percentile, systolic blood pressure (SBP) and diastolic blood pressure (DBP) ≥ 95th percentile and insulin resistance HOMA-IR ≥ 2.4. The -844 G/A and HindIII C/G PAI-1 polymorphisms were analyzed by PCR-RFLP.
Results
For the -844 G/A polymorphism, the G/A genotype (OR = 2.79; 95% CI, 1.11-7.08; p = 0.015) and the A allele (OR = 2.2; 95% CI, 1.10-4.43; p = 0.015) were associated with metabolic syndrome. The -844 G/A and A/A genotypes were associated with increase in plasma triglycerides levels (OR = 2.6; 95% CI, 1.16 to 6.04; p = 0.02), decrease in plasma HDL-cholesterol levels (OR = 2.4; 95% CI, 1.06 to 5.42; p = 0.03) and obesity (OR = 2.6; 95% CI, 1.17-5.92; p = 0.01). The C/G and G/G genotypes of the HindIII C/G polymorphism contributed to a significant increase in plasma total cholesterol levels (179 vs. 165 mg/dL; p = 0.02) in comparison with C/C genotype.
Conclusions
The -844 G/A PAI-1 polymorphism is related with the risk of developing metabolic syndrome, obesity and atherogenic dyslipidemia, and the HindIII C/G PAI-1 polymorphism was associated with the increase of total cholesterol levels in Mexican children.
Similar content being viewed by others
Background
Metabolic syndrome (MetS) is a common disorder caused by a combination of poor diet, sedentary lifestyle and genetic predisposition [1], the presence of MetS in children is the main risk factor that predisposes to the development of cardiovascular and metabolic diseases such as atherosclerosis and type 2 diabetes mellitus in adulthood [2]. The components of MetS include obesity, insulin resistance, hyperglycemia, atherogenic dyslipidemia, and hypertension [1, 3, 4]. Besides these components, a decrease in fibrinolytic capacity has been shown to contribute to the development of this syndrome, which has been generally attributed to increased levels of plasminogen activator inhibitor-1 (PAI-1) [5, 6].
PAI-1 is the main inhibitor in the plasminogen activation system (PAS), which comprises an inactive proenzyme, plasminogen, which can be converted into its active form plasmin by the action of physiological plasminogen activators (PAs). PAs degrades fibrin into soluble products, being PAI-1 one of the main regulators of fibrinolysis [7]. Increased PAI-1 levels in plasma are associated with the development of myocardial infarction and the formation/progression of chronic inflammatory diseases such as atherosclerosis and cardiovascular disease [8, 9]. Increased of PAI-1 levels also have been linked with risk factors such as obesity, insulin resistance, glucose intolerance, hypertension and dyslipidemia (low HDL plasma levels and hypertriglyceridemia), which together are components of metabolic syndrome [10–13].
The human PAI-1 gene is ~ 12.2 kb long, contains nine exons and 8 introns, and is located on chromosome 7q22. To date about 180 single nucleotide polymorphisms (SNP) in the PAI-1 gene have been described [14, 15]. Association studies have shown that polymorphisms located in the promoter region of PAI-1 gene shows a relationship with the concentrations of lipids (low HDL) in Mexico-American population [16, 17]. One of the polymorphisms in the promoter PAI-1 gene is the -844 G/A polymorphism, which has been associated with risk factors such as increased plasma levels of PAI-1, glucose, insulin resistance, triglycerides and low HDL, as well as with several diseases including deep vein thrombosis, coronary artery disease, rheumatoid arthritis and systemic lupus erithematosus [18–21]. Other SNP that is interesting is the HindIII C/G polymorphism located in the 3' untranslated region (UTR) of PAI-1 gene, which has been related to high levels of cholesterol and insulin in myocardial infarction patients [22].
Based on this knowledge, both PAI-1 polymorphisms are good candidates that might contribute to the pathological features associated to the MetS. Therefore, we designed this study to analyze allele and genotype frequencies of -844 G/A and HindIII C/G PAI-1 polymorphisms and its association with MetS and its components in a sample of Mexican Mestizo children.
Methods
Patients and healthy subjects
All children enrolled in the study were of Mexican Mestizo population born in the State of Guerrero, Mexico, with a family history of ancestors, at least back to the third generation born in our State. This cross-sectional study was carried out between June and December 2008. Participants were recruited of three schools in the urban area from Chilpancingo, Guerrero, Mexico. The total group included 100 children with age range 6-11 years, divided in two groups: a) 48 children diagnosed with MetS and b) 52 children metabolically healthy without any clinical or biochemical alteration. The children with one or two clinic or metabolic alterations were excluded.
Informed written consent was obtained from all parents or guardians before enrollment of children in the study. Approval for the study was obtained from the Research Ethics Committee of the University of Guerrero according to the ethical guidelines of 2008 Declaration of Helsinki.
Clinical and anthropometric measurements
Body weight was determined using a Tanita body composition monitor (Tanita BC-553, Arlington, USA) and height was measured to the nearest 0.1 cm using a stadiometer (Seca, Hamburg, Germany). From these measurements, body mass index was calculated (BMI = weight/height2, kg/m2). The circumferences were measured by duplicate using a diameter tape accurate to within ± 0.1 cm (Seca 201, Hamburg, Germany). Waist circumference was measured at the level of the umbilicus and the superior iliac crest. The measurement was made at the end of a normal expiration while the subjects stood upright, with feet together and arms hanging freely at the sides. Hip circumference was measured at the maximum point below the waist, without compressing the skin. The waist-to-hip ratio was calculated as waist/hip. The thickness of four skinfolds was measured to the nearest 0.1 mm, in duplicate, using skinfold caliper (Dynatronics Co, Salt Lake City, USA): triceps, biceps, subscapular and suprailiac. The duplicate measures were averaged.
Blood pressure (BP) was measured on the right arm of children seated and a rest for at least 5 min. Two consecutive measures were obtained at 1-min interval with an aneroid sphygmomanometer (Riester CE 0124, Jungingen, Germany). Hypertension was defined as the average of the two measurements where the systolic BP (SBP) or diastolic BP (DBP) is ≥ 95th percentile for age and gender was determined [23]. The classification of obesity was made using the 2000 Center for Disease Control and Prevention growth charts defined as normal weight 5th-85th percentiles and obesity ≥ 95th percentile [24].
Biochemical measurements and definitions
A blood sample was obtained from each child from antecubital venipuncture after overnight fast. Total serum cholesterol, triglycerides, HDL-cholesterol, LDL-cholesterol and glucose levels were obtained using a semi-automated equipment (COBAS MIRA), insulin levels were determined by immunoenzymatic assay (GenWay INS-EASIA kit).
The homeostasis model assessment of insulin resistance (HOMA-IR score) was used to determine insulin resistance in children; this score was calculated with the following formula: fasting serum insulin (μU/mL) × fasting plasma glucose (mmol/L)/22.5 taking scores ≥ 75th percentile (HOMA-IR ≥ 2.4) as the presence of insulin resistance.
We employed International Diabetes Federation proposal for metabolic syndrome definition in children aged 10-16 years old for blood glucose and lipid levels [25]. In this study, MetS was defined as the presence of three or more of the following criteria: fasting glucose levels ≥ 100 mg/dL, triglycerides ≥ 150 mg/dL, HDL-cholesterol < 40 mg/dL, obesity BMI ≥ 95th percentile, SBP and DBP ≥ 95th percentile and insulin resistance HOMA-IR ≥ 2.4.
Genotyping of -844 G/A and HindIII C/G PAI-1polymorphism
The genomic DNA (gDNA) was isolated from peripheral blood leukocyte according to the salting out method [26]. The -844 G/A and HindIII C/G PAI-1 single nucleotide polymorphisms were analyzed by polymerase chain reaction-restriction fragment length polymorphism (PCR-RFLP). Amplification of -844 PAI-1 promoter region was done in a thermal cycler (Techne, TC-312, Cambridge, UK) using the following oligonucleotides: 5'CAGGCTCCCACTGATTCTAC3' (Forward) and 5'GAGGGCTCTCTTGTGTCAAC3' (Reverse) [27]. PCR was carried out in a final volume of 20 μL containing 1 μg of gDNA, 20 μM of each oligonucleotide, 1.25 U/μL Taq DNA polymerase, supplied buffer enzyme 1×, MgCl2 2.5 mM, and 2.5 mM of each deoxynucleotide triphosphate (dNTP) (Invitrogen Life Technologies, Carlsbad, Ca). PCR reaction was performed by initial denaturation at 94°C for 3 min, 30 cycles of amplification at 94°C for 30 seconds for denaturation, 60°C for 30 seconds for annealing, and 72°C for 30 seconds for extension. Finally, 72°C for 1 min was used for ending extension, resulting in a 510 base pair amplified fragment analyzed on a 6% polyacrylamide gel stained with silver nitrate. Amplified fragments of -844 PAI-1 polymorphism were digested for 1 hour at 37°C with 3 U of XhoI (New England Biolabs, Beverly, Mass.) restriction enzyme. Afterward, restriction fragments were analyzed by electrophoresis on a 6% polyacrylamide gel stained with silver nitrate. The G/G wild-type genotype was digested and appeared as 364 and 146 bp fragments, whereas the A/A polymorphic genotype (absence of the XhoI site) migrated as a 510 bp fragment.
The HindIII polymorphism was detected using the following oligonucleotides: 5'GCCTCCAGCTACCGTTATTGTACA3' (Forward) and 5'CAGCCTAAACAACAGAGACCCC3' (Reverse) [28]. PCR was carried out in a final volume of 20 μL containing 1 μg of gDNA, 3 μM of each oligonucleotide, 1.25 U/μL Taq DNA polymerase, supplied buffer enzyme 1×, MgCl2 1.5 mM, and 2.5 mM of each dNTP (Invitrogen Life Technologies). PCR reaction was performed by initial denaturation at 94°C for 3 min, 30 cycles of amplification at 94°C for 30 seconds for denaturation, 60°C for 30 seconds for annealing, and 72°C for 30 seconds for extension. Finally, 72°C for 1 min was used for ending extension, resulting in a 755 bp amplified fragment analyzed on a 6% polyacrylamide gel stained with silver nitrate. Amplified fragments of Hind III PAI-1 polymorphism were digested for 1 hour at 37°C with 5 U of HindIII (New England Biolabs) restriction enzyme. Afterward, restriction fragments were analyzed by electrophoresis on 6% polyacrylamide gel stained with silver nitrate. The C/C wild-type genotype was digested and appeared as 567 and 188 bp fragments, whereas the G/G polymorphic genotype (absence of HindIII site) migrated as a 755 bp fragment. To confirm the results, genotyping of both polymorphisms were done in duplicate in all cases and were randomly selected only a few -844 and HindIII PAI-1 genotypes for sequencing.
Statistical analysis
Statistical analysis was performed using the statistical software STATA v 9.2. For the descriptive analysis, nominal variables were expressed as frequencies, continuous variables normally distributed as mean and standard deviation, and those not normally distributed were expressed as medians and percentile 5 and 95. We determined genotype and allele frequencies for the polymorphisms -844 and HindIII PAI-1 gene by direct counting, we performed chi-square test to compare proportions between groups (MetS vs. metabolically healthy) and to evaluate the Hardy-Weinberg equilibrium. The linkage disequilibrium between both SNPs was determined as D'.
The significance of the differences between the biochemical and anthropometric parameters by genotypes (G/G vs. GA + AA -844 and C/C vs. C/G + G/G HindIII PAI-1) was determined using student t test and by Mann Whitney. To evaluate the effect of polymorphism we used models of linear and logistic regression adjusted by gender and age. Differences were considered statistically significant at p < 0.05.
Results
The present study included 100 Mexican Mestizo children of both genders, aged 6 to 11 years. Children were classified into two groups made up of 52 children metabolically healthy and 48 children with MetS, according the criteria mentioned above. The prevalence of components de MetS in the cases group was: 33.04% for obesity + hyperglycemia + high triglycerides, 29.79% for obesity + hyperglycemia + high triglycerides + low HDL-cholesterol, 14.89% for obesity + hyperglycemia + low HDL-cholesterol and others combinations with minor prevalence (data no shown).
In this study, both polymorphisms evaluated were in Hardy-Weinberg equilibrium (χ 2 = 0.005; p = 1.0 for -844 G/A polymorphism, and χ 2 = 0.62; p = 0.66 for HindIII C/G polymorphism). The linkage disequilibrium (D') between both SNPs was 0.81. The distribution of allele and genotype frequencies between the two groups did not show significant differences for HindIII C/G polymorphism, but for the -844 G/A polymorphism we observed a significant differences in genotype (p = 0.034) and allele frequencies (p = 0.015) between the two groups, with an OR of 2.79 (95% CI, 1.11 to 7.08) for the G/A genotype and an OR of 2.2 (95% CI, 1.10 to 4.43) for A allele, which indicates that children who were carriers of the risk A allele, have 2.2 fold more susceptibility to present MetS, and in children carrying the G/A genotype the risk increases to 2.79 fold (Table 1).
Demographic, clinical and biochemical variables were compared by gender in all children. Only was observed a difference, the boys had higher fasting glucose levels than girls (median, 97 vs. 93 mg/dL; p = 0.04) (Table 2).
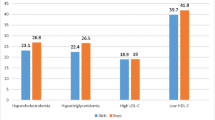
Demographic, clinical and biochemical variables were compared by genotypes of both PAI-1 polymorphisms according to a dominant genetic model. For the -844 G/A polymorphism the G/A and A/A genotypes were grouped for this genetic model, the -844 G/A + A/A group showed a high prevalence of obesity (60%; p = 0.01), an increase in thickness of the biceps (16 mm; p = 0.05), triceps (16 mm; p = 0.01) and subscapular skinfolds (15 mm; p = 0.03) and arm circumference (22 cm; p = 0.04), as well as decrease in HDL levels (39 mg/dL; p = 0.04) in comparison with G/G group (Table 3). To estimate in all children, the association of -844 G/A + A/A genotypes with demographic, clinical and biochemical variables that showed significant differences or tendencies, we used logistic regression models adjusted by age and gender. The -844 G/A + A/A genotypes were associated with increase in plasma triglycerides levels (OR = 2.6; 95% CI, 1.16 to 6.04; p = 0.02), decrease in plasma HDL-cholesterol levels (OR = 2.4; 95% CI, 1.06 to 5.42; p = 0.03) and obesity (OR = 2.6; 95% CI, 1.17-5.92; p = 0.01) (Table 4). However, we did not find a relationship with biceps, triceps and subscapular skinfolds as well as arm circumference (data no shown).
For the HindIII C/G polymorphism, when the C/G and G/G genotypes were grouped, the C/G + G/G group showed only an increase in plasma total cholesterol levels (179 mg/dL; p = 0.02) in comparison with C/C group (165 mg/dL).
Discussion
This study shows the association of two polymorphisms in the PAI-1 gene with the development of MetS and its components such as obesity and atherogenic dyslipidemia in a Mexican children population.
Regarding the distribution of genotype and allele frequencies of both polymorphisms, for the HindIII C/G polymorphism we found a high frequency of C allele, similar to previous reports in Mexican mestizo population and Caucasian population, however the G allele frequency was lower in Mexican population [18, 21, 29]. On the other hand, the -844 G/A polymorphism we observed that is distributed inversely to those reported in Caucasian populations, in which the A allele is more frequent than the G allele [12, 18, 19]. In our study this polymorphism had a high frequency of G allele and a lower frequency of A allele, consistent with already reported frequencies in previous studies in Mexican mestizo population [20, 30], suggesting that in the Mexican population there is a high frequency of allele G.
According to our results, the differences observed in the distribution of -844 G/A polymorphism may be attributed to the racial influence, which is central to the heterogeneous distribution of genetic polymorphisms. It is known that the Mexican population originated from a mixture of European (4.2 to 70.8%) and African (0.9 to 40.5%) populations with Amerindian groups (27.6 to 94.5%), giving origin to the Mexican mestizo population, which has a higher genetic diversity in the distribution of this and other polymorphisms [31]. This can explain the differences in the distribution of genotypic and allelic frequencies of our population with other populations in the world.
As an important finding, in our study we found significant differences in the distribution of genotype and allele frequencies of -844 G/A polymorphism in both groups, determining an OR of 2.2 for A allele, and an OR of 2.79 for G/A genotype, which indicates that children who carry the A allele are 2.2 fold more susceptible to develop MetS and children who are carriers of the G/A genotype have a 2.79 fold increased risk of developing the syndrome, compared to those who are carriers of G allele and G/G genotype. These results obtained in our study are similar to those reported in a previous study done in Caucasian population in which A/A genotype was associated with the susceptibility of developing MetS (OR, 4.87; p < 0.001) [12]. These consistent results reported in different populations may be due to the effect of the polymorphism on the levels of the protein, since it has been reported that the base change of G to A at position -844 of the promoter PAI-1 gene generates a binding site consensus sequence for Ets nuclear protein, which could be involved in regulating gene expression and influencing the increase in PAI-1 plasma protein levels [32, 33]. While for the HindIII C/G polymorphism not significant differences were found in genotype and allele distribution, but it has been reported that the base change of C to G at the 3' UTR of PAI-1 gene might plays an important role in the disruption of the translational regulation process and cause changes in the translational levels of messenger ribonucleic acid (mRNA) in both physiological and pathological conditions, resulting in an increase in PAI-1 plasma protein levels [34].
We described for first time in Mexican children that -844 G/A polymorphism contribute to a significant increase in subcutaneous fat, increasing the risk of developing obesity (OR, 2.6; p = 0.01) in children who are carriers of the G/A and A/A genotypes. A possible explanation for this finding could be that the -844 G/A polymorphism contribute to the large amount of PAI-1 produced by adipose tissue expansion, as well as the increase of obesity. Studies of PAI-1 knockout mice have shown an effect of PAI-1 on weight gain and increased adipose cellularity associated with high-fat dieting [35]. Besides, studies in which the PAI-1 gene was disrupted in ob/ob mice show a reduction of adiposity in these mice. This suggests that PAI-1 gene can control fat mass, although the mechanism of action is not yet known, may be PAI-1 gene can control fat mass at least in part, by inducing the proliferation of adipocytes through the effect on the expression of genes such as tumour necrosis factor alpha (TNF-α) and transforming growth factor beta (TGF-β), leptin and insulin [36].
In addition the effect on the increase in adipose tissue, there was an association of G/A and A/A genotypes of -844 G/A polymorphism with increased triglyceride levels and decreased HDL-C levels, which indicates that those children who are carriers of these genotypes, have an increase in the risk to develop atherogenic dyslipidemia compared with genotype G/G. In the case of HindIII C/G polymorphism, C/G and G/G genotypes were associated with a raise of total cholesterol explaining 8% of the variability of their plasma concentration, influencing along with -844 G/A polymorphism to the development of atherogenic profile that characterizes the MetS. It has been reported that a very-low-density lipoprotein (VLDL)-responsive element in the PAI-1 promoter could be responsible for the effect of plasma lipids on PAI-1 expression [14]. Therefore, the increase in PAI-1 levels may contribute to the development of obesity and atherogenic dyslipidemia, and PAI-1 may be a causal link between obesity and cardiovascular disease.
The -844 G/A and HindIII C/G PAI-1 single nucleotide polymorphisms have not been associated with PAI-1 levels. Several adult studies showed that an increase in the level of PAI-1 was related to the genotype PAI-1 4 G/5 G polymorphism [37, 38]. However, in children some information is available on the influence of the 4 G/5 G polymorphism on PAI-1 levels or with others obesity-related phenotypes. In Children with obesity, Estelles et al. [39] observed no influence of the 4 G/5 G polymorphism on PAI-1 levels. Moreover, no influence of the PAI-1 4 G/5 G polymorphism on lipid and glucose metabolism parameters was observed in Turkish obese children [40, 41].
A limitation of this study is the small number of sample, even though is a sample with children that were recruited with precise selection criteria and the control group did not have any of the components included in the definition of MetS. In addition, few studies of genetic association of PAI-1 gene with MetS have been conducted in children. Other limitation of this study is that lack of replication, the replication of genetic associations in independent populations is essential to reduce the number of false-positive results and to further define the role of these variants in the susceptibility to complex disease as MetS.
Although our study found an association of -844 G/A polymorphism with the MetS and its components such as obesity and a atherogenic dyslipidemia characterized by hypertriglyceridemia and low HDL-cholesterol, and the HindIII C/G polymorphism with increased plasma levels of total cholesterol, other of the limitations is that PAI-1 plasma levels were not measured; therefore the association of -844 G/A and HindIII C/G polymorphisms with PAI-1 levels remains uncertain in our population. Therefore it is necessary to determine PAI-1 plasma levels in future studies in Mexican children.
Conclusions
In summary, this study provide evidence that the -844 G/A PAI-1 polymorphism is related with the risk of developing MetS, obesity and atherogenic dyslipidemia, and the HindIII C/G PAI-1 polymorphism is associated with increased total cholesterol levels, which contributes to the pathogenesis of MetS.
Abbreviations
- MetS:
-
metabolic syndrome
- PAI-1:
-
plasminogen activator inhibitor-1
- PAS:
-
plasminogen activation system
- Pas:
-
physiological plasminogen activators
- HDL:
-
high density lipoprotein
- 3'UTR:
-
3'untranslated region
- BP:
-
blood pressure
- SBP:
-
systolic blood pressure
- DBP:
-
diastolic blood pressure
- LDL:
-
low density lipoprotein
- HOMA-IR:
-
homeostasis model assessment of insulin resistance
- gDNA:
-
genomic DNA
- PCR-RFLP:
-
polymerase chain reaction-restriction fragment length polymorphism
References
Huang PL: A comprehensive definition for metabolic syndrome. Dis Model Mech. 2009, 2: 231-237. 10.1242/dmm.001180.
Taslim S, Tai ES: The relevance of the metabolic syndrome. Ann Acad Med Singap. 2009, 38: 29-25.
Alberti KGMM, Zimmet P, Shaw J: Metabolic syndrome--a new world-wide definition. A Consensus Statement from the International Diabetes Federation. Diabet Med. 2006, 23: 469-480.
Bruce KD, Byrne CD: The metabolic syndrome: common origins of a multifactorial disorder. Postgrad Med J. 2009, 85: 614-621. 10.1136/pgmj.2008.078014.
Hamsten A, de Faire U, Walldius G, Dahlén G, Szamosi A, Landou C, Blombäck M, Wiman B: Plasminogen activator inhibitor in plasma: risk factor for recurrent myocardial infarction. Lancet. 1987, 2: 3-9.
Juhan-Vague I, Thompson SG, Jespersen J: Involvement of the hemostatic system in the insulin resistance syndrome. A study of 1500 patients with angina pectoris. The ECAT Angina Pectoris Study Group. Arterioscler Thromb. 1993, 13: 1865-1873. 10.1161/01.ATV.13.12.1865.
Aso Y: Plasminogen activator inhibitor (PAI)-1 in vascular inflammation and thrombosis. Front Biosci. 2007, 12: 2957-2966. 10.2741/2285.
Schneiderman J, Sawdey MS, Keeton MR, Bordin GM, Bernstein EF, Dilley RB, Loskutoff DJ: Increased type 1 plasminogen activator inhibitor gene expression in atherosclerotic human arteries. Proc Natl Acad Sci USA. 1992, 89: 6998-7002. 10.1073/pnas.89.15.6998.
Sobel BE: Increased plasminogen activator inhibitor-1 and vasculopathy. A reconcilable paradox. Circulation. 1999, 99: 2496-2498.
Morange PE, Lijnen HR, Alessi MC, Kopp F, Collen D, Juhan-Vague I: Influence of PAI-1 on adipose tissue growth and metabolic parameters in a murine model of diet-induced obesity. Arterioscler Thromb Vasc Biol. 2000, 20: 1150-1154. 10.1161/01.ATV.20.4.1150.
Naran NH, Chetty N, Crowther NJ: The influence of metabolic syndrome components on plasma PAI-1 concentrations is modified by the PAI-1 4 G/5G genotype and ethnicity. Atherosclerosis. 2008, 196: 155-163. 10.1016/j.atherosclerosis.2007.03.024.
Bouchard L, Vohl MC, Lebel S, Hould FS, Marceau P, Bergeron J, Pérusse L, Mauriège P: Contribution of genetic and metabolic syndrome to omental adipose tissue PAI-1 gene mRNA and plasma levels in obesity. Obes Surg. 2010, 20: 492-499. 10.1007/s11695-010-0079-1.
Ha H, Oh EY, Lee HB: The role of plasminogen activator inhibitor 1 in renal and cardiovascular diseases. Nat Rev Nephrol. 2009, 5: 203-211. 10.1038/nrneph.2009.15.
Binder BR, Christ G, Gruber F, Grubic N, Hufnagl P, Krebs M, Mihaly J, Prager GW: Plasminogen activator inhibitor 1: physiological and pathophysiological roles. News Physiol Sci. 2002, 17: 56-61.
Ma Z, Paek D, Oh CK: Plasminogen activator inhibitor-1 and asthma: role in the pathogenesis and molecular regulation. Clin Exp Allergy. 2009, 39: 1136-1144. 10.1111/j.1365-2222.2009.03272.x.
Arya R, Blangero J, Williams K, Almasy L, Dyer TD, Leach RJ, O'Connell P, Stern MP, Duggirala R: Factors of insulin resistance syndrome-related phenotypes are linked to genetic locations on chromosomes 6 and 7 in nondiabetic mexican-americans. Diabetes. 2002, 51: 841-847. 10.2337/diabetes.51.3.841.
Duggirala R, Blangero J, Almasy L, Dyer TD, Williams KL, Leach RJ, O'Connell P, Stern MP: A major susceptibility locus influencing plasma triglyceride concentrations is located on chromosome 15q in Mexican Americans. Am J Hum Genet. 2000, 66: 1237-1245. 10.1086/302849.
Adamski MG, Turaj W, Slowik A, Wloch-Kopec D, Wolkow P, Szczudlik A: A-G-4 G haplotype of PAI-1 gene polymorphisms -844 G/A, HindIII G/C, and -675 4 G/5G is associated with increased risk of ischemic stroke caused by small vessel disease. Acta Neurol Scand. 2009, 120: 94-100. 10.1111/j.1600-0404.2008.01127.x.
Lopes C, Dina C, Durand E, Froguel P: PAI-1 polymorphisms modulate phenotypes associated with the metabolic syndrome in obese and diabetic Caucasian population. Diabetologia. 2003, 46: 1284-1290. 10.1007/s00125-003-1170-0.
Torres-Carrillo NM, Torres-Carrillo N, Vázquez-Del Mercado M, Delgado-Rizo V, Oregón-Romero E, Parra-Rojas I, Muñoz-Valle JF: The -844 G/A PAI-1 polymorphism is associated with mRNA expression in rheumatoid arthritis. Rheumatol Int. 2008, 28: 355-360. 10.1007/s00296-007-0453-z.
Padilla-Gutiérrez JR, Palafox-Sánchez CA, Valle Y, Orozco-Barocio G, Oregón-Romero E, Vázquez-Del Mercado M, Rangel-Villalobos H, Llamas-Covarrubias MA, Muñoz-Valle JF: Plasminogen activator inhibitor-1 polymorphisms (-844 G > A and HindIII C > G) in systemic lupus erythematosus: association with clinical variables. Clin Exp Med. 2011, 11: 11-17. 10.1007/s10238-010-0099-0.
Dawson S, Hamsten A, Wiman B, Henney A, Humphries S: Genetic variation at the plasminogen activator inhibitor-1 locus is associated with altered levels of plasma plasminogen activator inhibitor-1 activity. Arterioscler Thromb. 1991, 11: 183-190. 10.1161/01.ATV.11.1.183.
National High Blood Pressure Education Program Working Group on High Blood Pressure in Children and Adolescents: The fourth report on the diagnosis, evaluation, and treatment of high blood pressure in children and adolescents. Pediatrics. 2004, 114: 555-576.
Centers for Disease Control and Prevention, National Center for Health Statistics: Clinical Growth Charts. [http://www.cdc.gov/growthcharts/clinical_charts.htm]
Zimmet P, Alberti G, Kaufman F, Tajima N, Arslanian S, Wong G, Bennett P, Shaw J, Caprio S: International diabetes federation task force on epidemiology and prevention of diabetes: the metabolic syndrome in children and adolescents. Lancet. 2007, 369: 2059-2061. 10.1016/S0140-6736(07)60958-1.
Miller SA, Dykes DD, Polesky HF: A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Res. 1988, 16: 1215-10.1093/nar/16.3.1215.
Henry M, Chomiki N, Scarabin PY, Alessi MC, Peiretti F, Arveiler D, Ferrières J, Evans A, Amouyel P, Poirier O, Cambien F, Juhan-Vague I: Five frequent polymorphisms of the PAI-1 gene: lack of association between genotypes, PAI activity, and triglyceride levels in a healthy population. Arterioscler Thromb Vasc Biol. 1997, 17: 851-858. 10.1161/01.ATV.17.5.851.
Grenett HE, Khan N, Jiang W, Booyse FM: Identification of the Hind III polymorphic site in the PAI-1 gene: analysis of the PAI-1 Hind III polymorphism by PCR. Genet Test. 2000, 4: 65-68. 10.1089/109065700316507.
Benza RL, Grenett H, Li XN, Reeder VC, Brown SL, Go RC, Hanson KA, Perry GJ, Holman WL, McGiffin DC, Kirk KA, Booyse FM: Gene Polymorphisms for PAI-1 Are Associated with the Angiographic Extent of Coronary Artery Disease. J Thromb Thrombolysis. 1998, 5: 143-150. 10.1023/A:1008882113177.
Torres-Carrillo N, Magdalena Torres-Carrillo N, Vázquez-Del Mercado M, Rangel-Villalobos H, Parra-Rojas I, Sánchez-Enríquez S, Francisco Muñoz-Valle J: Distribution of -844 G/A and Hind III C/G PAI-1 polymorphisms and plasma PAI-1 levels in Mexican subjects: comparison of frequencies between populations. Clin Appl Thromb Hemost. 2008, 14: 220-226. 10.1177/1076029607304747.
Rubi-Castellanos R, Martínez-Cortés G, Muñoz-Valle JF, González-Martín A, Cerda-Flores RM, Anaya-Palafox M, Rangel-Villalobos H: Pre-Hispanic Mesoamerican demography approximates the present-day ancestry of Mestizos throughout the territory of Mexico. Am J Phys Anthropol. 2009, 139: 284-294. 10.1002/ajpa.20980.
Grubic N, Stegnar M, Peternel P, Kaider A, Binder BR: A novel G/A and the 4 G/5G polymorphism within the promoter of the plasminogen activator inhibitor-1 gene in patients with deep vein thrombosis. Thromb Res. 1996, 84: 431-443. 10.1016/S0049-3848(96)00211-3.
Henry M, Chomiki N, Scarabin PY, Alessi MC, Peiretti F, Arveiler D, Ferrières J, Evans A, Amouyel P, Poirier O, Cambien F, Juhan-Vague I: Five frequent polymorphisms of the PAI-1 gene: lack of association between genotypes, PAI activity, and triglyceride levels in a healthy population. Arterioscler Thromb Vasc Biol. 1997, 17: 851-858. 10.1161/01.ATV.17.5.851.
Torres-Carrillo N, Torres-Carrillo NM, Martínez-Bonilla GE, Vázquez-Del Mercado M, Palafox-Sánchez CA, Oregón-Romero E, Bernard-Medina AG, Rangel-Villalobos H, Muñoz-Valle JF: Plasminogen activator inhibitor-1 C/G polymorphism in relation to plasma levels in rheumatoid arthritis. Clin Exp Med. 2009, 9: 223-228. 10.1007/s10238-009-0038-0.
Morange PE, Lijnen HR, Alessi MC, Kopp F, Collen D, Juhan-Vague I: Influence of PAI-1 on adipose tissue growth and metabolic parameters in a murine model of diet-induced obesity. Arterioscler Thromb Vasc Biol. 2000, 20: 1150-1154. 10.1161/01.ATV.20.4.1150.
Schäfer K, Fujisawa K, Konstantinides S, Loskutoff DJ: Disruption of the plasminogen activator inhibitor 1 gene reduces the adiposity and improves the metabolic profile of genetically obese and diabetic ob/ob mice. FASEB J. 2001, 15: 1840-1842.
Hoffstedt J, Andersson LL, Persson L, Isaksson B, Arner P: The common -675 4 G/5G polymorphism in the plasminogen activator inhibitor-1 gene is strongly associated with obesity. Diabetologia. 2002, 45: 584-587. 10.1007/s00125-001-0774-5.
Sartori MT, Vettor R, De Pergola G, De Mitrio V, Saggiorato G, Della Mea PD, Patrassi GM, Lombardi AM, Fabris R, Girolami A: Role of the 4 G/5G polymorphism of PAI-1 gene promoter on PAI-1 levels in obese patients: influence of fat distribution and insulin-resistance. Thromb Haemost. 2001, 86: 1161-1169.
Estelles A, Dalmau J, Falco C, Berbel O, Castello R, Espana F, Aznar J: Plasma PAI-1 levels in obese children-effect of weight loss and influence of PAI-1 promoter 4 G/5G genotype. Thromb Haemost. 2001, 86: 647-652.
Kinik ST, Ataç FB, Verdi H, Cetintaş S, Sahin FI, Ozbek N: The effect of plasminogen activator inhibitor-1 gene 4 G/5G polymorphism on glucose and lipid metabolisms in Turkish obese children. Clin Endocrinol. 2005, 62: 607-610. 10.1111/j.1365-2265.2005.02268.x.
Kinik ST, Ozbek N, Yuce M, Yazici AC, Verdi H, Ataç FB: PAI-1 gene 4 G/5G polymorphism, cytokine levels and their relations with metabolic parameters in obese children. Thromb Haemost. 2008, 99: 352-356.
Pre-publication history
The pre-publication history for this paper can be accessed here:http://www.biomedcentral.com/1471-2431/12/41/prepub
Acknowledgements
This work was supported by grants from PROMEP-SEP (UAGRO-EXB-057) and FOMIX-CONACYT-Gobierno del Estado de Guerrero 2010-01 (No. 147778).
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
IPR conceived and organized the study and prepared the manuscript. UDCM performed the genotyping and participated in the statistical analysis, interpreted the data and wrote the manuscript. LSG and AGC were responsible for patient enrollment and participated in the genetic analysis. JFMV, BIA and ECS participated in the design of the study, contributed interpreting the data and revising successive drafts of the manuscript. All authors read and approved the final manuscript.
Rights and permissions
Open Access This article is published under license to BioMed Central Ltd. This is an Open Access article is distributed under the terms of the Creative Commons Attribution License ( https://creativecommons.org/licenses/by/2.0 ), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
De la Cruz-Mosso, U., Muñoz-Valle, J.F., Salgado-Goytia, L. et al. Relationship of metabolic syndrome and its components with -844 G/A and HindIII C/G PAI-1 gene polymorphisms in Mexican children. BMC Pediatr 12, 41 (2012). https://doi.org/10.1186/1471-2431-12-41
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1471-2431-12-41