Abstract
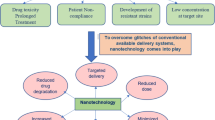
Tuberculosis (TB), caused by Mycobacterium tuberculosis, is one of the most devastating bacterial diseases to affect humans. M. tuberculosis is a robust pathogen that has evolved the capacity to survive and grow inside macrophage phagosomes. A cocktail of antibiotics has long been successfully used against M. tuberculosis but is becoming less effective owing to the emergence of multidrug resistance. The only available preventive vaccine, using Mycobacterium bovis bacille Calmette–Guérin, is considered to be ineffective against adult pulmonary TB, the most prevalent form of the disease. Here, we review the potential use of biodegradable nanoparticle-based anti-TB drug delivery systems that have been shown to be more effective against M. tuberculosis in animal models than conventional antibiotic treatment regimens. This technology also has substantial potential for vaccination and other therapeutic strategies against TB and other infectious diseases.



Similar content being viewed by others
References
Young, D., Perkins, M., Duncan, K. & Barry, C. E. 3rd. Confronting the scientific obstacles to global control of tuberculosis. J. Clin. Invest. 118, 1255–1265 (2008).
Dye, C. & Williams, B. G. The population dynamics and control of tuberculosis. Science 328, 856–861 (2010).
Russell, D. G., Barry, C. E. 3rd & Flynn, J. L. Tuberculosis: what we don't know can, and does, hurt us. Science 328, 852–856 (2010).
Chan, E. D. & Iseman, M. D. Multidrug-resistant and extensively drug-resistant tuberculosis: a review. Curr. Opin. Infect. Dis. 21, 587–595 (2008).
Zhang, Y. & Yew, W. W. Mechanisms of drug resistance in Mycobacterium tuberculosis. Int. J. Tuberc. Lung Dis. 13, 1320–1330 (2009).
Dye, C. Doomsday postponed? Preventing and reversing epidemics of drug-resistant tuberculosis. Nature Rev. Microbiol. 7, 81–87 (2009).
Bhatt, K. & Salgame, P. Host innate immune response to Mycobacterium tuberculosis. J. Clin. Immunol. 27, 347–362 (2007).
Vergne, I., Chua, J., Singh, S. B. & Deretic, V. Cell biology of Mycobacterium tuberculosis phagosome. Annu. Rev. Cell Dev. Biol. 20, 367–394 (2004).
Cooper, A. M. Cell-mediated immune responses in tuberculosis. Annu. Rev. Immunol. 27, 393–422 (2009).
Couvreur, P. & Vauthier, C. Nanotechnology: intelligent design to treat complex disease. Pharm. Res. 23, 1417–1450 (2006).
Zhang, L. et al. Nanoparticles in medicine: therapeutic applications and developments. Clin. Pharmacol. Ther. 83, 761–769 (2008).
Davis, M. E., Chen, Z. G. & Shin, D. M. Nanoparticle therapeutics: an emerging treatment modality for cancer. Nature Rev. Drug Discov. 7, 771–782 (2008).
Sosnik, A., Carcaboso, A. M., Glisoni, R. J., Moretton, M. A. & Chiappetta, D. A. New old challenges in tuberculosis: potentially effective nanotechnologies in drug delivery. Adv. Drug Deliv. Rev. 62, 547–559 (2010).
Khuller, G. K., Kapur, M. & Sharma, S. Liposome technology for drug delivery against mycobacterial infections. Curr. Pharm. Des 10, 3263–3274 (2004).
Pandey, R. & Khuller, G. K. Solid lipid particle-based inhalable sustained drug delivery system against experimental tuberculosis. Tuberculosis 85, 227–234 (2005).
Desjardins, M. & Griffiths, G. Phagocytosis: latex leads the way. Curr. Opin. Cell Biol. 15, 498–503 (2003).
Areschoug, T. & Gordon, S. Scavenger receptors: role in innate immunity and microbial pathogenesis. Cell. Microbiol. 11, 1160–1169 (2009).
Yoshida, A. et al. Selective delivery of rifampicin incorporated into poly(DL-lactic-co-glycolic) acid microspheres after phagocytotic uptake by alveolar macrophages, and the killing effect against intracellular Mycobacterium bovis Calmette–Guérin. Microbes Infect. 8, 2481–2491 (2006).
Hirota, K. et al. Delivery of rifampicin–PLGA microspheres into alveolar macrophages is promising for treatment of tuberculosis. J. Control. Release 142, 339–346 (2010).
Kisich, K. O. et al. Encapsulation of moxifloxacin within poly(butyl cyanoacrylate) nanoparticles enhances efficacy against intracellular Mycobacterium tuberculosis. Int. J. Pharm. 345, 154–162 (2007).
Onoshita, T. et al. The behavior of PLGA microspheres containing rifampicin in alveolar macrophages. Colloids Surf. B Biointerfaces 76, 151–157 (2010).
Pandey, R. & Khuller, G. K. Polymer based drug delivery systems for mycobacterial infections. Curr. Drug Deliv. 1, 195–201 (2004).
Shive, M. S. & Anderson, J. M. Biodegradation and biocompatibility of PLA and PLGA microspheres. Adv. Drug Deliv. Rev. 28, 5–24 (1997).
Anisimova, Y. V., Gelperina, S. I., Peloquin, C. A. & Heifets, L. B. Nanoparticles as antituberculosis drugs carriers: effect on activity against Mycobacterium tuberculosis in human monocyte-derived macrophages. J. Nanopart. Res. 2, 165–171 (2000).
Muttil, P. et al. Inhalable microparticles containing large payload of anti-tuberculosis drugs. Eur. J. Pharm. Sci. 32, 140–150 (2007).
Singh, R. & Lillard, J. W. Jr. Nanoparticle-based targeted drug delivery. Exp. Mol. Pathol. 86, 215–223 (2009).
Sharma, A., Sharma, S. & Khuller, G. K. Lectin-functionalized poly (lactide-co-glycolide) nanoparticles as oral/aerosolized antitubercular drug carriers for treatment of tuberculosis. J. Antimicrob. Chemother. 54, 761–766 (2004).
Pandey, R. et al. Poly (DL-lactide-co-glycolide) nanoparticle-based inhalable sustained drug delivery system for experimental tuberculosis. J. Antimicrob. Chemother. 52, 981–986 (2003).
Garcia-Contreras, L. et al. Inhaled large porous particles of capreomycin for treatment of tuberculosis in a guinea pig model. Antimicrob. Agents Chemother. 51, 2830–2836 (2007).
Ahmad, Z., Sharma, S. & Khuller, G. K. The potential of azole antifungals against latent/persistent tuberculosis. FEMS Microbiol. Lett. 258, 200–203 (2006).
Pandey, R. & Khuller, G. K. Nanoparticle-based oral drug delivery system for an injectable antibiotic – streptomycin. Evaluation in a murine tuberculosis model. Chemotherapy 53, 437–441 (2007).
Hussain, N., Jaitley, V. & Florence, A. T. Recent advances in the understanding of uptake of microparticulates across the gastrointestinal lymphatics. Adv. Drug Deliv. Rev. 50, 107–142 (2001).
Ahmad, Z. & Khuller, G. K. Alginate-based sustained release drug delivery systems for tuberculosis. Expert Opin. Drug Deliv. 5, 1323–1334 (2008).
Jain, R. A. The manufacturing techniques of various drug loaded biodegradable poly(lactide-co-glycolide) (PLGA) devices. Biomaterials 21, 2475–2490 (2000).
Gaumet, M., Gurny, R. & Delie, F. Localization and quantification of biodegradable particles in an intestinal cell model: the influence of particle size. Eur. J. Pharm. Sci. 36, 465–473 (2009).
Skeiky, Y. A. & Sadoff, J. C. Advances in tuberculosis vaccine strategies. Nature Rev. Microbiol. 4, 469–476 (2006).
Andersen, P. Vaccine strategies against latent tuberculosis infection. Trends Microbiol. 15, 7–13 (2007).
Fifis, T. et al. Size-dependent immunogenicity: therapeutic and protective properties of nano-vaccines against tumors. J. Immunol. 173, 3148–3154 (2004).
Xiang, S. D. et al. Pathogen recognition and development of particulate vaccines: does size matter? Methods 40, 1–9 (2006).
Hu, Y. et al. Cytosolic delivery of membrane-impermeable molecules in dendritic cells using pH-responsive core-shell nanoparticles. Nano Lett. 7, 3056–3064 (2007).
Verma, A. et al. Surface-structure-regulated cell-membrane penetration by monolayer-protected nanoparticles. Nature Mater. 7, 588–595 (2008).
Dhiman, N. & Khuller, G. K. Protective efficacy of mycobacterial 71-kDa cell wall associated protein using poly (DL-lactide-co-glycolide) microparticles as carrier vehicles. FEMS Immunol. Med. Microbiol. 21, 19–28 (1998).
Venkataprasad, N. et al. Induction of cellular immunity to a mycobacterial antigen adsorbed on lamellar particles of lactide polymers. Vaccine 17, 1814–1819 (1999).
Carpenter, Z. K., Williamson, E. D. & Eyles, J. E. Mucosal delivery of microparticle encapsulated ESAT-6 induces robust cell-mediated responses in the lung milieu. J. Control. Release 104, 67–77 (2005).
Kirby, D. J. et al. PLGA microspheres for the delivery of a novel subunit TB vaccine. J. Drug Target 16, 282–293 (2008).
Shi, S. & Hickey, A. J. PLGA microparticles in respirable sizes enhance an in vitro T cell response to recombinant Mycobacterium tuberculosis antigen TB10.4-Ag85B. Pharm. Res. 27, 350–360 (2010).
Lu, D. et al. Pulmonary immunization using antigen 85-B polymeric microparticles to boost tuberculosis immunity. AAPS J. 12, 338–347 (2010).
Muttil, P., Wang, C. & Hickey, A. J. Inhaled drug delivery for tuberculosis therapy. Pharm. Res. 26, 2401–2416 (2009).
Mollenkopf, H. J. et al. Enhanced protective efficacy of a tuberculosis DNA vaccine by adsorption onto cationic PLG microparticles. Vaccine 22, 2690–2695 (2004).
Johansen, P. et al. Anti-mycobacterial immunity induced by a single injection of M. leprae Hsp65-encoding plasmid DNA in biodegradable microparticles. Immunol. Lett. 90, 81–85 (2003).
Bivas-Benita, M. et al. Pulmonary delivery of chitosan-DNA nanoparticles enhances the immunogenicity of a DNA vaccine encoding HLA-A*0201-restricted T-cell epitopes of Mycobacterium tuberculosis. Vaccine 22, 1609–1615 (2004).
Bivas-Benita, M. et al. Pulmonary delivery of DNA encoding Mycobacterium tuberculosis latency antigen Rv1733c associated to PLGA–PEI nanoparticles enhances T cell responses in a DNA prime/protein boost vaccination regimen in mice. Vaccine 27, 4010–4017 (2009).
Akin, D. et al. Bacteria-mediated delivery of nanoparticles and cargo into cells. Nature Nanotech. 2, 441–449 (2007).
Cosma, C. L., Humbert, O., Sherman, D. R. & Ramakrishnan, L. Trafficking of superinfecting Mycobacterium organisms into established granulomas occurs in mammals and is independent of the Erp and ESX-1 mycobacterial virulence loci. J. Infect. Dis. 198, 1851–1855 (2008).
Garcia-Contreras, L. et al. Immunization by a bacterial aerosol. Proc. Natl Acad. Sci. USA 105, 4656–4660 (2008).
Ajdary, S. et al. Oral administration of BCG encapsulated in alginate microspheres induces strong TH1 response in BALB/c mice. Vaccine 25, 4595–4601 (2007).
Wong, Y. L. et al. Drying a tuberculosis vaccine without freezing. Proc. Natl Acad. Sci. USA 104, 2591–2595 (2007).
Sung, J. C., Pulliam, B. L. & Edwards, D. A. Nanoparticles for drug delivery to the lungs. Trends Biotechnol. 25, 563–570 (2007).
Singh, R. & Kostarelos, K. Designer adenoviruses for nanomedicine and nanodiagnostics. Trends Biotechnol. 27, 220–229 (2009).
Reddy, S. T., Swartz, M. A. & Hubbell, J. A. Targeting dendritic cells with biomaterials: developing the next generation of vaccines. Trends Immunol. 27, 573–579 (2006).
Liu, P. T. et al. Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science 311, 1770–1773 (2006).
Liu, P. T., Stenger, S., Tang, D. H. & Modlin, R. L. Cutting edge: vitamin D-mediated human antimicrobial activity against Mycobacterium tuberculosis is dependent on the induction of cathelicidin. J. Immunol. 179, 2060–2063 (2007).
Sharma, S., Verma, I. & Khuller, G. K. Antibacterial activity of human neutrophil peptide-1 against Mycobacterium tuberculosis H37Rv: in vitro and ex vivo study. Eur. Respir. J. 16, 112–117 (2000).
Toro, J. C. et al. Enhanced susceptibility of multidrug resistant strains of Mycobacterium tuberculosis to granulysin peptides correlates with a reduced fitness phenotype. Microbes Infect. 8, 1985–1993 (2006).
Bottger, E. C., Springer, B., Pletschette, M. & Sander, P. Fitness of antibiotic-resistant microorganisms and compensatory mutations. Nature Med. 4, 1343–1344 (1998).
Amaral, L., Martins, M. & Viveiros, M. Phenothiazines as anti-multi-drug resistant tubercular agents. Infect. Disord. Drug Targets 7, 257–265 (2007).
Kuijl, C. et al. Intracellular bacterial growth is controlled by a kinase network around PKB/AKT1. Nature 450, 725–730 (2007).
Lee, C. C., MacKay, J. A., Frechet, J. M. & Szoka, F. C. Designing dendrimers for biological applications. Nature Biotechnol. 23, 1517–1526 (2005).
Kumar, P. V., Agashe, H., Dutta, T. & Jain, N. K. PEGylated dendritic architecture for development of a prolonged drug delivery system for an antitubercular drug. Curr. Drug Deliv. 4, 11–19 (2007).
Wolf, A. J. et al. Initiation of the adaptive immune response to Mycobacterium tuberculosis depends on antigen production in the local lymph node, not the lungs. J. Exp. Med. 205, 105–115 (2008).
Acknowledgements
We thank A. Maleki, M. Gutierrez, A. Haas, B. Plikaytis and J. Posey for critically evaluating this Review, and J. Husley and the creative services of the CDC for help with figures.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary information S1 (table)
Some characteristic properties of polymers that can be used to produce biocompatible micro- and nanobeads. (PDF 883 kb)
Supplementary information S2 (table)
Salient features of antitubercular drug delivery systems (PDF 263 kb)
Related links
Rights and permissions
About this article
Cite this article
Griffiths, G., Nyström, B., Sable, S. et al. Nanobead-based interventions for the treatment and prevention of tuberculosis. Nat Rev Microbiol 8, 827–834 (2010). https://doi.org/10.1038/nrmicro2437
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrmicro2437
- Springer Nature Limited
This article is cited by
-
Targeting tuberculosis infection in macrophages using chitosan oligosaccharide nanoplexes
Journal of Nanoparticle Research (2019)
-
Striking the right immunological balance prevents progression of tuberculosis
Inflammation Research (2017)
-
The nanomaterial toolkit for neuroengineering
Nano Convergence (2016)
-
The path of anti-tuberculosis drugs: from blood to lesions to mycobacterial cells
Nature Reviews Microbiology (2014)
-
Nanosuspensions: a new approach for organ and cellular targeting in infectious diseases
Journal of Pharmaceutical Investigation (2013)