Abstract
This paper presents a preliminary study of SiC production by use of natural gas for reduction of silica. Direct reduction of SiO2 by gas mixtures containing CH4, H2, and Ar was studied at temperatures between 1273 K and 1773 K (1000 °C and 1500 °C). Silica in form of particles between 1 and 3 mm and pellets with mean grain size 50 µm were exposed to the gas mixture for 6 hours. Influence of temperature and CH4\H2 ratio was investigated. Higher temperature and CH4 concentration resulted in greater SiC production. Two kinds of SiC were found: one was deposited between SiO2 particles, the other one was deposited inside the SiO2 particles. Although the exact reaction mechanisms have not been determined, it is clear that gas-phase reactions play an important role in both cases. The reaction products were analyzed by Electron Probe Micro Analyzer.
Similar content being viewed by others
Introduction
About 90 pct of solar cells are made from solar grade silicon (SoG-Si) with the maximum impurity level below 1 ppm.[1] Forecasts presented by the U.S. Energy Information Administration show that the photovoltaic market will be one of the main sources of renewable energy.[2] Increasing demand for high-purity silicon forces producers to minimize the cost of production and develop new methods for obtaining high-purity SoG-Si. One of the new production routes for SoG-Si may be a two-step process involving production of silicon carbide using natural gas and high-purity quartz. The second step would be conversion of SiC to SoG-Si, however, this approach will not be discussed in this paper. Natural gas is a mixture of methane (80 to 95 pct), higher order hydrocarbons (2 to 15 pct ethane), CO2, and N2.[3] From a solar cell perspective, it is positive that metallic impurities (total <1 ppmw) and phosphorus (P < 0.5ppmw) are low.[4] An estimated boron concentration has not been found, but it is known to be present in natural gas.[5] Based on these findings, most impurities in the final product would be expected to come from the quartz, not from the source of carbon. Methane as a reducing agent for different metal oxides was investigated by various authors. Ostrovski and Zhang[6] reduced iron, manganese, chromium, and titanium oxides by CH4-H2-Ar gas mixtures. They found that the reduction processes were faster and took place at lower temperatures than the corresponding reactions with pure carbon. The reaction rates were reported to increase with increasing methane content for fixed hydrogen content, but beyond a certain saturation level further increases in methane content had only a small effect on the reaction rate. The authors suggested the mechanism as a multistep reaction where methane is first adsorbed on the oxide and then dissociates through CH3, CH2, and CH to adsorbed active carbon according to the following reaction:
Cad is adsorbed active carbon, fundamentally different from deposited solid carbon resulting from methane cracking. Cad has higher activity in comparison to graphite and this improves the thermodynamics of the process. In fact they found carbon deposition resulting from methane cracking to be a problem: The authors reported that the solid carbon deposited on the oxide surface reduces the activity of carbon and also blocks the access of reducing gas to the oxides.
ZnO reduction by methane as an alternative method for zinc production was investigated by Ebrahim and Jamshidi.[7] They used a thermo-gravimetric setup with continuous gas analysis by an online mass spectrometer. The ZnO was in the form of near spherical particles pressed to pellets of <1 pct porosity; the temperature regime investigated was 1113 K to 1203 K (840 °C to 930 °C). The gas mixture used was 20 to 60 pct methane with Ar. An increase in reaction rate was seen with both increasing temperatures and methane concentrations. The authors did not mention the effect of carbon deposition as a result of methane cracking at high temperatures. The same authors investigated reduction of BaSO4 to BaSO3 with methane. In this work they reported that temperatures above 1223 K (950 °C) are undesirable because of carbon black deposition.[8]
Alizade et al.[9] performed a kinetic study of NiO reduction by methane. They used a He-CH4 gas mixture at temperatures between 873 K and 998 K (600 °C and 725 °C) in a thermogravimetric setup. They used porous NiO pellets of powder with particle size 0.026 μm. Increased reaction speed and reduced reaction temperatures compared to a solid–solid reaction with carbon were observed. Complete conversion was achieved in 11 minutes at 998 K (725 °C) vs 120 minutes at 1273 K (1000 °C) with carbon. The effects of methane cracking and carbon deposition did not play a significant role at the low temperatures at which the experiments were performed. A simulation study on carbothermal reduction of SiO2 was performed by Lee et al.[10] using the HSC software. For methane and SiO2 in a 3:1 mol-fraction relationship, Si was predicted to be produced above 2273 K (2000 °C). The calculation showed a three-step reaction, with methane cracking below 1523 K (1250 °C) followed by quartz reacting with the free carbon to form SiC between 1523 K to 2273 K (1250 °C and 2000 °C), before the SiC decomposed to Si and C above 2273 K (2000 °C). Reduction of quartz by methane–argon gas mixture in the temperature range between 1573 K and 1773 K (1300 °C and 1400 °C) was studied experimentally by Beheshti and Ringdalen.[11] Two setups were used. First, top blowing reactor was applied where the charge (1 kg) containing the quartz particles (5 to 8 mm) was heated up in an induction furnace in a graphite crucible. At the target temperature, the gas mixture with gas flow 2 to 6 L/min was injected into the charge. A second run was carried out in a fixed bed reactor where 7 g of sample was used and the gasflow was 200 mL/min. In both attempts, no SiC formation was found. In the top blowing reactor, there was some problems with equal gas mixture distribution inside the charge. The author reported large solid carbon deposition in both cases.
This paper describes a preliminary study of conversion of SiO2 to SiC by direct use of natural gas. The main focus of this work was on experimental setup development and determination of conditions for successful SiO2 reduction.
Experimental
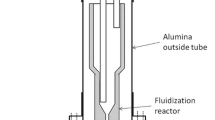
All the experiments were carried out using vertical tube furnace. The simple scheme of experimental setup is shown in Figure 1. As a lining material, alumina tubes were used (Alsint 99.7). The furnace was equipped with two separate cooling systems: one for the top lid and shell of the furnace, the second one is cooling the bottom lid and gas injection lance. The gas mixture was injected directly into the crucible through the tube tightly connected to the bottom of the crucible, both made from Alsint. In all but one experiment, the Alsint tube was located within a water-cooled lance in order to prevent methane cracking. To keep the charge material from falling into the gas, carrier tube an alumina plate with holes was located on the bottom of the crucible. This plate also helps in giving a more uniform gas distribution inside the crucible.
To control the gas composition, the mass flow controllers were used. The used gas compositions are listed in Table I. The temperature inside the reactor was controlled by a type S thermocouple inserted from the top of the furnace. The experimental conditions are listed in Table I. Samples were heated to 1273 K to 1773 K (1000 °C to 1500 °C) under the argon atmosphere. At the target temperature, the gas was switched to Ar-CH4-H2 or CH4-H2 mixture. After the experiment, no purge gas was used. The average heating rate was 12 °C/min. The pressure of the inlet gas was monitored during the whole experiment, which gives information about the possible carbon deposition and condensate formation. Due to safety issues, when pressure of inlet gas was above certain limit, experiments were stopped. Most of the clogging happened in case of using of pellets (due to fast condensate formation). Clogging caused by carbon formation was only observed in experiment 16, where the inflowing gas was not actively cooled. Quartz with particles between 1 and 3 mm and quartz pellets (1 and 3 mm) with the D 50 = 30 µm made from the same quartz were used as charge material. In addition, in experiments 15 and 16 β-cristobalite was used as a charge material. β-Cristobalite was obtained by heating the raw material to 1773 K (1500 °C) for 6 hours prior to the experiment. After heat treatment sample consisted of 90.8 wt pct of cristobalite and 9.2 wt pct of quartz, as confirmed by XRD. Porosity of materials is presented in Table II.
After the experiment, crucibles were mounted using epoxy resin. Samples were drilled out from crucibles, grained, and finally polished with 1 µm diamond paste. Electro Probe Micro Analyzer (EPMA) JEOL JXA 8500F was used for examining the samples.
Results
Quartz Samples
The cross-sections of crucibles presented in Figure 2 show typical three-layer structure found in experiments above 1273 K (1000 °C). The first layer is unreacted quartz where no reactions took place. The second layer is the condensation region where SiO gas needle shape condensates are agglomerated. Under the condensates layer so-called black quartz layer was found. In this area, all cracking of methane occurred. The solid carbon deposition on the edge and inside the quartz particles caused the change of its color. SiC formation was observed in those two lower layers.
Two kinds of SiC were produced during the experiment at 1673 K and 1773 K (1400 °C and 1500 °C). The first type of SiC was found to be formed in close distance to the quartz particles in region where tube-shape carbon was deposited. All SiO2 particles in this area are surrounded by layer of carbon deposition. Such a SiC has porous-compacted needles structure, and it occurs as agglomeration of needles, which is shown in Figure 3. SiC occurs more frequently in condensate area, than in the lower part (black quartz area) of crucible.
The second type of SiC formation was observed inside the particles of “black quartz.” Most of them have round shape, and they are located close to the cracks. Figure 4 shows that SiC inside the particle seems to be growing by consuming/transforming SiO2 into SiC. That round shape SiC has more dense structure than SiC formed outside of quartz particles. SiC formed inside the black SiO2 was mostly found in the black quartz layer below the SiO gas condensates layer and at the interface between these two layers. However, this kind of SiC was not observed at the very bottom of the crucible where most of the particles were covered by a thick layer of solid carbon.
Pellets
Cross-sections of crucibles where pellets were used as charge materials are presented in Figure 5. The figure shows clearly a layer of condensate located very close to the bottom of the crucible. It took much shorter time to create a dense condensate layer compared to the quartz particles. This is mostly due to the high porosity and surface area of pellets which allows CH4 to react with SiO2. Unfortunately, fast formation of condensates led to a rapid increase of inlet gas pressure and resulted in stopping the experiment.
Despite short reaction time, some SiC formation inside and outside of the pellets was observed in the bottom part of crucible. SiC looks like needle shape SiC from experiments where quartz particle was used. Light microscope picture presented in Figure 6 shows SiO2 pellets located between the condensation front and the area where methane cracking occurred. In this region, SiC was found appearing inside the condensate layer and also inside the pellet. Pellets above condensates did not contain any SiC.
Discussion
Obtained results indicate that three main processes\reaction occur in the crucible:
-
decomposition of methane resulting in generation of solid carbon and hydrogen,
-
SiO gas generation (which will react to produce SiC or condensate),
-
SiC formation.
Methane starts to decompose to carbon and hydrogen during heating according to the endothermic reaction:
Figure 7 presents the results of thermodynamic calculation for equilibrium gas composition during the cracking of methane. At temperature 1273 K (1000 °C) conversion should be completed and almost all methane converted to solid carbon and hydrogen.
The thermodynamic calculation for equilibrium gas composition during thermal decomposition of methane.[12] (Rest is assumed inert gas)
The process of CH4 cracking generates solid carbon which deposits on the silica particles and blocks gas access to the oxides. Measurement of temperature distribution inside the crucible for experiment at 1773 K (1500 °C) showed rapid temperature drop when mixture of methane and hydrogen gas was injected, both due to a cooled gas as well as the endothermic reaction.
The only one source of SiO gas in the studied system is the quartz, through one of the following reactions:
Reaction between SiC and SiO2 as potential rout for SiO gas formation is not considered since no SiC was present in the system below 1673 K (1400 °C). Figure 8 presents the results of thermodynamic calculation for equilibrium amount of species for experimental conditions. Two cases are considered; first is when methane reacts directly with silica and second one when methane cracks to solid carbon and hydrogen. In both cases, SiO gas formation stops around 1673 K (1400 °C) due to the lack of carbon source for further production. In addition, carbon deposition starts to appear at 673 K (400 °C) which blocks the CH4 access to the silica which prevent direct reduction of oxide.
The thermodynamic calculation for equilibrium amount of species for experimental conditions: (a) and (b) do not include the cracking of methane, (b) and (c) include the solid carbon occurrence[12] (Color figure online)
Two kinds of SiC formation were observed. At 1673 K and 1773 K (1400 °C and 1500 °C) compacted-needle-shape SiC around SiO2 particles was found. This kind of SiC was mostly found in the border between SiO gas condensates and black quartz area. It was not found in the upper part of condensates, and any region in crucible above it. It is believed that this SiC formation comes from reactions between the gas phases (SiO-CH4 or SiO-CO). SiC was found away from the SiO2 surface, usually in some distance from quartz. In addition, the quartz particle was covered by a thick layer of solid carbon, which leads to the suspicion that the Si in the SiC has its source in SiO gas rather than SiO2. Gas-phase reaction can be as follows:
A gas-phase reaction between CH4 and SiO is a possible route to produce SiC. Assuming that the SiO gas was formed by reaction between methane and quartz (Eq. [3]) the local partial pressure of methane will decrease as one meets the quartz surface. However, in some distance from the quartz particle methane and SiO partial pressure could be high enough for SiC formation. That fits to what was found in microscope pictures, that the formation of SiC takes place in some distance from quartz particles. Monsen et al.[13] produced SiC from gas phase consisting of methane and SiO gas. They suggested that SiC formation in their reactor follows Reaction [7]. Saito et al.[14] in their work on crystal growth of SiC whiskers from SiO-CO system produced needle-like SiC according to Eq. [8]:
They suggested that the growth of needle like whiskers occurs also according to Eq. [6] and the presence of carbonaceous solid is essential for the growth. SiC from reaction between quartz and methane containing gas should start on the particle surface as it was reported by Zhang and Ostrovski.[15] Nevertheless, such SiC deposition was not observed in following work. The other possible route to produce SiC in such conditions is reaction between SiO and C:
However, SiC was found to be surrounded by large quantities of deposited carbon. SiO gas could not penetrate it without forming SiC on that layer. A possible explanation in this case could be that the SiC was created at the initial stage of experiment and then covered by excess of carbon. The second kind of SiC formation inside of the quartz particles was observed mostly at 1773 K (1500 °C). It occurs inside a quartz particle far from its edge. It has a different shape than SiC found outside of particles, but has also a porous structure. A question that can be asked is how and why such SiC is formed inside the particles. One of the suggestions is that this is still porous region of SiO2 which has not been covered by solid carbon, and gas can react with SiO2 to form SiC. EMPA pictures rather show that this SiC is not directly from converted SiO2 but was formed through gas phase according to reactions mentioned before. In summary, SiC is expected to be formed from gas phases. It is believed that the mechanism of SiC formation corresponds to one or more of the reactions discussed above, however, the presented results do not allow for a precise determination of the main SiC reaction (Figure 9).
The temperature plays an important role when CH4 is used as reduction gas due to its thermal decomposition. Low temperature reduces the problem of extensive solid carbon deposition; however in experiments where the target temperature was below 1673 K (1400 °C) no SiC and no SiO gas were generated. The temperature was too low to start any reaction between gas mixture and charge material. At the low temperature experiments some quartz particles changed color and became grayish, probably as a result of some methane cracking and carbon deposition inside the quartz. However, no carbon deposition was detected by EMPA. At target temperatures above 1673 K (1400 °C) both types of SiC formation was found. At 1673 K and 1773 K (1400 °C and 1500 °C), the needle shape SiC was found usually in some distance from the quartz. Higher temperature caused SiC formation inside the SiO2 particles, which was not seen at lower temperature. The solid carbon deposition was also increased due to an increased temperature. It was found as a thick layer around of the particles in the bottom of crucible, and also in form of tubes in the upper part of the crucible. The effect of CH4/H2 ratio was preliminarily studied at 1273 K and 1773 K (1000 °C and 1500 °C), where 1:9 and 1:2 ratios were used. At 1273 K (1000 °C) no significant effect of gas composition was observed. Only visual observations of samples suggest that cracking of methane was more widespread when 1:2 CH4/H2 ratio was used. Quartz became more grayish especially in the upper part of the crucible. Various CH4/H2 ratios at high temperature experiments did not influence the SiC formation. However, above 1773 K (1500 °C) higher CH4 concentration gave more carbon deposition.
Conclusions
Preliminary results for usage of natural gas for reduction of SiO2 to SiC formation were presented. Application of water-cooled lance gave possibility to inject the cooled gas mixture to the crucible without prior cracking of methane. SiC was formed via gas phase between SiO gas and a source of carbon according to one of the reaction Eqs. [6] to [8], or a combination of more than one reaction. Based on presented results it is difficult to establish what is the main mechanism for SiC formation. Experiments ran at 1673 K and 1773 K (1400 °C and 1500 °C) where SiC was formed gave interesting results and should be investigated more deeply. The temperature, CH4-H2 ratios, particle size of charge material,l and total gas flow are parameters which seem to play an important role. The solid carbon deposition occurs to be the main reason for low SiC production, as it was observed that SiC was mostly created in areas where SiO2 particles were not covered by layer of C. Cristobalite and quartz pellets as charge material did not give satisfactory results due to fast SiO gas condensation reaction resulting in clogging the charge. Application of cristobalite particles instead of quartz particles resulted in low SiC production and big amount of condensates which led to stopping the experiments.
References
M. Tangstad, Metal Production in Norway (Akademika forlag, Trondheim, 2013).
www.eia.gov, 2013.
B. Kelley, Natural Gas and Reformer Catalyst (Midrex Direct Reduction Corporation, Charlotte, NC, 2000).
D. Wang, M. Deng, Y. Liu, Y. Liu, X. Li, R. Lu, Earth Sci. Front. 15(6), 124–32 (2008).
H. Dalaker: Methane and Natural Gas as Reducing Agent—A Review with the Aim to Reduce SiO 2 to Si, SINTEF Report, SINTEF A19858, ISBN 978-82-14-05143-8, 2011.
O. Ostrovski, G. Zhang, AIChE J. 52(1), 300–310 (2006).
H. Ale-Ebrahim, E. Jamshidi, Trans. IChemE Part A 79, 62–70 (2001).
E. Jamshidi, H.A. Ebrahim, Chem. Eng. Process. 47, 1567–77 (2008).
R. Alizadeh, E. Jamshid, H. Ale-Ebrahim, Chem. Eng. Technol. 30(8), 1123–28 (2007).
H.-C. Lee, S. Dhage, M.S. Akhtar, D.H. Kwak, W.J. Lee, C.Y. Kim, O.B. Yang, Curr. Appl. Phys. 10, 218–21 (2010).
R.K. Beheshti and E. Ringdalen: “Reduction and Carburization of Quartz by Methane”, SINTEF Internal Report, Project No. 801832, 2011.
HSC Chemistry 7.1 Computer Program for Thermodynamic Calculation.
B. Monsen, L. Kolbeinsen, S. Prytz, V. Myrvågnes, and K. Tang: INFACON XIII The Thirteenth International Ferroalloys Congress Proceedings, Kazakhstan, 2013, vol. 1, pp. 467–78.
M. Saito, S. Nagashima, A. Kato, J. Mater. Sci. Lett. 11, 373–76 (1992).
G. Zhang, O. Ostrovski, Metall. Mater. Trans. 31B, 129–39 (2000).
Author information
Authors and Affiliations
Corresponding author
Additional information
Manuscript submitted November 21, 2013.
Rights and permissions
About this article
Cite this article
Ksiazek, M., Tangstad, M., Dalaker, H. et al. Reduction of SiO2 to SiC Using Natural Gas. Metallurgical and Materials Transactions E 1, 272–279 (2014). https://doi.org/10.1007/s40553-014-0027-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40553-014-0027-4