Abstract
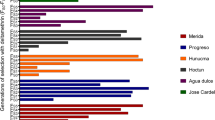
IN Culex pipiens, overproduction of nonspecific esterases is a common mechanism of resistance to organophosphate insecticides1,2. The esterases are attributed to closely linked loci named A and B according to substrate preference3–6, and over-production of all esterases B is due to gene amplification7,8. Distribution of electrophoretically distinct variants of overproduced esterases A and B is geographically restricted, with the exception of esterases A2 and B2, always found together throughout at least three continents (Fig. 1). To determine whether this situation is due to migration or to a high mutation rate, esterase B structural genes and their flanking regions were compared by sequence and/or restriction fragment length polymorphism analysis. Whereas structural genes were similar, flanking regions of electrophoretically dissimilar esterases B varied considerably. In contrast, flanking sequences of esterases B2 from different geographical locations (Africa, Asia, North America) were identical. These results suggest that amplified esterase B2 genes originated from an initial event that has subsequently spread organophosphate insecticide resistance by migration.
Similar content being viewed by others
References
Fournier, D. et al. Pest. Biochem. Physiol. 27, 211–217 (1987).
Mouchès, C. et al. Proc. natn. Acad. Sci. U.S.A 84, 2113–2116 (1987).
Callaghan, A. thesis. Univ. of London (1989).
Villani, F., White, G. B., Curtis, C. F. & Miles, S. J. Bull. ent. Res. 73, 153–170 (1983).
Pasteur, N., Iseki, A. & Georghiou, G. P. Biochem. Genet. 19, 909–919 (1981).
Wirth, M. C., Marquine, M., Georghiou, G. P. & Pasteur, N. J. med. Entomol. 27, 202–206 (1990).
Mouchès, C. et al. Science 233, 778–780 (1986).
Raymond, M. et al. Biochem. Genet. 27, 417–423 (1989).
Mouchès, C. et al. Proc. nantn. Acad. Sci. U.S.A. 87, 2574–2578 (1990).
de Stordeur, E. Biochem. Genet. 14, 481–493 (1976).
Curtis, C. F. & Pasteur, N. Bull. ent. Res. 71, 153–161 (1981).
Raymond, M. et al. J. med. Ent. 24, 24–27 (1987).
Magnin, M. thesis, Univ. Paris VI (1986).
Andreadis, T. G. J. Am. Mosq. Control Assoc. 4, 256–260 (1988).
Asahina, S. Jap. J. Med. Sci. Biol. 23, 255–258 (1970).
Highton, R. B. & Van Someren, E. C. C. Bull. WHO 42, 334–335 (1970).
Aquadro, C. F., Desse, S. F., Bland, M. M., Langley, C. H. & Laurie-Ahlberg, C. C. Genetics 114, 1165–1190 (1986).
Kreitman, M. & Aguadé. M. Proc. natn. Acad. Sci. U.S.A. 83, 3562–3566 (1986).
Aguadé, M. Genetics 119, 135–140 (1988).
Simmons, G. M., Kreitman, M., Quattlebaum, W. F. & Miyashita, N. Evolution 43, 393–409 (1989).
Pasteur, N. & Georghiou, G. P. J. Econ. Entomol. 82, 347–353 (1989).
Magnin, M., Marboutin, E. & Pasteur N. J. med. Ent. 25, 99–104 (1988).
Hemingway, J., Callaghan, A. & Amin, A. Med. Vet. Ent. 3, 445–446 (1989).
Beyssat-Arnaouty, V., Mouchès, C., Georghiou, G. P. & Pasteur, N. J. Am. Mosq. Control Assoc. 5, 196–200 (1989).
Urbanelli, S., Bullini, L. & Villani, F. Bull. ent. Res. 75, 291–304 (1985).
Pasteur, N., Sinègre, G. & Gabinaud, A. Biochem. Genet. 19, 499–508 (1981).
Villani, F. & Hemingway, J. J. Pest. Biochem. Physiol. 27, 218–228 (1987).
Sanger, F., Nicklen, S. & Coulson, A. R. Proc. natn. Acad. Sci. U.S.A. 74, 5463–5467 (1977).
Georghiou, G. P. & Pasteur, N. J. Econ. Ent. 73, 489–492 (1980).
Georghiou, G. P., Metcalf, R. L. & Glidden, F. E. Bull. WHO 35, 691–708 (1966).
Raymond, M. et al. C.r. Acad. Sci. Paris 300, 509–512 (1985).
Beyssat-Arnaouty, V. thesis, Univ. Montpellier II (1989).
Southern, E. M. J. molec. Biol. 98, 503 (1975).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Raymond, M., Callaghan, A., Fort, P. et al. Worldwide migration of amplified insecticide resistance genes in mosquitoes. Nature 350, 151–153 (1991). https://doi.org/10.1038/350151a0
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/350151a0
- Springer Nature Limited
This article is cited by
-
Population structure and insecticide response of Gammarus spp. in agricultural and upstream forested sites of small streams
Environmental Sciences Europe (2023)
-
Variation in Esterase Activity Among Different Aedes aegypti L. Populations from the Dooars and Terai Regions of West Bengal, India
Proceedings of the Zoological Society (2018)
-
Spatial variation of insecticide resistance in the dengue vector Aedes aegypti presents unique vector control challenges
Parasites & Vectors (2016)
-
Past and new challenges for malaria control and elimination: the role of operational research for innovation in designing interventions
Malaria Journal (2015)
-
Phenotypic effects of concomitant insensitive acetylcholinesterase (ace-1 R ) and knockdown resistance (kdr R ) in Anopheles gambiae: a hindrance for insecticide resistance management for malaria vector control
Parasites & Vectors (2014)