Abstract
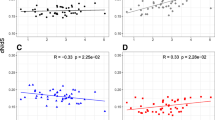
Assuming that new mutations arise mainly during DNA replication, sequence evolution in mammals has been seen as ‘male driven’ (ref. 1) because of the many more cell divisions in spermatogenesis than in oogenesis. Molecular support for this idea has been obtained from the observation of higher substitution rates in genes on the Y than on the X chromosome of primates and rodents2–4, which are species with male heterogamety, but has not been confirmed by the reciprocal analysis of organisms with female heterogamety. The recent suggestion that an intrinsic reduction in the X-chromosome mutation rate may be confounded with male effects in previous comparisons5, and the paradoxical finding of low levels of polymorphism on the primate Y chromosome6–8 indicate that the idea of male-biased mutation rate needs to be re-examined. We have analysed the molecular evolution of the gene CHD, which is present on the Z and W sex chromosomes of birds. The substitution rate at synonymous positions, as well as in intron DNA, was considerably higher on the Z chromosome than on the female-specific W chromosome, with an estimated male-to-female bias in mutation rate (αm) of 3.9–6.5. Thus, evolution appears to be male driven in birds—a situation that supports a neutral model of molecular evolution.
Similar content being viewed by others
References
Miyata, T., Hayashida, H., Kuma, K., Mitsuyasa, K. & Yasunaga, T. Male-driven molecular evolution: a model and nucleotide sequence analysis. Cold Spring Harbor Symp. Quant. Biol. 52, 863–867 (1987).
Shimmin, L.C., Chang, B.H.J. & Li, W.-H. Male-driven evolution of DNA sequences. Nature 362, 745–747 (1993).
Shimmin, L.C., Chang, B.H.J. & Li, W.-H. Contrasting rates of nucleotide substitution in the X-linked and Y-linked zinc finger genes. J. Mol. Evol. 39, 569–578 (1994).
Huang, W., Chang, B.H.J., Gu, X., Hewett-Emmett, D. & Li, W.-H. Sex differences in mutation rate in higher primates estimated from AMG intron sequences. J. Mol. Evol. 44, 463–465 (1994).
McVean, G.T. & Hurst, L.D. Evidence for a selectively favourable reduction in the mutation rate of the X-chromosome. Nature 386, 388–392 (1997).
Malaspina, P. et al. The human Y chromosome shows a low level of DNA polymorphism. Ann. Hum. Genet. 54, 297–305 (1990).
Dorit, R.L., Akashi, H. & Gilbert, W. Absence of polymorphism at the ZFY locus in the human Y chromosome. Science 268, 1183–1185 (1995).
Burrows, W. & Ryder, O.A. Y-chromosome variation in great apes. Nature 385, 125–126 (1997).
Ellegren, H. First gene on the avian W chromosome (CHD) provides a tag for universal sexing of non-ratite birds. Proc. R. Soc. Lond B Biol. Sci. 263, 1635–1641 (1996).
Griffiths, R., Daan, S. & Dijkstra, C. Sex identification in birds using two CHD genes. Proc. R. Soc. Lond. B Biol. Sci. 263, 1251–1256 (1996).
Delmas, V., Stokes, D. G. & Perry, R. P. A mammalian DNA-binding protein that contains a chromo-domain and an SNF2/SWI2-like helicase domain. Proc. Natl. Acad. Sci. USA 90, 2414–2418 (1994).
Stokes, D.G. & Perry, R.P. DNA-binding and chromatin localization properties of CHD1. Mol. Cell. Biol. 15, 2745–2753 (1995).
Li, W.-H. & Graur, D. Fundamentals of Molecular Evolution (Sinauer, Sunderland, Massachusetts, 1991).
Wolfe, K.H. & Sharp, P.M. Mammalian gene evolution: nucleotide sequence divergence between mouse and rat. J. Mol. Evol. 37, 441–456 (1993).
Chang, B.H.J., Shimmin, L.C., Shyue, S-K., Hewett-Emmett, D. & Li, W.-H. Weak male-driven molecular evolution in rodents. Proc. Natl. Acad. Sci. USA 91, 827–831 (1994).
Jones, R.C. & Lin, M. Spermatogenesis in birds. Oxf. Rev. Reprod. Biol. 15, 233–260 (1993).
Sibley, C.G. & Ahlquist, J.E. Phylogeny and Classification of Birds: A Study in Molecular Evolution (Yale University Press, New Haven, Connecticut, 1990).
Pamilo, P. & Bianchi, N.O. Evolution of the Zfx and Zfy genes: rates and interdependence between the genes. Mol. Biol. Evol. 10, 271–281 (1993).
Tajima, F. & Nei, M. Estimation of evolutionary distance between nucleotide sequences. Mol. Biol. Evol. 1, 269–285 (1984).
Kumar, S., Tamura, K. & Nei, M. MEGA: Molecular Evolutionary Genetic Analysis, version 1.0 (Pennsylvania State University, University Park, Pennsylvania, 1993).
Li, W.-H. A statistical test of phylogenies estimated from sequence data. Mol. Biol. Evol. 6, 424–435 (1989).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ellegren, H., Fridolfsson, AK. Male–driven evolution of DNA sequences in birds. Nat Genet 17, 182–184 (1997). https://doi.org/10.1038/ng1097-182
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/ng1097-182
- Springer Nature America, Inc.
This article is cited by
-
Evolution of the germline mutation rate across vertebrates
Nature (2023)
-
Multivariate selection and the making and breaking of mutational pleiotropy
Evolutionary Ecology (2022)
-
Pedigree-based and phylogenetic methods support surprising patterns of mutation rate and spectrum in the gray mouse lemur
Heredity (2021)
-
Origin of Sex-Biased Mental Disorders: An Evolutionary Perspective
Journal of Molecular Evolution (2021)
-
Postcopulatory sexual selection reduces Z-linked genetic variation and might contribute to the large Z effect in passerine birds
Heredity (2019)