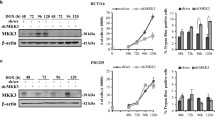
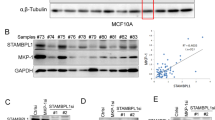
Abstract
MDM2 is an E3 ligase that promotes ubiquitin-mediated destruction of p53. Cellular stresses such as DNA damage can lead to p53 activation due in part to MDM2 destabilization. Here, we show that the stability of MDM2 is regulated by an ubiquitin-like NEDD8 pathway and identify NEDP1 as a chemotherapy-induced isopeptidase that deneddylates MDM2, resulting in MDM2 destabilization concomitant with p53 activation. Concordantly, RNAi-mediated knockdown of endogenous NEDP1 blocked diminution of MDM2 levels and increased chemoresistance of tumor cells. These findings unveil the regulation of MDM2 stability through NEDP1 as a common molecular determinant governing chemotherapy-induced p53-dependent cell death.




Similar content being viewed by others
References
Bode AM, Dong Z . (2004). Post-translational modification of p53 in tumorigenesis. Nat Rev Cancer 4: 793–805.
Chan Y, Yoon J, Wu JT, Kim HJ, Pan KT, Yim J et al. (2008). DEN1 deneddylates non-cullin proteins in vivo. J Cell Sci 121: 3218–3223.
Gan-Erdene T, Nagamalleswari K, Yin L, Wu K, Pan ZQ, Wilkinson KD . (2003). Identification and characterization of DEN1, a deneddylase of the ULP family. J Biol Chem 278: 28892–28900.
Hetfeld BK, Peth A, Sun XM, Henklein P, Cohen GM, Dubiel W . (2008). The COP9 signalosome-mediated deneddylation is stimulated by caspases during apoptosis. Apoptosis 13: 187–195.
Huang HY, West RB, Tzeng CC, van de Rijn M, Wang JW, Chou SC . (2008). Immunohistochemical and biogenetic features of diffuse-type tenosynovial giant cell tumors: the potential roles of cyclin A, P53, and deletion of 15q in sarcomatous transformation. Clin Cancer Res 14: 6023–6032.
Itahana K, Mao H, Jin A, Itahana Y, Clegg HV, Lindstrom MS et al. (2007). Targeted inactivation of Mdm2 RING finger E3 ubiquitin ligase activity in the mouse reveals mechanistic insights into p53 regulation. Cancer Cell 12: 355–366.
Jones J, Wu K, Yang Y, Guerrero C, Nillegoda N, Pan ZQ et al. (2008). A targeted proteomic analysis of the ubiquitin-like modifier nedd8 and associated proteins. J Proteome Res 7: 1274–1287.
Jones SN, Roe AE, Donehower LA, Bradley A . (1995). Rescue of embryonic lethality in Mdm2-deficient mice by absence of p53. Nature 378: 206–208.
Khosravi R, Maya R, Gottlieb T, Oren M, Shiloh Y, Shkedy D . (1999). Rapid ATM-dependent phosphorylation of MDM2 precedes p53 accumulation in response to DNA damage. Proc Natl Acad Sci USA 96: 14973–14977.
Koschny R, Holland H, Koschny T, Vitzthum HE . (2006). Comparative genomic hybridization pattern of non-anaplastic and anaplastic oligodendrogliomas—a meta-analysis. Pathol Res Pract 202: 23–30.
Lau L, Hansford LM, Cheng LS, Hang M, Baruchel S, Kaplan DR et al. (2007). Cyclooxygenase inhibitors modulate the p53/HDM2 pathway and enhance chemotherapy-induced apoptosis in neuroblastoma. Oncogene 26: 1920–1931.
Lee MH, Lee SW, Lee EJ, Choi SJ, Chung SS, Lee JI et al. (2006). SUMO-specific protease SUSP4 positively regulates p53 by promoting Mdm2 self-ubiquitination. Nat Cell Biol 8: 1424–1431.
Levav-Cohen Y, Haupt S, Haupt Y . (2005). Mdm2 in growth signaling and cancer. Growth Factors 23: 183–192.
Lowe SW . (1995). Cancer therapy and p53. Curr Opin Oncol 7: 547–553.
Mendoza HM, Shen LN, Botting C, Lewis A, Chen J, Ink B et al. (2003). NEDP1, a highly conserved cysteine protease that deNEDDylates Cullins. J Biol Chem 278: 25637–25643.
Meulmeester E, Maurice MM, Boutell C, Teunisse AF, Ovaa H, Abraham TE et al. (2005). Loss of HAUSP-mediated deubiquitination contributes to DNA damage-induced destabilization of Hdmx and Hdm2. Mol Cell 18: 565–576.
Montes de Oca Luna R, Wagner D, Lozano G . (1995). Rescue of early embryonic lethality in mdm2-deficient mice by deletion of p53. Nature 378: 203–206.
Morimoto M, Nishida T, Nagayama Y, Yasuda H . (2003). Nedd8-modification of Cul1 is promoted by Roc1 as a Nedd8-E3 ligase and regulates its stability. Biochem Biophys Res Commun 301: 392–398.
Pan ZQ, Kentsis A, Dias DC, Yamoah K, Wu K . (2004). Nedd8 on cullin: building an expressway to protein destruction. Oncogene 23: 1985–1997.
Paunu N, Lahermo P, Onkamo P, Ollikainen V, Rantala I, Helen P et al. (2002). A novel low-penetrance locus for familial glioma at 15q23-q26.3. Cancer Res 62: 3798–3802.
Rabut G, Peter M . (2008). Function and regulation of protein neddylation. ‘Protein modifications: beyond the usual suspects’ review series. EMBO Rep 9: 969–976.
Saville MK, Sparks A, Xirodimas DP, Wardrop J, Stevenson LF, Bourdon JC et al. (2004). Regulation of p53 by the ubiquitin-conjugating enzymes UbcH5B/C in vivo. J Biol Chem 279: 42169–42181.
Stommel JM, Wahl GM . (2004). Accelerated MDM2 auto-degradation induced by DNA-damage kinases is required for p53 activation. EMBO J 23: 1547–1556.
Suzuki H, Ouchida M, Yamamoto H, Yano M, Toyooka S, Aoe M et al. (2008). Decreased expression of the SIN3A gene, a candidate tumor suppressor located at the prevalent allelic loss region 15q23 in non-small cell lung cancer. Lung Cancer 59: 24–31.
Toledo F, Wahl GM . (2006). Regulating the p53 pathway: in vitro hypotheses, in vivo veritas. Nat Rev Cancer 6: 909–923.
Watson IR, Blanch A, Lin DC, Ohh M, Irwin MS . (2006). Mdm2-mediated NEDD8 modification of TAp73 regulates its transactivation function. J Biol Chem 281: 34096–34103.
Wu JT, Lin HC, Hu YC, Chien CT . (2005). Neddylation and deneddylation regulate Cul1 and Cul3 protein accumulation. Nat Cell Biol 7: 1014–1020.
Wu K, Yamoah K, Dolios G, Gan-Erdene T, Tan P, Chen A et al. (2003). DEN1 is a dual function protease capable of processing the C terminus of Nedd8 and deconjugating hyper-neddylated CUL1. J Biol Chem 278: 28882–28891.
Xirodimas DP . (2008). Novel substrates and functions for the ubiquitin-like molecule NEDD8. Biochem Soc Trans 36: 802–806.
Xirodimas DP, Saville MK, Bourdon JC, Hay RT, Lane DP . (2004). Mdm2-mediated NEDD8 conjugation of p53 inhibits its transcriptional activity. Cell 118: 83–97.
Xirodimas DP, Sundqvist A, Nakamura A, Shen L, Botting C, Hay RT . (2008). Ribosomal proteins are targets for the NEDD8 pathway. EMBO Rep 9: 280–286.
Yamoah K, Wu K, Pan ZQ . (2005). in vitro cleavage of Nedd8 from cullin 1 by COP9 signalosome and deneddylase 1. Methods Enzymol 398: 509–522.
Zhang XC, Chen J, Su CH, Yang HY, Lee MH . (2008). Roles for CSN5 in control of p53/MDM2 activities. J Cell Biochem 103: 1219–1230.
Zhou L, Watts FZ . (2005). Nep1, a Schizosaccharomyces pombe deneddylating enzyme. Biochem J 389: 307–314.
Acknowledgements
We thank Lynn Cheng, Joanne Lau, Roxana Sufan and Ryan Russell for their technical assistance. We thank Drs Uri Tabori and Loretta Lau for helpful discussions. We thank Drs Stephen Meyn and Paul Bradshaw for providing AT reagents. This work was supported by funds from the Canadian Cancer Society Research Institute (CCSRI 018460 to MO and 018054 to MSI). IRW is a recipient of the Hospital for Sick Children Foundation Student Scholarship and the Canadian Institutes of Health Research (CIHR) Canada Graduate Scholarship. OR is a recipient of the CIHR Fellowship. MO and MSI are Canada Research Chairs.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Supplementary Information accompanies the paper on the Oncogene website (http://www.nature.com/onc)
Rights and permissions
About this article
Cite this article
Watson, I., Li, B., Roche, O. et al. Chemotherapy induces NEDP1-mediated destabilization of MDM2. Oncogene 29, 297–304 (2010). https://doi.org/10.1038/onc.2009.314
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2009.314
- Springer Nature Limited
Keywords
This article is cited by
-
Neddylation of insulin receptor substrate acts as a bona fide regulator of insulin signaling and its implications for cancer cell migration
Cancer Gene Therapy (2024)
-
p53 regulation by ubiquitin and ubiquitin-like modifications
Genome Instability & Disease (2022)
-
The KBTBD6/7-DRD2 axis regulates pituitary adenoma sensitivity to dopamine agonist treatment
Acta Neuropathologica (2020)
-
Protein neddylation: beyond cullin–RING ligases
Nature Reviews Molecular Cell Biology (2015)
-
NEDDylation controls the target specificity of E2F1 and apoptosis induction
Oncogene (2013)