Abstract
This study was conducted to evaluate dietary protein and carbohydrate requirement of juvenile Hawaiian limpets Cellana sandwincensis. A total of 64 juvenile limpets (3.12 ± 0.86 g) were fed five different dietary protein levels ranging from 270 to 470 g kg−1 for 90 days. Carbohydrate and lipid levels were held constant at 180 and 49.7 g kg−1, respectively. Weight gain and growth rates of the animals did not differ significantly (P > 0.05) among the protein levels ranging from 270 g kg−1 (0.30 % day−1) to 470 g kg−1 (0.23 % day−1). Next, opihi were fed four diets with protein levels from 210 to 500 g kg−1 with a constant carbohydrate level at 120 g kg−1. Weight gain and specific growth rates of opihi increased with increasing dietary protein from 210 to 350 g kg−1, and significantly (P < 0.05) decreased at the 500 g kg−1 diet. Highest weight gain, growth rates, and protein efficiency ratio were achieved at 350 g kg−1. Elevated carbohydrate levels (180–370 g kg−1) produced a significant difference (P < 0.05) in growth. The fastest growth rates of animals were obtained with 270 g kg−1 (0.27 % day−1) and 320 g kg−1 (0.26 % day−1). The weight gain of animals fed 180 and 370 g kg−1 carbohydrate diets were significantly (P < 0.05) lower than those of animals fed 270 and 320 g kg−1. We conclude that about 350 g kg−1 protein and 320 g kg−1 carbohydrate levels could be used for opihi.
Similar content being viewed by others
Introduction
The Hawaiian limpet Cellana sandwicensis or yellow opihi is an economically important species with high potential for aquaculture in Hawaii. However, there are no practical aquaculture techniques for this species. Our previous study developed a feed which would sustain life and support a good growth rate for opihi (Hua and Ako 2012). We found that diet consisting of fishmeal, soybean meal, krill meal, and Porphyra was eaten at the highest rate and produced a good specific growth rate (0.44 % day−1).
Protein is a base component for muscle growth and sufficient quantity must be supplied to promote growth. No previous studies have been done on the protein requirements of opihi. Abalone was considered as reference for opihi species because abalone is an herbivorous marine gastropod, so we are comfortable with a working hypothesis that opihi and abalone diets should have a degree of similarity. There have been many studies of dietary protein requirements for abalone. Various studies reported different dietary protein requirements for optimal growth of abalone species (Britz 1996; Gomez-Montes et al. 2003; Mai et al. 1995; Coote et al. 2000; Sales et al. 2003). The differences in quantitative dietary protein level among different studies may have been due to the mismatch of the amino acid profiles of the diets with the amino acid profiles of the animal tissue.
Dietary carbohydrate could act as good energy sources for abalone H. kamtschatkana (Taylor 1997), sparing the amount of protein needed. Providing an adequate amount of lipid or carbohydrate in the diet would minimize the use of expensive protein (National Research National (NRC) 1993). Many studies have used lipid as an energy source and the optimal dietary protein to lipid ratio levels have been studied in fish (Satpathy et al. 2003; Alam et al. 2003), and abalone (Britz and Hecht 1997). For limpets, studies have showed that amylase activity was very high in the digestive system of the patellacean limpets including Patella vulgate, Cellana radiata, and Collisella pelta (Branch 1981). On the other hand, lipase activity has not been found in limpets, and studies have shown that lipases are very low in the digestive gland of abalone (Knauer et al. 1996; Fleming et al. 1996). Thongrod et al. (2003) used modified starch and fish oil to determine the optimum ratio of carbohydrate versus lipid levels for the growth of tropical abalone H. asinia. They found that the growth performance of abalone was relatively good when they were fed a diet containing a higher proportion of carbohydrate, up to 470 g kg−1, whereas higher lipid levels (>58.0 g kg−1) did not help abalone growth. The literature suggests that there is certainty in where abalones obtain their energy from. Study by Brizt confirmed that adjusting lipids did not affect growth rate. Carbohydrates are inexpensive and could minimize the cost of opihi feed. Thus, carbohydrate was considered as the second nutrient after dietary protein to be worth pursuing for opihi.
In the present study, the growth performance of opihi was examined using a practical diet at varying protein and carbohydrate levels using the best performance diet including of fishmeal, soy meal, krill meal, and Porphyra.
Materials and methods
Experimental diets
Formulations of dietary protein and carbohydrate levels are shown in Table 1. Two protein feeding trials were conducted. For the preliminary protein trial, five different dietary protein levels of 270, 320, 370, 420, and 470 g kg−1 were tested for 90 days. A package of protein ingredients of fish meal, defatted soybean, and krill meal, including Porphyra was used as a base diet because these ingredients were found to have the best performance in both palatability and supporting good growth of opihi (Hua and Ako 2012). Wheat flour was used as starch and diatomacetous earth was used as filler in the diets as protein weight was decreased. Based on the results of this trial and due to unexpected issue of low survival rate due to removal for weighing, the second protein feeding trial was repeated with four different formulated dietary protein levels consisting of 210, 300, 350, and 500 g kg−1.
For carbohydrate trial, a constant protein level of 270 g kg−1 protein with low lipid level of 35.0 g kg−1 was employed. Four different dietary carbohydrate levels of 180, 270, 320, and 370 g kg−1 were formulated and tested for 90 days. Wheat flour was used as carbohydrate source and diatomacetous earth was used to fill out in the diets as the carbohydrate level was decreased. Protocols for diet preparation in this study were done as described in the previous study (Hua and Ako 2012).
Experimental animals
Animals (3.12 ± 0.86 g) used for this study were collected from remote shoreline areas in Oahu Island, Hawaii, USA. After collection, the animals were immediately transferred to the laboratory and placed in aquaria with plastic liners, with algae seeded with benthic diatoms from Hawaii Institute of Marine Biology. An aquarium biofilter system (586 L h−1) was used to simulate water movement. They were held for about 3 days of acclimation. Thereafter, nine opihi were selected for each experimental group and their initial weight and shell length were measured. For the preliminary trial, each individual was placed into its own plastic colander (20 cm diameter). The colanders were put in tubs, nine colanders per tub. Animals that died in the first 3 days after placement into the test system were replaced. We observed a few more mortalities due to removing the animals from the colanders for weighing. Therefore, softer containers made of soft screen polyethylene plastic, cone shaped, 13 cm diameter, 17 cm height, were used in the second protein trial (Fig. 1). The experiment was done in recirculating system at a flow rate of 3 L min−1. Water temperature ranged from 24 to 26 °C and salinity was maintained at 35 mg L−1.
Feeding regime and feeding evaluation were as in our previous description (Hua and Ako 2012). The animals were fed one time per day at feeding rate about 0.73 ± 0.53 %, at 18:00 daily, as animals did not eat during the day. It was noticed that the animals normally did not eat well for the first few days after putting them into the test system, therefore feeding rate of animals for 3 days after stocking and 3 days after replacement were not counted in our calculations. With this taken into consideration, as animals were conditioned to the artificial feed used in the feed trials, the following data were calculated:
Sampling and analytical methods
The growth of animals in weight (g) and shell length (cm) was measured monthly. The growth was expressed in terms of specific growth rate (SGR), weight gain, and shell length increase. The shell length was measured with an electronic digital caliper (0.01 mm) and weight determined with an electronic scale (0.01 g error) every 4 weeks
where Wf is final weight, Wi is initial weight, and T is total day of the experiment.
All diets of protein trial 1 were subjected to proximate analysis (AOAC 2000) at the Aquatic Feed and Nutrition Laboratory, Oceanic Institute, Hawaii, USA. Two replicates were done for each diet.
Because of our hypothesis that previous studies had showed wide spreads in optimal protein levels due to inappropriate amino acid balance for abalone; the amino acid profiles of opihi tissue were determined to have an optimal balance in our trials. The amino acid profiles of opihi tissue and of the dietary protein in trial 1 were determined by Dr. Ju at the Aquatic Feed and Nutrition Laboratory, Oceanic Institute, Hawaii, USA, according to the described method of Ju et al. (2008). The results are presented as A/E ratio (Table 2). Most of the essential amino acids of diets were identical and/or close to the amino acid profile of opihi tissue except for Arg and Thr which were lower in the experimental diets compared to tissue.
Solid leaching of diets
Weight matter leaching was examined for the second dietary protein trial. The experiment was conducted in an experimental test system. Three pieces of each feed (1.0 cm2/piece) served as for replication, three replications per diet. They were weighed to obtain the initial weights of the three replications, then placed in an empty colander without opihi for 12 h. Stability of feed was assessed according to the equation: Water absorbed (%) = {(FW−IW)/IW} × 100. Where IW is initial weight of feed, and FW is the final weight of feed of the same diet after being immersed in water. The retained feed was rinsed with fresh water and transferred to a drying oven for 12 h at 100 °C to obtain dry weight. To obtain the initial dry weight of each diet, three other pieces of each diet were weighed and dried in the oven, this dry weight served as initial dried weight control. This data were used to determine leaching losses due to soaking.
Data analysis
Collected data were subjected to one-way ANOVA, followed by Tukey test to compare the weight gain (g), specific growth rate (% body weight/day), feed consumption rate (% dry matter/body weight/day), and feed conversion ratio (dry feed eaten/weight gain) using SPSS (version 11.5; SPSS Inc., Chicago. IL, USA). The data on weight gain (%) were subjected to the quadratic regression analysis model (Mai et al. 1995; Sales et al. 2003) and the optimal protein requirement of opihi was estimated.
Results
Survival
A low survival rate in the first protein trial could affect the strength of results. Mortality (25–50 %) was due in part to handling during weighing when the animals were removed from the colander, and was not affected by dietary protein. They died after weighing, we assume, due to physical injury and/or stress. A higher survival rate was obtained in the second protein trial using the softer containers, ranging from 89 to 100 %.
Growth
Growth performance of opihi fed with different dietary protein levels is shown in Table 3. For the first protein trial, statistical analysis by One-way ANOVA followed by Tukey–Kramer showed that there was no significant (P > 0.05) effect of various protein levels (270–470 g kg−1) on the weight gain and the specific growth rates of animals, although this dropped off insignificantly at higher protein levels from 420 to 470 g kg−1.
There was a significant (P < 0.05) difference in weight gain and specific growth rates of animals in the second dietary protein trial over 60 days; possibly due to better methods and more practice. Weight gain-tracked specific growth rates were progressively increased to maximum at 350 g kg−1 protein diet, and significantly higher than those animals fed with 500 g kg−1, but did not differ significantly compared to the 210 and 300 g kg−1 diets.
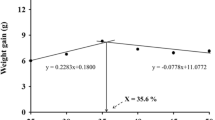
The growth response of opihi in terms of weight gain (%) of animals in dietary protein trial 2 was fitted into quadratic models (Fig. 2). The best fit for the estimation of optimal protein level could be described as Y = −0.0003x 2 + 0.234x−21.8 (R 2 = 0.96). The trend of growth showed that maximum weight gain appeared to be about 350 g kg−1 dietary protein.
There was a significant difference in feed consumption of animals. Feed consumption for animals that were fed with 500 g kg−1 dietary protein was significantly lower (P < 0.05) than those animals that were fed with dietary protein of 210, 300, and 350 g kg−1 in the second protein trial. The highest feed efficiency ratio tracked the weight gain of those animals that were fed with the 350 g kg−1 dietary protein and was significantly higher than 500 g kg−1 diet.
The percentage of solids remaining among the diets was 98.0 ± 1.13, 96.8 ± 1.39, 97.8 ± 1.170, and 98.5 ± 0.32 % for 210, 300, 350, and 500 g kg−1 of dietary protein, respectively. Statistical analysis for solid leaching rates showed that there was no significant difference (P > 0.05) in percentage of remaining dry weight between the dietary protein levels in trial 2.
Carbohydrate requirements
Growth performances of opihi that were fed at various carbohydrate levels are shown in Table 4. Statistical analysis by One-way ANOVA showed that there was a significant (P < 0.05) difference in weight gain and the specific growth rates of opihi among the diets, then a Tukey test showed that the weight gain of animals on the 180 and 370 g kg−1 diets was significantly (P < 0.05) lower than animals fed 270 and 320 g kg−1 carbohydrate. However, there was no significant difference in specific growth rate of those animals that were fed with diets between the 180 and 270 and 320 g kg−1 carbohydrate diets. The mean FCR values show that opihi are efficient converters of feed to body weight. Survival was about 90–100 % for all diets after 2 months, however, the survival rates were 67–78 % after 3 months. The mortality was due handling stress from the second weighing, when we removed them from the colander walls.
Response of opihi in weight gain to dietary carbohydrate levels was then fitted to quadratic models (Fig. 3). It shows that the weight gains of opihi progressively increased and reached their maximum value at a carbohydrate level of about 270 g kg−1, which could probably be described as Y = −0.0012x 2 + 0.64x−56.7 with the correlation value of R 2 = 0.91.
Statistical analysis for solid leach rates showed that there was no significant difference (P > 0.05) in percentage of remaining dry weight between the two carbohydrate diets. There was about 95.2 ± 1.90 and 98.3 ± 0.20 % remaining dry matter for the dietary carbohydrate levels of 180 and 320 g kg−1, respectively, after they were immersed for 12 h. The results suggest that dry matter loss was not a major issue among the different carbohydrate levels.
Discussion
Determination of optimum dietary protein level which produced the maximum growth of opihi was our first goal in this nutritional study. Our previous study showed that a diet containing fishmeal, soymeal with krill meal, and Porphyra was preferentially consumed and sustained opihi life well (Hua and Ako 2012). This diet was then used as a base diet in the present study and the results support that these dietary protein ingredients would be a practical-formulated feed for the aquaculture of opihi.
The high mortality rate in our first trial raised questions about the statistical strength of our findings about the optimum protein requirement for opihi in that trial. At this stage, it is believed that all the handling and nutritional variables must be taken care of and there should have been only a few mortalities. However, in these longer term experiments 25–50 % total mortalities occurring after animals were removed from the colanders for weighing. Another kind of soft container (Fig. 1) was designed to overcome the colander problems. Animals were removed easily for weighing during the course of experiment by deforming the soft plastic screen. This method improved survival rates significantly to 89–100 %, improving the statistical strength of the results of our second trial.
The results of this study showed that the growth of opihi did not correlate to increased dietary protein levels. Statistical analysis by One-way ANOVA showed that there was no significant (P > 0.05) difference in weight gain and SGR of opihi among the diets from 270 to 470 g kg−1 protein. As stated above, no statistically significant difference in growth rates among the diets may be due to a low survival of animals. Therefore, it is premature to draw any conclusion from the Trial 1 and the data may be use as a preliminary for following trial. Better and significant data were obtained in Trial 2 (Table 3; Fig. 2). The highest weight gain was achieved with the 350 g kg−1 protein diet, but there was no significant difference in weight gain with those animals fed with 210 and 300 g kg−1 diets. This indicated that opihi could satisfy their needs with a low protein level above 210 g kg−1. The trend of the growth rate suggests that when the protein level is too high it may be detrimental to growth. In fact, the results from the 500 g kg−1 protein group in the second trial showed poor growth, significantly lower than the other diets, indicating that excess protein is not easily metabolized by opihi.
The second-order polynomial regression model is a way graphical model which allows us to choose an optimal protein level among statistically insignificant differences. In our present study, the 350 g kg−1 is the choice by polynomial analysis although it is not statically different from 210 and 300 g kg−1 diets (Fig. 2). The pattern of protein requirement for opihi in our study was similar to other studies for abalone species. Mai et al. (1995) also found that the optimal protein level for maximum growth of abalone H. discus hannai was about 356 g kg−1 dietary protein level, while statistical analysis revealed there was no significant difference in weight gain of abalone that were fed dietary protein from 202 to 353 g kg−1 and about 323 g kg−1 for H. tuberculata (within 202–400 g kg−1). Another study by Sales et al. (2003) stated that the 360 g kg−1 protein produced the best growth; however, no statically significant difference occurred between protein levels ranging from 290 to 480 g kg−1.
The specific growth rate of opihi ranged from 0.13 to 0.28 % BW/day for dietary protein trial 2. These growth rates are close to the growth rate (approximately 0.2–0.4 % BW/day) for abalone H. discus hannai found by Uki et al. (1985), but lower compared to the growth rate (0.94–1.11 % BW/day) of H. midae reported by Britz (1996). However, may not be valid to compare these values directly between opihi and abalone or even among the abalone species because the apparent requirement may vary due to many different conditions such as difference in diets, experimental species, digestible protein, feed intake, and the management of feeding regimes.
Feed consumption rate seemed to be responsible for growth rate. We have earlier found that feed consumption was correlated with the growth response of opihi (Hua and Ako 2012). Matching the amino acid profile of diets and animal tissue is an important issue, it was believed that it is not sufficient to support optimum growth, because the growth of animals involves many factors including feed intake. In the present study, feed intake tended to be positively related to the growth rate of opihi. The lower feed consumption of animals that were fed 500 g kg−1 corresponded to the decrease in the weight gain of animals. Overall, the feed consumption rates were low as compared to our previous study. On the other hand, a lower feed consumption rate in the second trial may be due to a lower carbohydrate level.
The results of our present study demonstrated that various carbohydrate levels in the diet affected growth performances of opihi. Statistical analysis showed that there was a significant (P < 0.05) difference in both weight gain and SGR of opihi between the diets (Table 4). There was no significant difference between the weight gain of animal that fed with 270 and 320 g kg−1 diets which indicates that the opihi could satisfy their need for carbohydrate with either of the two diets. Polynomial analysis suggests that 270 g kg−1 carbohydrate diet would be an optimal level. On the other hand, the cost of feed would probably be higher at the lower carbohydrate levels. Carbohydrate is an inexpensive ingredient. It is noted that non-nutrient ingredients being used as a filter in diet are more expensive than carbohydrate sources. Therefore, the 320 g kg−1 carbohydrate could be used as a final level for commercial opihi feed.
A lower carbohydrate level (180 g kg−1) may not be sufficient for adequate energy utilization by opihi and part of the energy needed may be catabolized from protein, therefore it may reduce the optimal use of dietary protein for somatic growth. Livingstone and de Zwaan (1983) reported that when insufficient supply of carbohydrate in diet, the animal can be readily synthesized via the catabolism of proteins and lipids. It is unclear why a significantly lower growth rate was seen in animals fed 370 g kg−1 carbohydrate. For abalone, literatures have suggested that the abalone would be able to utilize various carbohydrate sources, for example; dextrin (Uki and Watanabe 1989), mixtures of dextrin, wheat flour, sucrose and potato starch (Lee et al. 1998), modified starch (Thongrod et al. 2003), and wheat flour and alginate (Cho et al. 2008; Cho 2010), but provide no quantitative comparisons. It is probably due to high amylase contents in the digestive tract system of abalone (Fleming et al. 1996 and Knauer et al. 1996). Similarly, amylases activity was high in limpet species (Branch 1981). On the other hand, Britz and Hecht (1997) found that increasing the lipid levels of abalone feed decreased the growth rate of abalone. This result indicates a more efficient use of carbohydrates as compared to lipids as an energy source by abalone. This makes sense because abalones mostly eat seaweed and amylase activity is high in abalone (Fleming et al. 1996). Thus, the 320 g kg−1 carbohydrate diet would be an optimal level for good growth of opihi in our present study.
In conclusion, this is the first reported study on macronutrient requirements for growth of opihi using an practical artificial diet. The protein level of 350 and 320 g kg−1 carbohydrate could be used for generating a commercial feed for opihi if there are no other factors of concern rather than the feed cost.
References
Alam MdS, Teshima SI, Koshio S, Uyan O, Ishikawa M (2003) Effects of dietary protein and lipid levels on growth and body composition of juvenile Japanese flounder, Paralichthys olivaceus, fed diets containing intact proteins or crystalline amino acids. J Appl Aquac 14:115–131
AOAC international (2000) Official methods of analysis of the association of official analytical chemists, 17th edn. Association of Analytical Communities, Gaithersburg, MD, USA
Branch GM (1981) The biology of limpets: physical factors, energy flow, and ecological interactions. Oceanogr Mar Biol Ann Rev 19:235–380
Britz PJ (1996) Effect of dietary protein level on growth performance of South African abalone. Haliotis midae, fed fishmeal-based semi-purified diets. Aquaculture 140:55–61
Britz PJ, Hecht T (1997) Effect of dietary protein and energy level on growth and body composition of South African abalone Haliotis midae. Aquaculture 156:195–210
Cho SH (2010) Effect of fishmeal substitution with various animal and/or plant protein sources in the diet of the abalone Haliotis discus hannai Ino. Aquac Res 41:587–593
Cho SH, Park J, Yoo JH (2008) Effect of casein substitution with fishmeal, soybean meal and crustacean meal in the diet of the abalone Haliotis discus hannai Ino. Aquac Nutr 14:61–66
Coote TA, Hone PW, Van Barneveld RW, Maguire GT (2000) Optimal protein level in a semipurified diet for juvenile greenlip Haliotis laevigatu. Aquac Nutr 6:213–220
Fleming AE, Van Bameveld R, Hone PW (1996) The development of artificial diets for abalone: a review and future directions. Aquaculture 140:5–53
Gomez-Montes L, Garcia-Esquivel Z, D’Abramom LR, Shimada A, Vasquez-Pelaez C, Viana MT (2003) Effect of dietary protein:ratio on intake, growth and metabolism of juvenile green abalone Haliotis fulgens. Aquaculture 220:769–780
Hua TN, Ako H (2012) Enabling studies for aquaculture of the Hawaiian Opihi, the Limpet Cellana. World Aquac Soc 12:40–44
Ju ZY, Forster I, Conquest L, Dominy W, Kuo WC, Horgen FD (2008) Determination of microbial community structures of shrimp floc cultures by biomarkers and analysis of floc amino acid profiles. Aquac Res 39:118–134
Knauer J, Britz PJ, Hetcht T (1996) Comparative growth performance and digestive enzyme activity of juvenile South African abalone, Haliotis midae, fed on diatoms and a practical diet. Aquaculture 140:75–85
Lee SM, Yun SJ, Min KS, Yoo SK (1998) Evaluation of dietary carbohydrate sources for juvenile abalone Haliotis discus hannai. J Aquac 11:133–140
Livingstone DR, de Zwaan A (1983) Carbohydrate metabolism of gastropods. In: Hochachke PW (ed) The mollusca, Vol. 1. Metabolic biochemistry and molecular biomechanics. Academic, New York, pp 177–242
Mai K, Mercer JP, Donlon J (1995) Comparative studies on the nutrition of two species of abalone, Haliotis tuberculata L. and Haliotis discus hannai Ino. IV. Optimum dietary protein level for growth. Aquaculture 136:165–180
National Research Council (1993) Nutrient Requirements of Fish. The National Academy Press, Washington, D.C., pp 345–346
National Research Council (NRC) (1981) Nutrient Requirements of coldwater Fishes, No. 16. The National Academy Press, Washington, D.C
Sales J, Truter PJ, Birtz PJ (2003) Optimum dietary crude protein level for growth in South African abalone (Haliotis midae L.). Aquac Nutr 9:85–89
Satpathy BB, Mukherjee D, Ray AK (2003) effects of dietary and lipid levels on growth, feed conversion and body composition in rohu, Labeo rohita (Hamilton), fingerlings. Aquac Nutr 9:17–24
Taylor B (1997) Protein utilization, hormone treatment and nutrient metabolism as they apply to culture of Haliotis kamtschatkana. PhD thesis, the University of British Columbia, Canada
Thongrod S, Tamtina M, Chairatb C, Boonyaratpalin M (2003) Lipid to carbohydrate ratio in donkey’s ear abalone Haliotis asinina, Linne diets. Aquaculture 225:165–174
Uki N, Watanabe T (1989) Review of nutritional requirements of abalone (Haliotis spp.) and development of more efficient artificial diets. In: Shepherd SA, Tegner MJ, Guzmiin Del SA (eds) Abalone of the World. Fisheries, biology and culture. Fishing News Books, Oxford, pp 504–517
Uki N, Kemuyama A, Watanabe T (1985) Nutritional evaluation of several protein sources in diets for abalone Haliotis discus hannai. Bull Jpn Soc Sci Fish 51:1835–1839
Acknowledgments
This study was supported financially by the Center for Tropical and Subtropical Aquaculture, USA, Grant #@2009-210 and the Vietnam Education Foundation.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Hua, N.T., Ako, H. Dietary protein and carbohydrate requirement of juvenile Hawaiian limpet (Cellana sandwicensis Pease, 1861) fed practical diet. Int Aquat Res 8, 323–332 (2016). https://doi.org/10.1007/s40071-016-0145-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40071-016-0145-y