Abstract
Phosphorus (P) ore is an expensive and limited resource that will be depleted in a few decades if the current global consumption rate continues. Japan, one of the most developed countries in the world, relies completely on imported phosphate rock for P. Potassium (K) ore, which is equally important for continuous development, is also becoming increasingly expensive. The recovery of P and K is therefore important for continuous and sustainable development. In this study, concentrated P (obtained from eluent) and K (obtained through alkali leaching of rice straw charcoal) were recovered as potassium magnesium phosphate (PMP) through the controlled addition of magnesium (Mg). A PMP crystal was produced when an equimolar (with respect to P) Mg solution was added to the leaching solution (rich in P and K) at a pH range of 11–12. Thus, the production of PMP in a crystalline form demonstrates the huge scope for the recycling of limited resources.
Similar content being viewed by others
Introduction
The global human population is expanding at a rapid rate, requiring more food and resulting in increased demand for chemical fertilizers. The raw materials used to produce chemical fertilizers are not in danger of being depleted in the short term; however, because of population growth the raw materials required for fertilizer production will be consumed at a rapid rate. The three main elements of fertilizer are nitrogen (N), phosphorus (P) and potassium (K). K and P are obtained from underground resources and regional reserves that are unevenly distributed. Japan relies solely on imported raw materials for chemical fertilizer production, which results in high prices for chemical fertilizers in this country.
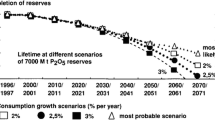
Potash ore, the raw material for potassium production, is unevenly distributed around the globe, with 53 % of total global potash reserves located in Canada (US Geological Survey 2012). The majority of potash produced in 2008 was from Canada (31 %), Russia (19 %) and Belarus (14 %). Based on current usage trends, potash reserves will last for nearly a century (Avnimelech et al. 1992). The majority of phosphate produced in 2008 was from unevenly distributed resources in Morocco (70 %), Iraq (8 %), China (5.2 %) and Algeria (3.0 %).
Depletion of phosphate rock is estimated to occur sooner than depletion of potash ore, based on the assumption that the amount of fertilizer used will increase by 3 % annually (Kuroda et al. 2005). With the geometric increase in populations throughout the world and the increased usage of these two resources by developing countries, current supplies of P and K will likely be exhausted in 2060 and 2120, respectively (Kuroda et al. 2005). It is imperative to develop a sustainable method for recycling these resources.
Since neither K nor P is produced in Japan, the development of such recycling technology would be very useful in the field of fertilizer production and overall social and industrial development in this country. Although the need for recycling of K is high, there have been few studies on potential methods of K recovery (Zhou et al. 2007). Zhou et al. (2007) recovered 44.3 % K from a dried stem–leaf sample of water hyacinth during the formation of KC4H5O6 by adding tartaric acid. Utilization of agricultural waste or plant biomass is necessary for a sustainable society. Biomass resources have been used as bio-fuels or as industrial raw materials in recent years. In the present study, we have developed an adsorbent using agricultural waste biomass. We have made a P adsorbent from orange juice residue. In this study, we examined the solidification of P as a potassium salt by adjusting the solubility. Rice straw charcoal was used to supply K. The potassium–magnesium complex salt of P was produced by the addition of magnesium and by adjusting the pH. This compound was easily solidified. The use of P and K together raises the prospect of producing potassium magnesium phosphate (PMP) if magnesium is also added (Eq. 1). Precipitated or crystallized PMP can be used as fertilizer and can positively benefit the agricultural sector (Xu et al. 2011).
The typical K content of rice straw is 1.7 % (Summer and Williams 2001). This amount of K can be used if it is recovered in an efficient and cost-effective way. K can be leached from rice straw using an alkaline solution, especially when the rice straw is in carbonized form. In our previous study, we found that a bio-adsorbent prepared from orange juice residue exhibited very high capacity to adsorb P. The residue-adsorbed P was successfully desorbed using NaOH solution (Biswas et al. 2008). Therefore, the alkaline solution (rich in P after the desorption process) could be easily used for leaching K from rice straw charcoal. From the above viewpoint we set our objective for this study, which was to prepare PMP by adding magnesium to P- and K-rich solution obtained from extraction of K from rice straw charcoal by the P-rich alkaline desorption liquid.
Methods
Analytical methods
The concentrations of phosphate, and ammonium were determined by the molybdenum blue ascorbic acid method and indophenol method using ultraviolet–visible spectrophotometry (Jasco, V-630BIO). The concentrations of potassium, magnesium and sodium ions were measured by a polarized Zeeman atomic absorption spectrophotometer (HITACHI Z-2000). Collected white precipitates were dissolved in 3.0 M HCl to calculate percentages of phosphorous, potassium, magnesium, and sodium. While scanning electron microscope is used to observe the surface morphology of the crystals. Characterization of the white precipitate was carried out by X-ray diffraction analysis equipment. The morphology and chemical composition of the dried crystals were determined using scanning electron microscope (SEM: JEOL, JCM-5100) and energy dispersive X-ray spectroscopy (EDX: HITACHI S 3000) apparatus at an emission voltage of 15 kV. All experiments were conducted twice and the samples were analyzed to ensure reproducibility of experimental data.
Materials
Sodium di-hydrogen phosphate (Cat. No. 32379-00), Magnesium chloride (Cat. No. 103) were obtained from Kanto Chemical Co., Inc. while, sodium hydroxide (No. 198-104 13765) was obtained from Wako Pure Chemical Industries, Ltd. All other chemicals and reagents were of analytical grade and used without any purification. Rice straw was obtained from a local farm and cut into pieces 2–3 cm long. Pieces were then air dried for 20 days and placed in a small crucible which was then placed in a large crucible. The space between the small and large crucible was filled with powdered graphite. The crucible was then heated at an initial temperature of 540 °C and the temperature was gradually increased over an 1 h period to 800 °C in an electric furnace. After cooling, the carbonized rice straw was crushed into particles of less than 150 μm in size. Figure 1 and Table 1 show the results of the XRD and EDX analyses. The adsorbent was made from immobilized Zr from unused bio-material using the method described by Biswas et al. (2008). This adsorbent was saturated in 1000 mg/L sodium di-hydrogen phosphate solution. The capacity of the saturated adsorbent was approximately 0.8–1.1 mol-P/kg.
Methods
Desorption of phosphorus
Batch-wise desorption tests were carried out using the adsorbent saturated with phosphorus. The pH of the desorption liquid was adjusted to between 11 and 13 using 1 M NaOH and 1 M HCl solution at a solid-to-liquid (S/L) ratio of 33. The solutions were then stirred for 24 h at 40 rpm. Beforehand the effect of solvent pH and that of S/L ratio (from 15 to 33) were examined. The concentration relationships between phosphorus, potassium and magnesium were adjusted to be greater than the solubility product of magnesium phosphate potassium. Simultaneous recovery of both phosphorus and the potassium was resulted to obtain a solid product. However, it was necessary to clarify the elute concentrations under various conditions for obtaining a precipitate.
Therefore, by changing the amount of adsorbent in the solvent, it was possible to determine the concentration. In order to achieve a leaching at a lower pH, desorption operation was conducted again under the same condition using the same adsorbent.
Behavior of the potassium extraction
To determine the behavior of potassium extraction from rice straw charcoal, batch-wise extraction tests were carried out at varying pH and stirring time at a specified S/L ratio. The pH of the extracted potassium solution was adjusted to between 11 and 13 using 1 M NaOH and 1 M HCl. The solvent and rice straw charcoal solution which had S/L ratio of 80 was stirred for 1 h at 40 rpm. The elution test was carried out at a specific S/L ratio and at varying time (from 5 to 90 min) to determine the effect of elution time.
Solidification
During potassium extraction from the rice straw charcoal using phosphorous desorption liquid after separation of the desorption liquid, potassium was extracted by mixing this solution at an 25 S/L. The whole solution was stirred at 40 rpm for 24 h. The suspensions were filtered through filter paper type 5C (ADVANTEC Co.) and the equilibrium concentration was measured. After that different amount of 100 mM magnesium chloride solution was added to fixed amount of obtained solution (rich in P and K). This was performed to determine optimum molar ratio of magnesium added. However, the subtle change in phosphorus concentration due to the increase in volume can be ignore. The PMP precipitates along with rice straw charcoal was washed with deionized water, dried in oven at 60 °C for 24 h and finally analyzed by EDX. The concentration of P was measured using ion-chromatography (Mettler Toledo, Japan) and the concentrations of magnesium, potassium and sodium were determined using an atomic absorption spectrophotometer.
Results and discussion
Desorption of P with NaOH
In our previous study (Biswas et al. 2008), high P desorption rates from zirconium-immobilized orange waste residue were observed when a 0.2 M NaOH solution was used as the desorption eluent. The adsorption and desorption reactions resulted from the exchange between the hydroxyl groups and different phosphate species on the coordinated site of the Zr. If desorption of P was conducted using a high concentration NaOH solution, evaporation of the solvent would be required to solidify tri-sodium phosphate. Moreover, using a relatively low concentration of NaOH solution, KMP precipitate can be easily obtained because of its low solubility product (Taylor et al. 1963). In this study, 0.05–0.1 M NaOH solutions were used to avoid the effect of sodium in the formation of the KMP precipitate/crystal. The pH of the desorption liquid was adjusted in the range of 12.5–13 when mixed with the absorbent.
The changes in the desorption rate at varying S/L ratios (from 15 to 33) are shown in Fig. 2. Desorption increased because of the repetitive operation at every S/L ratio tested. Repetitive operations applied of the new alkali solution were conducted after separation of adsorbent. However, it was found that total desorption increased slightly with the decrease in the S/L ratio. This desorption rate corresponded to the range of 250–300 mg/L of P. Table 2 shows the saturation adsorption capacity (for P) of various adsorbents. From this table it is evident that the saturated adsorption capacity of the adsorbent of this study is higher than many other adsorbents used in other studies.
K extraction from rice straw charcoal
Figure 3 shows the relationship between the K concentrations extracted from different rice straw charcoal prepared at different carbonization temperatures. The concentration of extracted K was lower at relatively low carbonization temperature. The highest concentration of K was obtained from the rice straw charcoal prepared at a carbonization temperature of 540 °C. However, at carbonization temperatures higher than 500 °C, KCl was found to occur in rice straw charcoal. Figure 4 depicts changes in the extraction of K from rice straw charcoal over time. This figure also shows that about 20 min is required to reach equilibrium. After this time no significant increase in extraction efficiency is observed.
The change in K extraction from rice straw charcoal with changing pH has been illustrated in Fig. 5. The amount of extracted K from rice straw charcoal was found to be higher at lower pH (pH <3) whereas the extraction was almost constant at 60–70 % at pH between 3 and 13. The pH was found to increase after equilibrium, which might be attributed to basic substances eluted from the rice straw charcoal. In addition, a KMP precipitate was produced when the pH was 10.5–12. The results indicated that K extraction from rice straw charcoal is possible using the alkaline desorption liquid containing concentrated P. Figure 6 shows the effects of the S/L ratio on the K extraction rate. The K extraction rate was maintained at 60–70 % at different L/S ratios and at initial pH values between 10 and 13. This low K extraction efficiency can be attributed to the fact that it is difficult to dissolve KCl, which was formed during high temperature carbonization. The K concentrations were 2000 and 1200 mg/L for S/L ratios of 25 and 12.5, respectively.
Solidification of P and K
Analyses of the precipitate were conducted by energy dispersive X-ray spectrometry and by measurement of P, K, Na and Mg in the liquid. Table 3 shows the concentrations of P, K, Na and Mg in the solution while Table 4 shows the relative amounts of each of the elements. The rate of P extraction was greater than 90 % (Table 3). This high rate of P extraction indicated that the P concentration in the desorption liquid was appropriate for use in the solidification process. In addition, 90 % of the added Mg was removed. The K concentration in the treatment liquid remained high, which might require other methods of recycling. The ratio of K–Mg–P in the PMP salt was almost equimolar.
Conclusion
In this study, the K component of rice straw charcoal in agricultural waste was used for the solidification of P in an alkaline desorption solution. The P adsorbent was made from orange juice production residues, a previously unused waste biomass. Therefore, the present study offers the potential for recovery of fertilizer resources from biomass-based agricultural waste. This study recommended the formation of a P complex solid salt containing K and Mg. Using agricultural waste the fertilizer components could be recovered, which contributes to the development of recycling processes.
Potassium magnesium phosphate (PMP) was obtained from recovered P and K along with Mg in bench-scale experiments. In this study, a solution of 0.1 M NaOH (pH 13) was used for P desorption from the orange waste residue adsorbent. This ensured that: (1) concentrated P was obtained and (2) the adsorbent could be used repeatedly for adsorption of P from solution. This study demonstrated that using this method, P desorption rates of 70–80 % were obtained, which was sufficient to achieve the desired concentration of P. The study also revealed that a higher concentration of K was extracted from rice straw charcoal at lower pH values, while the extraction efficiency ranged between 60 and 70 % at pH values ranging from 3 to 13. The result indicated that PMP precipitation is an efficient method for yielding a multinutrient product through simultaneous removal of K and P.
References
Avnimelech Y, Bazelet M, Weber B (1992) Replacement of sodium in home and industrial uses by potassium: importance and possibilities. Potassium Ecosyst Biochem Flux Cation Agro For Syst: 377–382
Biswas BK, Inoue K, Ghimire KN, Harada H, Ohto K, Kawakita H (2008) Removal and recovery of phosphorus from water by means of adsorption onto orange waste gel loaded with zirconium. Bioresour Technol 99:8685–8690
Biswas BK, Inoue K, Harada H, Ohto K, Kawakita H (2009) Leaching of phosphorus from incinerated sewage sludge ash by means of acid extraction followed by adsorption on orange waste gel. Environ Sci 21:1753–1760
Kuroda A, Noboru T, Kato J, Ohtake H (2005) Development of technologies to save phosphorus resources in response to phosphate crisis. J Environ Biotechnol 4:87–94
Kuzawa K, Jung YJ, Kiso Y, Yamada T, Nagai M, Lee TG (2006) Phosphate removal and recovery with a synthetic hydrotalcite as an adsorbent. Chemosphere 62:45–52
Li H, Ru J, Yin W, Liu X, Wang J, Zhang W (2009) Removal of phosphate from polluted water by lanthanum doped vesuvianite. J Hazard Mater 168:326–330
Loblich KR (1985) US Patent No. US4496526A
Lŭ J, Liu H, Zhao X, Sun L, Jiuhui Q (2013) Adsorptive removal of phosphate by a nanostructured Fe–Al–Mn trimetal oxide adsorbent. Powder Technol 233:146–154
Summer MD, Williams J (2001) Proceeding: rice straw management update. UCCE, Yuba
Taylor AW, Gurney EL, Frazier AW (1963) Solubility products of magnesium ammonium and magnesium potassium phosphates. T Faraday Soc 59:1580–1584
US Geological Survey (2012) Mineral Commodity Summaries: 118–122
Xiong JB, Mahmood Q (2010) Adsorptive removal of phosphate from aqueous media by peat. Desalination 259(1–3):59–64
Xu K, Wang C, Wang X, Qian Y (2011) Simultaneous removal of phosphorus and potassium from synthetic urine through the recipitation of magnesium potassium phosphate hexahydrate. Chemosphere 84:207–212
Xu K, Wang C, Wang X, Qian Y (2012) Laboratory experiments on simultaneous removal of K and P from synthetic and real urine for nutrient recycle by crystallization of magnesium–potassium–phosphate–hexahydrate in a draft tube and baffle reactor. Chemosphere 88(2):219–223
Zhou W, Zhu Duanwei, Tan Liangfeng (2007) Extraction and retrieval of potassium from water hyacinth, (Eichhornia crassipes). Bioresour Technol 98:226–231
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Koutarou, A., Seigo, U., Harada, H. et al. Simultaneous solidification of potassium and phosphorus using rice straw charcoal and saturated phosphorus adsorbent. Int J Recycl Org Waste Agricult 4, 67–72 (2015). https://doi.org/10.1007/s40093-015-0086-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40093-015-0086-2