Abstract
Introduction
Invasive candidiasis (IC) can be a life-threatening infection in immunocompromised patients, particularly those with cancer, hematologic diseases and/or hematopoietic stem cell transplantation (HSCT) recipients. The objective of this study was to evaluate the effectiveness of micafungin in patients with hematologic malignancies or HSCT recipients, relevant to clinical presentation of IC, in real-life practice in Greece.
Methods
ASPIRE was a phase IV, multicenter, non-interventional, prospective cohort study, conducted at ten tertiary hospitals in Greece, in adults with hematologic disease. Micafungin treatment for IC or prophylaxis for Candida infection was administered per standard clinical practice until a clinical outcome (success or failure) was reached. Treatment success was defined by the EORTC/MSG criteria for invasive fungal infections (IFI) and was assessed by the investigator. Treatment discontinuation and safety were also evaluated.
Results
One hundred forty-three patients were enrolled. Median age was 62; 85 (59.4%) patients were male, and 133 (93.0%) had Greek ethnicity. One hundred twenty-six (88.1%) patients had hematologic malignancies, and 21 (14.7%) had received HSCT. Prophylaxis was administered to 74 (51.7%) patients [median (range) dose: 50 (50–150) mg/day] with no signs of IFI. Overall, 52 (36.4%) patients with possible IFI at baseline received micafungin treatment [100 (50–125) mg/day] versus 12 (17.2%) with probable [100 (75–150) mg/day] and 5 (3.5%) with confirmed [125 (100–150) mg/day] IFI. Treatment success was 91.6% (95% CI 85.80–95.59; n = 131) overall and 90.5% (n = 67) in patients receiving prophylaxis. Median time on treatment was 13 days. Treatment discontinuation (n = 26; 18.2%) was not related to adverse events. No treatment-related serious adverse events were reported.
Conclusion
Micafungin treatment for IC or prophylaxis for Candida infection was effective and well tolerated in patients with hematologic disorders in clinical practice in Greece. These results demonstrate that micafungin could be used more widely for prophylaxis. Further work is required to determine the efficacy and safety of micafungin for the management of IFIs in hematologic settings.
Funding
Astellas Pharma Inc.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Invasive fungal infections (IFIs) are a major cause of nosocomial infections leading to morbidity and mortality. Immunocompromised patients, in particular those with cancer, hematologic diseases and/or hematopoietic stem cell transplantation (HSCT) recipients, carry a high risk of developing life-threatening opportunistic IFIs, such as invasive candidiasis (IC), aspergillosis and mucormycosis [1,2,3]. While advances such as organ transplantation, cancer therapies and intensive care unit interventions have improved the prognosis in these patients, these interventions have also increased susceptibility to IFIs [4, 5]. In patients with hematologic malignancies and IC, studies have reported an overall mortality risk of up to 38% with an attributable mortality of 19% [6]. Overall, IC is responsible for 2–3% of nosocomial infections in Europe [7].
Guidelines for the management of IC from the European Society of Clinical Microbiology and Infectious Diseases (ESCMID) strongly recommend an echinocandin (e.g., anidulafungin, caspofungin, micafungin) [6, 8, 9]. In adult patients with hematologic malignancies, guidelines only moderately support the use of liposomal amphotericin B and provide marginal support for fluconazole [6]. Of the recommended echinocandin therapies, micafungin is strongly recommended for both targeted therapy in patients with malignancies receiving allogeneic HSCT and for prophylaxis therapy in patients receiving allogeneic HSCT [6].
Micafungin inhibits the synthesis of 1, 3-β-d-glucan, an essential component of the fungal cell wall, leading to osmotic instability and eventual cell death [10, 11]. Several clinical trials involving micafungin have demonstrated its efficacy in IC, reporting response rates ranging from 71–90% [12,13,14,15]. Micafungin has also shown efficacy when used as a prophylactic agent for fungal infections in neutropenic patients [6, 16]. In addition, micafungin has a favorable safety and tolerability profile with a low potential for drug-drug interactions compared with caspofungin and fluconazole [17,18,19]. In addition, micafungin does not require a loading dose (unlike other echinocandins and azoles) and does not require dose adjustment in patients with renal or mild to moderate hepatic impairment [4, 10, 20].
Relatively few published data are available concerning current clinical practice patterns in Greece regarding fungal infections in patients with hematologic conditions and in HSCT recipients. Despite increasing interest in the clinical and therapeutic aspects of fungal infections in patients with hematologic disease, recent observational trials focus primarily on gathering evidence on prevalence and microbiologic characteristics [21,22,23]. To our knowledge, there are no published data from prospective, multicenter, observational studies regarding treatment with new-generation antifungal agents in patients with hematologic disorders in real-life practice in Greece.
The aim of this multicenter, non-interventional, prospective study was to evaluate the effectiveness of micafungin administration in standard clinical practice among patients with hematologic malignancies, including patients who underwent HSCT. This article reports clinical presentation at treatment initiation, clinical outcomes, treatment duration and tolerability associated with micafungin treatment in Greece.
Methods
Study Design and Treatment
The ASPIRE study (protocol: GR-MYC-NI-002) was a phase IV, multicenter, non-interventional, prospective cohort study conducted between June 2013 and March 2014 at ten tertiary hospitals in Greece. The study was designed to evaluate the efficacy of micafungin in standard clinical practice. Due to the non-interventional nature of this study, it was not registered in local or international clinical trial domains. The institutional review board or independent ethics committee of each participating hospital approved the study design, and the study was performed in accordance with the principles of the 1964 Declaration of Helsinki and its later amendments, International Conference on Harmonisation Guidelines and local ethical and legal requirements. Informed consent was obtained prior to patients’ participation in the study.
Inclusion criteria included being > 18 years of age and having a diagnosis of hematologic disease (including patients who underwent HSCT) with a performance status of 0–2 (on the Eastern Cooperative Oncology Group scale). Written informed consent was obtained from all patients or their legal representative(s). As micafungin is not recommended for use in patients with severe hepatic impairment, and caution is advised in those with renal impairment, patients with severe renal insufficiency (estimated creatinine clearance < 20 ml/min at baseline or likely to require dialysis during the study) and those with moderate or severe liver dysfunction at baseline [defined as aspartate aminotransferase, alanine aminotransferase or alkaline phosphatase levels greater than two times the upper limit of normal (ULN) or a total bilirubin level greater than two times the ULN] were excluded from this trial.
Patients were classified (four levels) according to their clinical presentation at baseline, according to the investigator’s discretion, using the revised European Organisation for Research and Treatment of Cancer (EORTC)/Mycoses Study Group (MSG) criteria for IFI: no signs (at risk), possible, probably and proven [2]. For possible IFI, patients should have appropriate host factors (e.g., allogeneic HSCT) and clinical evidence of IFI, but no mycologic evidence. Cases of probable IFI require the presence of a host factor, together with clinical features and mycologic evidence. Proven IFI must be confirmed by microscopic analysis on sterile samples or recovery of yeast or mold by culture of blood or sterile samples [2]. Established risk factors for IFI were recorded for patients at treatment initiation [24,25,26,27,28,29]. Concomitant antineoplastic treatments received during the study period or during the 3-month period before study inclusion were recorded. In addition, for patients who did not receive micafungin as monotherapy to treat their IFI, the therapeutic agents included in the combination therapy were recorded.
The assignment of a patient to a particular therapeutic strategy was not decided in advance by the protocol. All patients received micafungin, administered at the discretion of the investigator, as per standard clinical practice in accordance with the approved prescribing information. Micafungin is approved in Europe as an intravenous infusion over approximately 1 h:100 mg/day for treatment and 50 mg/day for prophylaxis [20]. The observational period lasted until a clinical outcome (success or failure) was recorded.
Outcome Measures
The primary objective was treatment success, as assessed by investigators, relative to clinical presentation at baseline. Treatment success was defined by EORTC/MSG criteria and the daily clinical decision-making of investigators. Secondary objectives included describing the profile of clinical presentation on micafungin treatment initiation, the duration of micafungin treatment, micafungin discontinuation rate and safety. The latter included monitoring adverse drug reactions (ADRs), serious adverse events (SAEs) and clinical laboratory measurements (temperature; neutrophil count; liver and renal laboratory tests, including alkaline phosphatase, creatinine, aspartate aminotransferase, alanine aminotransferase, total bilirubin and γ-glutamyl transferase). The average daily dose of micafungin was also recorded.
As this was an observational study performed under real-world clinical conditions, there was no schedule of procedures. Other than screening, all study assessments and follow-ups were performed at the discretion of the investigation in line with daily clinical practice at each center.
Statistical Analyses
The planned sample size, 108 patients, was calculated to provide an adequate precision level [95% confidence interval (CI) of ± 10%] for measuring clinical outcome (success versus failure), following a conservative statistical approach regarding the expected success rate (50%, as an expected success rate based on a previously published study could not be determined). Prior to study initiation, the protocol was amended to enroll 190 patients to increase precision; however, recruitment was not sufficient to complete enrollment within the planned study time lines. All patients were included in the analyses irrespective of study withdrawal.
Descriptive analyses are stratified by clinical presentation at baseline. Results are expressed as means ± standard deviation (SD) and median plus range for all continuous variables and as counts and proportions (along with Clopper-Pearson exact 95% CIs for categorical variables). Missing values were presented without imputation for all analyses. Statistical analysis was performed using SAS® 9.3 (SAS Institute, Inc., Cary, NC, USA).
Results
Patient Disposition and Clinical Presentation at Baseline
A total of 143 patients were enrolled in the study to receive treatment with micafungin (Table 1). Fourteen patients (10%) did not complete the study because of lack of treatment availability at the treatment center’s pharmacy (n = 6), death (n = 5), lack of efficacy (n = 1), adverse events (AEs) not related to micafungin (n = 1) and possible aspergillosis (n = 1). The most frequently observed comorbidities were related to cardiovascular (35.0%) and endocrine/metabolic (24.5%) systems. The median age of the study population was 62 (range: 22–89) years; 59.4% of patients were male, and the majority were of Greek ethnicity (93.0%).
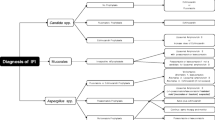
At baseline, 74 (51.7%) patients received micafungin as prophylaxis (no signs of IFI), while 52 (36.4%), 12 (8.4%) and 5 (3.5%) patients received treatment for possible, probably and proven IFI, respectively (Fig. 1). Patients with proven IFI had Candida albicans recovered from their last cultures (urine culture n = 2; blood culture, sputum culture, tissue culture, each n = 1) prior to micafungin administration.
Overall, 122 (85.3%) patients had hematologic malignancies and 21 (14.7%) underwent HSCT. Of the HSCT recipients [12 (8.4%) autologous and 9 (6.3%) allogenic], 17 (11.9%) had hematologic malignancy and 4 (2.8%) underwent HSCT for other hematologic disorders (Table 1). Over half (51.0%) of patients were newly diagnosed and the most common hematologic disorder was acute myelogenous leukemia (40.6%). Three patients had Hodgkin’s lymphoma; of these, two had hematologic disease, two had hematologic malignancy and possible IFI, and one had hematologic malignancy and HSCT. Common patient risk factors at baseline included systemic antibiotics (78.3%, n = 112), neutropenia (59.4%, n = 85) and fever (54.5%, n = 78) (Fig. 2). Mean temperature and neutrophil counts were 38.2 (SD ± 0.5) °C and 260.0 (SD ± 351.5) cells/mm3, respectively. The mean number of risk factors per patient was 3.6 (SD ± 1.6) with over 25% of patients having five or more risk factors.
Treatment Exposure
The median duration of micafungin treatment for IC or prophylaxis for Candida infection was 13 [range: 0–77 (treatment was initiated on day 0)] days, and the mean average daily dose of micafungin per patient was 82.5 (SD ± 26.9) mg (Table 2). Seventy-four patients with no signs of IFI received micafungin as prophylactic therapy [mean average daily dose: 63.9 (SD ± 22.0)] mg. Micafungin was administered as monotherapy to 97.9% of patients and was first-line treatment in 75.5% of patients. In patients that received micafungin as second-line treatment (21.7%, n = 31), fluconazole was the most common first-line therapy (54.9%). Patients receiving combination antifungal therapy (2.1%; n = 3) received nystatin, posaconazole or amphotericin B in addition to micafungin.
Clinical Outcome
Overall, 91.6% (95% CI: 85.80–95.59; n = 131) of patients had treatment success with micafungin therapy (Table 3). Success rates between patients in each clinical presentation group were comparable. The highest success rate was observed in patients with proven IFI (100%; n = 5), followed by patients with possible (96.2%; n = 50), no signs of (90.5%; n = 67) and probable (75.0%, n = 9) IFI. Due to a lack of drug availability, an outcome could not be evaluated for five patients (3.5%). Only seven (4.9%) patients had treatment failure due to persistent febrile episodes and neutropenia (n = 1), no improvement in the patient's symptoms (n = 2), persistent fever with limited micafungin efficacy (n = 1), development of pleural effusion probably due to fungal infection (n = 1) or death due to lack of efficacy (n = 1).
Treatment Status on the Last Day of IC Therapy
On the last day of treatment, 26 (18.2%) patients had discontinued micafungin treatment and switched to another antifungal therapy. Common reasons for micafungin treatment discontinuation included patient discharge (n = 8) and lack of drug availability (n = 6). Only one patient stopped therapy because of limited efficacy (Fig. 3).
Safety
Over the course of the study, events of interest were reported on ADR report forms for 22 (15.4%) patients and included off-label use (n = 21) and lack of drug efficacy (n = 1). Overall, eight deaths (all SAEs, not considered related to micafungin) occurred during the study; five during treatment with micafungin and three following the end of treatment. One case of lack of efficacy was reported and classified as serious; this included death but was not related to micafungin. The mean body temperature on the last day of treatment was 36.9 (SD ± 0.8)°C, and the mean neutrophil count increased to 3901 (SD ± 6283) cells/mm3. There were no notable changes in liver and renal function.
Discussion
The study presented here demonstrated that micafungin is effective for the treatment of suspected and proven IC and as antifungal prophylaxis in patients with hematologic disease or in HSCT recipients in a clinical setting in Greece. Treatment success was observed in each patient group regardless of stratification by baseline clinical presentation or underlying disease. In addition, micafungin was well tolerated in these populations with no treatment-related SAEs reported.
The overall success rate (91.6%) in this study was higher than that observed in previous studies. In a prospective multicenter study conducted in Japan in patients with hematologic disorders and invasive aspergillosis and candidiasis, the total response rate to micafungin was 68.0% [30]. Similarly, as an empirical antifungal agent in febrile neutropenic patients, micafungin had a 61.7% and 64.4% success rate in an observational and randomized study, respectively [31, 32]. The higher success rate may be due to differences in the clinical profiles of patient populations, differences in the dosages administered or the use of micafungin in prophylaxis. In addition, our study only investigated candidiasis, as micafungin is not licensed for treatment of aspergillosis in Europe. Together, the overall success rates reported in these clinical and real-world studies support the ESCMID’s recommendation for micafungin treatment in patients with hematologic disorders/HSCT [6].
As our study was observational, the assignment of a patient to a particular therapeutic strategy was not decided in advance by the protocol, and micafungin was administered according to the physician’s decision, as well as per standard clinical practice according to its label. Given the high mortality in patients with IC, guidelines and the literature support prophylaxis for IFI as a rational strategy in high-risk patients, including those who are immunocompromised [33, 34]. In a meta-analysis of 38 trials including > 7000 patients, antifungal prophylaxis was associated with a treatment-related reduction in overall mortality among subgroups of high-risk patients with prolonged neutropenia and in HSCT recipients, providing support for this approach [35].
Prophylaxis therapy is recommended for allogenic HSCT recipients who carry a particularly high risk of IFIs [6]. Although azoles are widely used for antifungal prophylaxis after HSCT, they are associated with hepatic toxicity and drug-drug interactions [36]. In addition, antifungal resistance has become a global problem, especially with Candida glabrata, which may be responsible for as many as 18.2% IC infections worldwide [37, 38]. Numerous trials have demonstrated the benefit of micafungin prophylaxis in HSCT recipients [16, 36, 39, 40]. In a prospective, randomized, double-blind comparative study of micafungin versus fluconazole, administered as antifungal prophylaxis during the neutropenic phase of HSCT, treatment success of micafungin was 80.0% versus 73.5% for fluconazole (95% CI: 0.9–12; P = 0.03) [36]. In HSCT recipients, prophylaxis with micafungin has also been shown to have similar efficacy to fluconazole [16]. In the current study, micafungin was effective for prophylaxis in patients at risk of IFI (90.5%). In agreement with our findings, recent studies have documented the benefit of micafungin prophylaxis in patients at high risk of IFI, regardless of HSCT [39,40,41].
In Europe, micafungin is licensed for prophylaxis in HSCT recipients and in patients who are expected to have neutropenia (absolute neutrophil count < 500 cells/μl) for ≥ 10 days, at a dose of 50 mg/day (patients weighing > 40 kg weight and aged ≥ 4 months) and at a dose of 100–200 mg for treatment of IC based on clinical response and body weight [20]. Among the patients in the ‘no signs of IFI’ group in ASPIRE, 52.7% had neutropenia at the onset of treatment, suggesting the presence of other risk factors. In ASPIRE, no controls were used for dose. The average dose administered to patients with no signs of IFI was 63.9 mg/day, with 15% of patients in this group receiving 100 mg/day. A retrospective study of micafungin prophylaxis prescribing practice in Germany found that 50% of physicians administered 50 mg/day and 50% administered 100 mg/day with success rates of 79.2% (n = 42) and 98.1% (n = 52), respectively [39]. Therefore, although clinical practice treatment indications for antifungals may deviate from the strict criteria used in clinical trials, additional benefits may also be observed.
In the present study, micafungin was shown to be well tolerated with no treatment-related SAEs reported. Liver injury or renal dysfunction was not observed during study monitoring. This is in agreement with previous studies including a retrospective observational study in patients with hematologic malignancies, where there was no evidence of liver or renal toxicity with micafungin prophylaxis at doses of up to 300 mg (2–3 doses per week) [40, 42]. However, cases of hepatic dysfunction with micafungin have been reported. Due to insufficient data available, micafungin is currently not recommended for patients with severe hepatic impairment [20]. In addition, although micafungin has demonstrated efficacy and safety at high doses, the potential emergence of echinocandin resistance should be considered in future clinical trials [43].
The primary reasons for treatment discontinuation were patient discharge and lack of drug availability; no treatment-related AEs leading to treatment discontinuation were recorded. In general, rates of discontinuation due to AEs are usually lower for echinocandins than azoles, with the exception of fluconazole [41].
The strengths of the ASPIRE study include a heterogeneous population with different underlying diseases, stratification of baseline characteristics and study data by clinical presentation at baseline (EORTC/MSG criteria), administration of micafungin monotherapy in the majority of patients (97.9%), administration of micafungin as both prophylaxis and targeted treatment, and a real-world setting. The study also has potential limitations. There was no standard definition of treatment success, which was assessed by the investigator, relative to clinical presentation at the start of treatment. In addition, clinical outcome was evaluated on the last day of study treatment with no further follow-up period after the end of study treatment and was defined by the investigators based on the EORTC/MSG criteria and their judgment as relevant for clinical decision-making. Other limitations include low enrollment in the probable and proven IFI groups, low enrollment of patients with HSCT at baseline and monitoring of only SAEs and ADRs instead of all AEs. Finally, a combination of antifungal prophylaxis and treatment was used in this study.
Conclusion
In summary, in this multicenter study in Greece, micafungin was effective and well tolerated in clinical practice as prophylaxis and treatment for IC in patients with hematologic disease or with HSCT. This study demonstrates that micafungin could be more widely used for prophylaxis and provides evidence of micafungin use at doses up to 150 mg/day. However, further work is required to determine the efficacy and safety of micafungin for the management of IFI in a hematologic setting.
References
Ascioglu S, Rex JH, de Pauw B, Bennett JE, Bille J, Crokaert F, et al. Defining opportunistic invasive fungal infections in immunocompromised patients with cancer and hematopoietic stem cell transplants: an international consensus. Clin Infect Dis. 2002;34(1):7–14.
De Pauw B, Walsh TJ, Donnelly JP, Stevens DA, Edwards JE, Calandra T, et al. Revised definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group. Clin Infect Dis. 2008;46(12):1813–21.
Blyth CC, Gilroy NM, Guy SD, Chambers ST, Cheong EY, Gottlieb T, et al. Consensus guidelines for the treatment of invasive mould infections in haematological malignancy and haemopoietic stem cell transplantation, 2014. Intern Med J. 2014;44(12b):1333–499.
de la Torre P, Reboli AC. Micafungin: an evidence-based review of its place in therapy. Core Evid. 2014;9:27–39.
Pfaller MA, Diekema DJ. Epidemiology of invasive candidiasis: a persistent public health problem. Clin Microbiol Rev. 2007;20(1):133–63.
Ullmann AJ, Cornely OA, Donnelly JP, Akova M, Arendrup MC, Arikan-Akdagli S, et al. ESCMID* guideline for the diagnosis and management of Candida diseases 2012: developing European guidelines in clinical microbiology and infectious diseases. Clin Microbiol Infect. 2012;18(Suppl 7):1–8.
Marchetti O, Bille J, Fluckiger U, Eggimann P, Ruef C, Garbino J, et al. Epidemiology of candidemia in Swiss tertiary care hospitals: secular trends, 1991–2000. Clin Infect Dis. 2004;38(3):311–20.
Cornely OA, Bassetti M, Calandra T, Garbino J, Kullberg BJ, Lortholary O, et al. ESCMID* guideline for the diagnosis and management of Candida diseases 2012: non-neutropenic adult patients. Clin Microbiol Infect. 2012;18(Suppl 7):19–37.
Hope WW, Castagnola E, Groll AH, Roilides E, Akova M, Arendrup MC, et al. ESCMID* guideline for the diagnosis and management of Candida diseases 2012: prevention and management of invasive infections in neonates and children caused by Candida spp. Clin Microbiol Infect. 2012;18(Suppl 7):38–52.
Denning DW. Echinocandin antifungal drugs. Lancet. 2003;362(9390):1142–51.
Yanada M, Kiyoi H, Murata M, Suzuki M, Iwai M, Yokozawa T, et al. Micafungin, a novel antifungal agent, as empirical therapy in acute leukemia patients with febrile neutropenia. Intern Med. 2006;45(5):259–64.
Kuse ER, Chetchotisakd P, da Cunha CA, Ruhnke M, Barrios C, Raghunadharao D, et al. Micafungin versus liposomal amphotericin B for candidaemia and invasive candidosis: a phase III randomised double-blind trial. Lancet. 2007;369(9572):1519–27.
Ostrosky-Zeichner L, Kontoyiannis D, Raffalli J, Mullane KM, Vazquez J, Anaissie EJ, et al. International, open-label, noncomparative, clinical trial of micafungin alone and in combination for treatment of newly diagnosed and refractory candidemia. Eur J Clin Microbiol Infect Dis. 2005;24(10):654–61.
Pappas PG, Rotstein CM, Betts RF, Nucci M, Talwar D, De Waele JJ, et al. Micafungin versus caspofungin for treatment of candidemia and other forms of invasive candidiasis. Clin Infect Dis. 2007;45(7):883–93.
Queiroz-Telles F, Berezin E, Leverger G, Freire A, van der Vyver A, Chotpitayasunondh T, et al. Micafungin versus liposomal amphotericin B for pediatric patients with invasive candidiasis: substudy of a randomized double-blind trial. Pediatr Infect Dis J. 2008;27(9):820–6.
Park S, Kim K, Jang JH, Kim SJ, Kim WS, Chung DR, et al. Randomized trial of micafungin versus fluconazole as prophylaxis against invasive fungal infections in hematopoietic stem cell transplant recipients. J Infect. 2016;73(5):496–505.
Andes D. Optimizing antifungal choice and administration. Curr Med Res Opin. 2013;29(Suppl 4):13–8.
Nivoix Y, Ubeaud-Sequier G, Engel P, Leveque D, Herbrecht R. Drug-drug interactions of triazole antifungal agents in multimorbid patients and implications for patient care. Curr Drug Metab. 2009;10(4):395–409.
Kofla G, Ruhnke M. Pharmacology and metabolism of anidulafungin, caspofungin and micafungin in the treatment of invasive candidosis: review of the literature. Eur J Med Res. 2011;16(4):159–66.
Astellas Pharma B.V. MYCAMINETM (micafungin sodium) for injection SmPC. 2016. https://www.medicines.org.uk/emc/print-document?documentId=20997. Accessed June 2016.
Gamaletsou MN, Drogari-Apiranthitou M, Denning DW, Sipsas NV. An estimate of the burden of serious fungal diseases in Greece. Eur J Clin Microbiol Infect Dis. 2016;35(7):1115–20.
Gamaletsou MN, Walsh TJ, Zaoutis T, Pagoni M, Kotsopoulou M, Voulgarelis M, et al. A prospective, cohort, multicentre study of candidaemia in hospitalized adult patients with haematological malignancies. Clin Microbiol Infect. 2014;20(1):O50–57.
Klingspor L, Tortorano AM, Peman J, Willinger B, Hamal P, Sendid B, et al. Invasive Candida infections in surgical patients in intensive care units: a prospective, multicentre survey initiated by the European Confederation of Medical Mycology (ECMM) (2006-2008). Clin Microbiol Infect. 2015;21(1):87 (e1–e10).
Jantunen E, Salonen J, Juvonen E, Koivunen E, Siitonen T, Lehtinen T, et al. Invasive fungal infections in autologous stem cell transplant recipients: a nation-wide study of 1188 transplanted patients. Eur J Haematol. 2004;73(3):174–8.
Martino R, Subira M, Rovira M, Solano C, Vazquez L, Sanz GF, et al. Invasive fungal infections after allogeneic peripheral blood stem cell transplantation: incidence and risk factors in 395 patients. Br J Haematol. 2002;116(2):475–82.
Pappas PG, Andes D, Schuster M, Hadley S, Rabkin J, Merion RM, et al. Invasive fungal infections in low-risk liver transplant recipients: a multi-center prospective observational study. Am J Transpl. 2006;6(2):386–91.
Patterson TF. Advances and challenges in management of invasive mycoses. Lancet. 2005;366(9490):1013–25.
Segal BH. Aspergillosis. N Engl J Med. 2009;360(18):1870–84.
Singh N, Paterson DL. Aspergillus infections in transplant recipients. Clin Microbiol Rev. 2005;18(1):44–69.
Tamura K, Urabe A, Yoshida M, Kanamaru A, Kodera Y, Okamoto S, et al. Efficacy and safety of micafungin, an echinocandin antifungal agent, on invasive fungal infections in patients with hematological disorders. Leuk Lymphoma. 2009;50(1):92–100.
Park JS, Kim DH, Choi CW, Jeong SH, Choi JH, Kim K, et al. Efficacy and safety of micafungin as an empirical antifungal agent for febrile neutropenic patients with hematological diseases. Acta Haematol. 2010;124(2):92–7.
Jeong SH, Kim DY, Jang JH, Mun YC, Choi CW, Kim SH, et al. Efficacy and safety of micafungin versus intravenous itraconazole as empirical antifungal therapy for febrile neutropenic patients with hematological malignancies: a randomized, controlled, prospective, multicenter study. Ann Hematol. 2016;95(2):337–44.
Kullberg BJ, Arendrup MC. Invasive Candidiasis. N Engl J Med. 2015;373(15):1445–566.
Pappas PG, Kauffman CA, Andes DR, Clancy CJ, Marr KA, Ostrosky-Zeichner L, et al. Clinical practice guideline for the management of candidiasis: 2016 update by the infectious diseases Society of America. Clin Infect Dis. 2016;62(4):e1–50.
Bow EJ, Laverdiere M, Lussier N, Rotstein C, Cheang MS, Ioannou S. Antifungal prophylaxis for severely neutropenic chemotherapy recipients: a meta analysis of randomized-controlled clinical trials. Cancer. 2002;94(12):3230–46.
van Burik JA, Ratanatharathorn V, Stepan DE, Miller CB, Lipton JH, Vesole DH, et al. Micafungin versus fluconazole for prophylaxis against invasive fungal infections during neutropenia in patients undergoing hematopoietic stem cell transplantation. Clin Infect Dis. 2004;39(10):1407–16.
Arendrup MC. Update on antifungal resistance in Aspergillus and Candida. Clin Microbiol Infect. 2014;20(Suppl 6):42–8.
Rodloff C, Koch D, Schaumann R. Epidemiology and antifungal resistance in invasive candidiasis. Eur J Med Res. 2011;16(4):187–95.
Heimann SM, Vehreschild MJ, Meintker L, Heinz W, Schroeder T, von Bergwelt-Baildon M, et al. Different doses of micafungin for prophylaxis of invasive fungal diseases in hemato-oncological high-risk patients: a web-based non-interventional trial in four large university hospitals in Germany. Transpl Infect Dis. 2014;16(6):968–74.
Neofytos D, Huang YT, Cheng K, Cohen N, Perales MA, Barker J, et al. Safety and efficacy of intermittent intravenous administration of high-dose micafungin. Clin Infect Dis. 2015;61(Suppl 6):S652–61.
Wang JF, Xue Y, Zhu XB, Fan H. Efficacy and safety of echinocandins versus triazoles for the prophylaxis and treatment of fungal infections: a meta-analysis of RCTs. Eur J Clin Microbiol Infect Dis. 2015;34(4):651–9.
Cornely OA, Pappas PG, Young JA, Maddison P, Ullmann AJ. Accumulated safety data of micafungin in therapy and prophylaxis in fungal diseases. Expert Opin Drug Saf. 2011;10(2):171–83.
Shields RK, Nguyen MH, Clancy CJ. Clinical perspectives on echinocandin resistance among Candida species. Curr Opin Infect Dis. 2015;28(6):514–22.
Acknowledgements
We thank the participants of the study.
Funding
The study and article processing fees were funded by Astellas Pharma Inc. All authors had full access to all of the data in this study and take complete responsibility for the integrity of the data and accuracy of the data analysis.
Medical Writing and Editorial Assistance
George Kouvatseas of HEADS Ltd. provided assistance on the statistical analyses. Medical writing support was provided by Kinnari Patel, PhD, of Bioscript and funded by Astellas Pharma Inc.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole and have given their approval for this version to be published.
Author Contributions
All authors provided critical revision of the publication for intellectual content and meet the criteria for authorship. Author contributions included: Christina Papadaki, Konstantinos Anargyrou, Alexandros Spyridonidis, Ioannis Baltadakis, Helen A. Papadaki, Maria Angelopoulou, Vasiliki Pappa, George Vassilopoulos: investigation; Maria Kotsopoulou: investigation and supervision; Kleoniki Liakou: conceptualization, methodology, project administration and supervision; Manto Tzanetakou: conceptualization and methodology; Marina Moustaka: supervision and visualization.
Disclosures
Konstantinos Anargyrou received fees from Astellas Pharma Inc. for staff training outside the submitted work. Ioannis Baltadakis received fees from Astellas Pharma Inc. during the conduct of the study and fees and non-financial support outside of the submitted work. Maria Angelopoulou received fees from Gilead for an advisory board and fees from Janssen, Takeda, Genesis, AbbVie, Novartis and Roche for advisory boards and lectures. Kleoniki Liakou is an employee of Astellas Pharma Inc. Manto Tzanetakou is an employee of Astellas Pharma Inc. Marina Moustaka is an employee of Astellas Pharma Inc. Maria Kotsopoulou, Christina Papadaki, Alexandros Spyridonidis, Helen A. Papadaki, Vasiliki Pappa and George Vassilopoulos have nothing to disclose.
Compliance with Ethics Guidelines
The institutional review board or independent ethics committee of each participating hospital approved the study design (University General Hospital “Attikon,” Chaidari; 251 Airforce Hospital, Athens; General Hospital of Athens “Laikon,” Athens; CTHP “Metaxa,” Piraeus; University General Hospital of Heraklion, Heraklion; General Hospital of Athens “Euaggelismos,” Athens; University General Hospital of Larissa, Larissa; University General Hospital of Patras, Patra; General Hospital of Athens “G. Gennimatas,” Athens; General Hospital of Athens “Laikon,” Athens). Informed consent was obtained prior to patients’ participation in the study, according to the International Conference on Harmonisation (ICH) and good clinical practice and to the regulatory and legal requirements. The study was performed in accordance with the principles of the 1964 Declaration of Helsinki and its later amendments, ICH Guidelines and local ethical and legal requirements.
Data Availability
Access to anonymized individual participant level data will not be provided for this trial as it meets one or more of the exceptions described on https://www.clinicalstudydatarequest.com under “Sponsor Specific Details for Astellas.”
Open Access
This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Author information
Authors and Affiliations
Corresponding author
Additional information
Enhanced digital features
To view enhanced digital features for this article go to https://doi.org/10.6084/m9.figshare.7660652.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Kotsopoulou, M., Papadaki, C., Anargyrou, K. et al. Effectiveness and Safety of Micafungin in Managing Invasive Fungal Infections among Patients in Greece with Hematologic Disorders: The ASPIRE Study. Infect Dis Ther 8, 255–268 (2019). https://doi.org/10.1007/s40121-019-0236-3
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40121-019-0236-3