Abstract
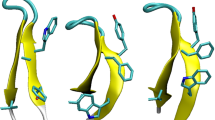
It is difficult to determine the structures of protein folding intermediates because folding is a highly cooperative process. A disulphide-bonded peptide pair, designed to mimic the first crucial intermediate in the folding of bovine pancreatic trypsin inhibitor, contains secondary and tertiary structure similar to that found in the native protein. Peptide models like this circumvent the problem of cooperativity and permit characterization of structures of folding intermediates.
Similar content being viewed by others
References
Roder, H., Elöve, G. A. & Englander, S. W. Nature 335, 700–704 (1988).
Udgaonkar, J. B. & Baldwin, R. L. Nature 335, 694–699 (1988).
Creighton, T. E. J. molec. Biol. 113, 275–293 (1977).
Creighton, T. E. & Goldenberg, D. P. J. molec. Biol. 179, 497–526 (1984).
Creighton, T. E. Prog. Biophys. molec. Biol. 33, 231–297 (1978).
Creighton, T. E. J. molec. Biol. 87, 603–624 (1974).
States, D. J., Creighton, T. E., Dobson, C. M. & Karplus, M. J. molec. Biol. 195, 731–739 (1987).
Wagner, G. & Wüthrich, K. J. molec. Biol. 155, 347–366 (1982).
States, D. J., Dobson, C. M., Karplus, M. & Creighton, T. E. J. molec. Biol. 174, 411–418 (1984).
Goldenberg, D. P. Biochemistry 27, 2481–2489 (1988).
Creighton, T. E. in Protein Folding (ed. Jaenicke, R.) 427–446 (Elsevier/North-Holland Biomedical, Amsterdam, 1980).
Creighton, T. E. J. molec. Biol. 113, 313–328 (1977).
Creighton, T. E. J. molec. Biol. 144, 521–550 (1980).
Creighton, T. E. J. molec. Biol. 87, 603–624 (1974).
Creighton, T. E., Kalef, E. & Arnon, R. J. molec. Biol. 123, 129–147 (1978).
Kosen, P. A., Creighton, T. E. & Blout, E. R. Biochemistry 22, 2433–2440 (1983).
Deisenhofer, J. & Steigemann, W. Acta crystallogr. B31, 238–250 (1975).
Wlodawer, A., Walter, J., Huber, R. & Sjölin, L. J. molec. Biol. 180, 301–329 (1984).
Shoemaker, K. R., Fairman, R., York, E. J., Stewart, J. M. & Baldwin, R. L. in Proc. 10th Am. Peptide Symp. (ed. Marshall, G. R.) 15–20 (ESCOM, Leiden, 1988).
Wüthrich, K. NMR of Proteins and Nucleic Acids (Wiley-Interscience, New York, 1986).
Bundi, A. & Wüthrich, K. Biopolymers 18, 285–297 (1979).
Creighton, T. E. J. molec. Biol. 96, 777–782 (1975).
Creighton, T. E. J. phys. Chem. 89, 2452–2459 (1985).
Goldenberg, D. P. & Creighton, T. E. Biopolymers 24, 167–182 (1985).
Marks, C. B., Naderi, H., Kosen, P. A., Kuntz, I. D. & Anderson, S. Science 235, 1370–1373 (1987).
Hollecker, M. & Creighton, T. E. J. molec. Biol. 168, 409–437 (1983).
Anfinsen, C. B., Haber, E., Sela, M. & White, F. H. Jr Proc. natn. Acad. Sci. U.S.A. 47, 1309–1314 (1961).
Saxena, V. P. & Wetlaufer, D. B. Biochemistry 9, 5015–5022 (1970).
Ptitsyn, O. B. & Rashin, A. A. Biophys. Chem. 3, 1–20 (1975).
Karplus, M. & Weaver, D. L. Nature 260, 404–406 (1976).
Richmond, T. J. & Richards, F. M. J. molec. Biol. 119, 537–555 (1978).
Kim, P. S. & Baldwin, R. L. A. Rev. Biochem. 51, 459–489 (1982).
Kim, P. S. & Baldwin, R. L. Nature 307, 329–334 (1984).
Dyson, H. J. et al. Nature 318, 480–483 (1985).
Shoemaker, K. R., Kim, P. S., York, E. J., Stewart, J. M. & Baldwin, R. L. Nature 326, 563–567 (1987).
Creighton, T. E. J. molec. Biol. 119, 507–518 (1978).
Rose, G. D. & Roy, S. Proc. natn. Acad. Sci. U.S.A. 77, 4643–4647 (1980).
Kuwajima, K. J. molec. Biol. 114, 241–258 (1977).
Ptitsyn, O. B. J. Protein Chem. 6, 273–293 (1987).
Hartman, R., Schwaner, R. C. & Hermans, J. J. molec. Biol. 90, 415–429 (1974).
Auer, H. E. & Patton, E. Biophys. Chem. 4, 15–21 (1976).
Jullien, M. & Baldwin, R. L. J. molec. Biol. 145, 265–280 (1981).
Rose, G. D. J. molec. Biol. 134, 447–470 (1979).
Go, M. & Nosaka, M. Cold Spring Harb. Symp. quant. Biol. 52, 915–924 (1987).
Zehfus, M. H. & Rose, G. D. Biochemistry 25, 5759–5765 (1986).
Fronticelli-Bucci, C. & Bucci, E. Biochemistry 14, 4451–4458 (1975).
Reutimann, H., Luisi, P. L. & Holmgren, A. Biopolymers 22, 107–111 (1983).
Mutter, M. in Proc. 10th Am. Peptide Symp. (ed. Marshall, G. R.) 349–353 (ESCOM, Leiden, 1988).
Lerner, R. A. Nature 299, 592–596 (1982).
Walter, G. & Doolittle, R. F. Genet. Eng. 5, 61–91 (1983).
Berzofsky, J. A. Science 229, 932–940 (1985).
Richardson, J. S. Meth. Enzym. 115, 359–380 (1985).
Lee, B. & Richards, F. M. J. molec. Biol. 55, 379–400 (1971).
Edelhoch, H. Biochemistry 6, 1948–1954 (1967).
DeMarco, A. J. magn. Reson. 26, 527–528 (1977).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Oas, T., Kim, P. A peptide model of a protein folding intermediate. Nature 336, 42–48 (1988). https://doi.org/10.1038/336042a0
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/336042a0
- Springer Nature Limited
This article is cited by
-
Thermodynamics of helix formation in small peptides of varying length in vacuo, in implicit solvent, and in explicit solvent
Journal of Molecular Modeling (2019)
-
Solvent effects on the structures and vibrational features of zwitterionic dipeptides: L-diglycine and L-dialanine
Journal of Molecular Modeling (2015)
-
Structure of a protein in a kinetic trap
Nature Structural & Molecular Biology (1995)
-
A kinetic explanation for the rearrangement pathway of BPTI folding
Nature Structural & Molecular Biology (1995)
-
A third native one-disulphide intermediate in the folding of bovine pancreatic trypsin inhibitor
Nature Structural Biology (1995)