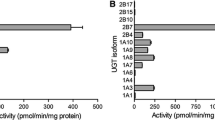
Abstract
Purpose. Nitrocatechol COMT inhibitors are a new class of bioactive compounds, for which glucuronidation is the most important metabolic pathway. The objective was to characterize the enzyme kinetics of nitrocatechol glucuronidation to improve the understanding and predicting of the pharmacokinetic behavior of this class of compounds.
Methods. The glucuronidation kinetics of seven nitrocatechols and 4-nitrophenol, the reference substrate for phenol UDP-glucuronosyltrans-ferase activity, was measured in liver microsomes from creosote-treated rats and determined by non-linear fitting of the experimental data to the Michaelis-Menten equation. A new method that combined densitometric and radioactivity measurement of the glucuronides separated by HPTLC was developed for the quantification.
Results. Apparent Km values for the nitrocatechols varied greatly depending on substitution pattern being comparable with 4-nitrophenol (0.11 mM) only in the case of 4-nitrocatechol (0.19 mM). Simple nitrocatechols showed two-fold Vmax values compared with 4-nitrophenol (68.6 nmol min−1 mg−1), while all disubstituted catechols exhibited much lower glucuronidation rate. Vmax/Km values were about 10 times higher for monosubstituted catechols compared to disubstituted ones. The kinetic parameters for COMT inhibitors were in the following order: Km nitecapone >> entacapone > tolcapone; Vmax nitecapone > entacapone > tolcapone; Vmax/Km tolcapone > nitecapone > entacapone.
Conclusions. Nitrocatechols can in principle be good substrates of UGTs. However, substituents may have a remarkable effect on the enzyme kinetic parameters. The different behaviour of nitecapone compared to the other COMT inhibitors may be due to its hydrophilic 5-substituent. The longer elimination half-life of tolcapone in vivo compared to entacapone could not be explained by glucuronidation kinetics in vitro.
Similar content being viewed by others
REFERENCES
T. Keränen, A. Gordin, M. Karlsson, K. Korpela, P. J. Pentikäinen, H. Rita, E. Schultz, L. Seppälä, and T. Wikberg. Eur. J. Clin. Pharmacol. 46:151-157 (1994).
S. Kaakkola, A. Gordin, M. Järvinen, T. Wikberg, E. Schultz, E. Nissinen, P. J. Pentikäinen, and H. Rita. Clin. Neuropharmacol. 13:436-447 (1990).
J. Dingemanse, K. Jorga, G. Zurcher, M. Schmitt, G. Sedek, M. Da Prada, and P. van Brummelen. Br. J. Clin. Pharmacol. 40:253-262 (1995).
T. Lotta, J. Taskinen, R. Bäckström, and E. Nissinen. J. Comput.-Aided Mol. Design, 6:235-272 (1992).
P.-C. Wang, N. T. Buu, O. Kuchel, and J. Genest. J. Lab. Clin. Med. 101:141-151 (1983).
T. Wikberg, A. Vuorela, P. Ottoila, and J. Taskinen. Drug Metab. Dispos. 21:81-92 (1993).
T. Wikberg and J. Taskinen. Drug Metab. Dispos. 21:325-333 (1993).
L. Nylund, P. Heikkilä, M. Hämeilä, L. Pyy, K. Linnainmaa, and M. Sorsa. Mutation Res. 265:223-236 (1992).
L. Luukkanen, E. Elovaara, P. Lautala, J. Taskinen, and H. Vainio. Pharmacol. Toxicol. 80:152-158 (1997).
O. Lowry, N. Rosebrough, L. Farr, and R. Randall. J. Biol. Chem. 193:265-275 (1951).
P. Lautala, H. Salomies, E. Elovaara, and J. Taskinen. J. Planar Chromatogr. 9:413-417 (1996).
D. J. Clarke and B. Burchell. The Uridine Diphosphate Glucuronosyl-transferase Multigene Family: Function and Regulation. In F. C. Kauffman (ed.), Conjugation-Deconjugation Reactions in Drug Metabolism and Toxicity, Springer-Verlag, Berlin Heidelberg, 1994, pp. 3-29.
H. Yin, G. Bennett, and J. P. Jones. Chem.-Biol. Interact. 90:47-58 (1994).
A. Temellini, M. Franchi, L. Giuliani, and G. M. Pacifici. Xenobiotica 21:171-177 (1991).
T. Wikberg, P. Ottoila, and J. Taskinen. Eur. J. Drug Metab. Pharmacokinet. 18:359-367 (1993).
J. Magdalou, Y. Hochman, and D. Zakim. J. Biol. Chem. 257:13624-13629 (1982).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lautala, P., Kivimaa, M., Salomies, H. et al. Glucuronidation of Entacapone, Nitecapone, Tolcapone, and Some Other Nitrocatechols by Rat Liver Microsomes. Pharm Res 14, 1444–1448 (1997). https://doi.org/10.1023/A:1012133008134
Issue Date:
DOI: https://doi.org/10.1023/A:1012133008134