Abstract
Trace elements in otoliths of sea bass (Dicentrarchus labrax L.) and sea bream (Sparus aurata L.) from fish farms and coastal wild populations in the western Mediterranean Sea were analysed by inductively coupled plasma-mass spectrometry. Results showed that concentrations of Mg, K, and Mn differed significantly between wild and farmed sea bass, while concentrations of Mg, K, Mn, Fe, Zn, Sr, and Ba varied significantly between wild and farmed sea bream. Discriminate analysis and cross-validation classification showed that the trace element profile in otoliths can be used to separate farmed fish from wild stocks with high accuracy on both sea bass (individuals correctly classified: 90.7 %) and sea bream (individuals correctly classified: 96.6 %). Moreover, trace elements in otoliths resulted to be useful to discriminate among wild fish stocks within each species.
Similar content being viewed by others
Introduction
Mediterranean aquaculture has risen during the last decades mainly producing European sea bass Dicentrarchus labrax L. (176,970 t in 2015) and gilthead sea bream Sparus aurata L. (181,442 t in 2015) in off-shore fish farms (FAO 2016). Both species are also of high commercial interest for coastal Mediterranean fisheries (FAO 2014). However, the rapid expansion of aquaculture and the problems concerning escape events, have led to environmental problems on coastal ecosystems (Dempster et al. 2007). Escaped sea bass and sea bream disperse from farms, potentially mixing with local stocks and leading to potential negative genetic and ecological consequences through interbreeding, predation, competition for food or habitat, and the transmission of pathogens to native populations (Arechavala-Lopez et al. 2011, 2012b, 2013b, 2014; Šegvić-Bubić et al. 2014). However, conflicts with local fishermen and fish markets have also arisen, regarding escape events at farms. For instance, if the number of escapees is high, they may bias estimates of wild populations if not accounted for (Fiske et al. 2005), but also fisheries landings may be unbalanced, decreasing the captures and incomes from target species (Dimitriou et al. 2007; Toledo-Guedes et al. 2014a, b; Arechavala-Lopez et al. 2015). Furthermore, frauds mislabelling the farmed fish occasionally occur at markets, because of the price premium commanded by wild fish, affecting the guarantee of fish quality for the consumer (Bell et al. 2007; Morrison et al. 2007). Therefore, there is a necessity to develop verifiable and accurate methods to distinguish farmed from wild fish, which will help to increase the existing knowledge about the potential environmental and economic problems of escapees.
In the Mediterranean, diverse studies on sea bass and sea bream have quantified differences among wild and farmed fish through genetic analysis, chemical and molecular compositions, morphology, and organoleptic characteristics (Arechavala-Lopez et al. 2013a, and references therein). However, a wide range of applications, accuracies, time-consuming, and economic costs were showed among these techniques. Based on the assumption that farmed fish is grown in a different environment, stocking densities, and feeding regimes compared with wild fish, method based on the features of otoliths is regarded as very successful for stock discrimination (Campana and Neilson 1985; Campana et al. 2000). Otoliths are considered metabolically inert and grow throughout the life of the fish. Due to the trace element, concentrations in otoliths are a function of physiology and environment, they have been used as an indicator of fish populations inhabiting different environments or stock identity (e.g. Geffen et al. 1998; Thorrold et al. 1998; Campana et al. 2000; Gillanders and Kingsford 2000; Sanchez-Jerez et al. 2002; Rooker et al. 2003). Specifically, some studies have successfully attempted to discriminate through trace elements in otoliths, the wild and farm origin of Atlantic salmon Salmo salar (Veinott and Porter 2005; Perrier et al. 2011), trout Salmo trutta and Oncorhyncus mykiss (Zitek et al. 2010), and yellowtail kingfish Seriola lalandi (Gillanders and Joyce 2005). In addition, other studies used the differences on morphology and shape contours of the otoliths to successfully distinguish the wild or farm origin on Atlantic salmon (Hindar and L’Abéelund 1992), cod Gadus morhua (Higgins et al. 2010), but also on sea bass and sea bream (Arechavala-Lopez et al. 2012a). However, there is a lack of studies on the latter Mediterranean fish species, regarding the trace elements profiles in otoliths. Therefore, the aim of this study was to develop otolith trace elemental signatures of sea bass and sea bream to discriminate between wild and farmed stocks in the Mediterranean.
Materials and methods
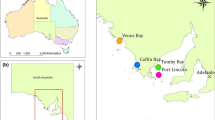
Fish sampling was carried out in the province of Alicante, southeast coast of Spain, and western Mediterranean Sea. A total of 30 sea bass (standard length, SL: 40.73 ± 0.81 cm) and 30 sea bream (SL: 35.43 ± 0.21 cm) were sampled from two neighbouring open-sea fish farms (F1 and F2), while 24 sea bass (SL: 51.81 ± 1.87 cm) and 29 sea bream (SL: 44.67 ± 1.65 cm) were sampled from local fish markets (i.e. Santa Pola and Guardamar del Segura; W1 and W2 respectively) (Fig. 1). The study area supports important fish farming and fisheries activities (trawl and artisanal fleets) of highly social and economic importance (García-Rodriguez et al. 2006). After fish sampling, both sagittal otoliths were collected through dissection using a stainless knife and disposable wooden chopsticks which were previously rinsed with ethanol 96°. The rest of the laboratory equipment was previously cleaned by soaking for 4 h with a chelating agent (EDTA 0.5 %) to prevent possible metal contaminations, and then moved to an acid wash in super-pure HNO3 (10 %) for 24 h. Later, the equipment was carefully rinsed with 18 ohm reverse osmosis water and left to soak in sterilized conditions between sampled individuals. The collected otoliths were rinsed with 18 ohm reverse osmosis water to rehydrate the otolith covering matter for 24 h and weighted. Afterwards, each otolith was dipped in H2O2 (3 %) for 5 min, then in HNO3 (1 %) for 20 s, and, finally, thoroughly rinsed in 18 ohm reverse osmosis water. The otoliths were then left to dry completely and stored in 10 mL test tubes. Otoliths digestion was achieve by submerging them in 2 mL ultra-pure HNO3 (10 %) and left overnight. Then, 8 mL of 18 ohm reverse osmosis water was added to each tube to raise the volume to 10 mL in each tube and drop the acid concentration to 2 % in every sample. Collecting and/or handling effects are likely to be minimal for all elements in our study based on similar treatment of fish after capture, chemical similarity, and the sterilized conditions.
Inductively coupled plasma-mass spectrometry (ICP-MS) was applied for trace elements determination on digested otoliths, which is the most commonly utilized technique for the otolith microchemical analysis. ICP-MS is a routine method widely used to analysed trace elements in otoliths of many fish species, which allows simultaneous determination of most elements within the periodic table with limits of detection below one part per billion (e.g. Campana et al. 1994; Campana and Gagne 1995; Campana 1999; Thresher 1999; Campana and Thorrold 2001; Sturgeon et al. 2005). Each sample was then analysed in triplicate, obtaining a mean value and a standard deviation per element and sample, which were standardized with the specific otolith mass (Campana et al. 2007). Mean dried otolith weights (±SD) of wild and farmed sea bass were 0.067 ± 0.019 and 0.056 ± 0.028 g, respectively. Mean dried otolith weights (±SD) of wild and farmed sea bream were 0.065 ± 0.023 and 0.051 ± 0.021 g, respectively. The following element isotopes were analysed: lithium (7Li), beryllium (9Be), boron (11B), sodium (23Na), magnesium (24Mg), aluminium (27Al), phosphorous (31P), potassium (39K), scandium (45Sc), vanadium (51V), manganese (55Mn), iron (56Fe), cobalt (59Co), nickel (60Ni), copper (65Cu), zinc (66Zn), strontium (88Sr), palladium (105Pd), silver (107Ag), tin (116Sn, 118Sn), barium (137Ba), rhenium (185Re), mercury (202Hg), titanium (205Ti), and lead (208Pb). These trace elements were quantified on the basis of peak areas and comparison with a calibrated curve obtained with the corresponding standards. Only those elements that were above the limit of detection of the ICP-MS in all or most (>90 %) of the samples were considered suitable for statistical analysis. Then, concentrations of 7Li, 9Be, 121Sb, 185Re, 202Hg, and 205Ti were below the limit of detection of the ICP-MS for both fish species. Concentrations of 11B, 23Na, 31P, and 52Cr were not detected in more than 10 % of the samples both in sea bass and sea bream. Moreover, the standard reference material was found inaccurate for 11B, 23Na, 45Sc, 51V, 105Pd, and 116,118Sn, and therefore, those elements were discarded too. Then, a total of 13 trace elements were finally selected for further analysis: 24Mg, 27Al, 39K, 55Mn, 56Fe, 59Co, 60Ni, 65Cu, 66Zn, 88Sr, 107Ag, 137Ba, and 208Pb. Non-parametric Mann–Whitney U test was used to test the variations in each trace element from fish otoliths (dependent variables), regarding the wild or farm origin of studied fish species (independent factors). Canonical discriminant function analysis and cross-validation classification were used to determine the efficacy of the trace elements composition in otoliths to discriminate between wild and farmed fish groups. Analyses of variances were carried out using the statistical software IBM SPSS Statistics 20 and discriminant analyses were carried out using the statistical software Canoco 4.5.
Results
Concentrations of some trace elements differed significantly between wild and farmed individuals (Table 1). Non-parametric analysis (Mann–Whitney U test) determined that concentrations of 24Mg (Z = −4.613, p = 0.001), 39K (Z = −5.988, p = 0.001) and 55Mn (Z = −5.971, p = 0.001) in otoliths differed significantly between wild and farmed sea bass (Fig. 2; Table 1). Concentrations of 24Mg (Z = −3.154, p = 0.002), 27Al (Z = −2.175, p = 0.030), 39K (Z = −3.093, p = 0.002), 55Mn (Z = −3.305, p = 0.001), 56Fe (Z = −2.723, p = 0.006), 66Zn (Z = −4.109, p = 0.001), 88Sr (Z = −4.215, p = 0.001), and 137Ba (Z = −3.972, p = 0.001) were significantly different between wild and farmed sea bream otoliths (Fig. 3; Table 1). The rest of the trace elements analysed did not showed any significant difference (p < 0.05) between fish origins for both fish species (Figs. 2, 3; Table 1). Considering the 13 selected trace elements, canonical discriminant analysis for sea bass otoliths showed that the 87.9 % of total variance among sampling groups was explained by two main axes (Fig. 4a). Among the elements in sea bass otoliths, concentrations of 55Mn, 39K, and 137Ba were the most contributors for discrimination between wild and farmed sea bass individuals (Fig. 4a). Concentrations of 60Ni, 56Fe, 65Cu, 66Zn, 59Co, 27Al, and 107Ag were the most contributors on variations between wild sea bass sampled stocks, but no variations were found between farmed stocks (Fig. 4a). Cross-validation classification regarding trace elements profiles in sea bass otoliths showed that the 90.7 % of sampled individuals were correctly classified according to their wild or farm origin, while only the 61.1 % were correctly classified within sea bass sampling groups (Table 2). For sea bream otoliths, canonical discriminant analysis showed that the 73.5 % of total variance among sampling groups was explained by two main axes (Fig. 4b). Among the elements in sea bream otoliths, concentrations of 55Mn, 66Zn, 88Sr, and 137Ba were the most contributors on variations among wild and farmed individuals (Fig. 4b). Concentrations of 56Fe, 208Pb, 24Mg, and 137Ba in sea bream otoliths were the most contributors on differences between wild stocks, while no variations were detected between farmed stocks (Fig. 4b). Cross-validation classification regarding trace elements profiles in sea bream otoliths showed that the 96.6 % of sampled individuals were correctly classified according to their wild or farm origin, while the 89.8 % were correctly classified within sampling groups (Table 2).
Scatter plot from canonical discriminant analysis for trace elements in otoliths of wild and farmed sea bass and sea bream groups. W1: group 1 of wild fish; W2: group 2 of wild fish; F1: group 1 of farmed fish; F2: group 2 of farmed fish. a Sea bass: axe X (PC1) explained the 51.5 % of total variance; axe Y (PC2) explained the 36.4 % of the total variance. b Sea bream: axe X (PC1) explained the 37.2 % of total variance; axe Y (PC2) explained the 36.3 % of the total variance
Discussion
The otolith grows throughout the life of the fish and the addition of new material will cause the average composition of the otolith to change with time and environment characteristics (Veinott and Porter 2005). Our data support that differences between wild and farm environments can be detected through the variations on trace elements compositions in sea bass and sea bream otoliths. In agreement, specific trace elements incorporated into the growing surface of the fish otolith reflect the physical and chemical characteristics of the ambient water, although not necessarily in a simplistic manner, the otolith elemental composition can serve as an environmentally induced tag of groups of fish (Campana et al. 2000). However, before an otolith elemental concentration can be applied as a biological tracer of stock mixing, it must be shown to differ among stocks or geographic locations (Campana et al. 2000). Regarding the trace elements of sea bass otoliths in this study, 24Mg, 39K, and 55Mn concentrations were detected significantly higher in farmed fish otoliths than in wild ones. The previous studies showed significant differences between the farmed and wild origin in muscle and liver of sea bass for those specific elements, but also showed considerable divergence among the results, either accordance, discrepancies, or no significant results (Orban et al. 2002; Monti et al. 2005; Santaella et al. 2007; Fuentes et al. 2010; Mnari et al. 2010). However, a wider range of trace elements were found on the previous studies with significant differences on muscle and liver of sea bass from different origins, such as 11Na, 13Al, 20Ca, 29Cu, 33As, 34Se, 48Cd, 56Fe, 66Zn, and 82Pb (Alasalvar et al. 2002; Orban et al. 2002; Monti et al. 2005; Fernandes et al. 2007; Santaella et al. 2007; Ferreira et al. 2010; Fuentes et al. 2010; Mnari et al. 2010). For sea bream otoliths analysed in this study, concentrations of 24Mg, 27Al, 39K, 55Mn, 56Fe, 66Zn, and 137Ba were significantly higher in farmed fish, while 88Sr was detected with higher concentrations in wild sea bream. The previous studies on sea bream muscle did not find significant differences on the trace elements analysed (Carpene et al. 1998; Minganti et al. 2010), except for 33As and 80Hg that higher concentrations were detected in both cases on wild individuals compared with farmed ones (Minganti et al. 2010). Divergent results might be explained by the existence of interannual variability in elemental composition of otoliths which may confound spatial interpretations and origin determinations (Gillanders 2002).
Thus, elemental signatures of Mg, Mn, Sr, or Ba may indicate migratory patterns (coastal and estuarine areas) of wild fish compared with stabled farmed sea bass and sea bream, but also differences on temperature and oxygen conditions among fish groups throughout their lives (Elsdon and Gillanders 2004; Gillanders 2005; Limburg et al. 2015). However, the use of combined elemental signatures is known to allow for greater distinction among fish than only one element, being preferable the use all elements at once, e.g., a multivariate elemental composition (Campana et al. 2000). The statistical evaluation of the data showed that differences in otolith chemistry enabled discrimination and retrospective assignment of fish to their wild or farm origin with high accuracy for both sea bass (90.7 % of individuals correctly classified) and sea bream (96.6 % of individuals correctly classified). Despite the fact that most of the previous studies gave contrasting results, most were also able to distinguish wild and farmed fish with great accuracy through a multivariate approach that accounted for a wide range of elements, indicating that the trace elemental profile might be more appropriate than the presence or absence of a specific quantity of an element (Arechavala-Lopez et al. 2013a). Multivariate approaches have been successfully applied for discriminating the wild and farm origin through trace elements in otoliths of other fish species (Gillanders and Joyce 2005; Veinott and Porter 2005; Zitek et al. 2010; Perrier et al. 2011), but also on sea bass and sea bream using other physical and chemical parameters, such as body morphology, otolith shape, lipids and fatty acids compositions, stable isotopes, genetic markers, etc. (Arechavala-Lopez et al. 2013a; Šegvić-Bubić et al. 2014). Many of these parameters were highly recommended to identify escaped individuals from farms within wild stocks. However, it remains unknown for how long these parameters persist post-escape, which will influence their accuracy (Arechavala-Lopez et al. 2013a). Either physical parameters or biochemical components might change on escaped fish along the time, since they are directly influenced by the surrounding environment. However, diet has no significant effect on the incorporation of elements into fish otoliths (Marohn et al. 2009); hence, the main cause of variations of those parameters in a short-medium term might be any modification on habitat characteristics. The use of molecular genetic markers is likely to be the most suitable, non-destructive, and highly informative tool for genetic discrimination of wild and farmed fish, despite the heterogeneous mixed gene pool in wild sea bass and sea bream populations in the Mediterranean (Arechavala-Lopez et al. 2013a; Šegvić-Bubić et al. 2014). Unfortunately, genetic techniques are among the most expensive and time-consuming techniques currently available, and require specific knowledge making them unavailable to many sectors (Arechavala-Lopez et al. 2013a). Nevertheless, unique otolith fingerprints do not require genetic diversity among fish groups or populations, therefore combining chemical, morphological, and genetic data could enhance the discriminatory power of each technique.
Analysis of the chemical composition of otoliths has also been widely used as a technique to differentiate among fish stocks within the same species, reflecting differences in the chemical composition of the individual fish habitat or geographic-associated fish origin (Campana 1999; Thresher 1999). Gillanders et al. (2001) suggested that it is difficult to detect substantial differences in trace elemental signatures among different populations of wild fish in the Mediterranean, since they move among various coastal habitats. However, our results showed a clear discrimination between wild stocks for sea bass (85.7–90 % of wild fish correctly classified) and for sea bream (93.3–100 % of fish correctly classified). On the contrary, it was not possible to distinguish with accuracy between farmed stocks for both species. These results might reflect the proximity between farms with similar water chemistries in both areas, but also the similarities among fish culture techniques in the Mediterranean off-shore farms, in terms of net-pen environments, stocking densities, and feeding regimes. Thus, the analysis of trace elements composition in otoliths of sea bass and sea bream can be suggested as a useful method to distinguish between wild fish stocks from different geographical areas, but also to identify escaped fish from farms in the wild. Further researches are necessary to better understand the potential use of the trace elements in otoliths on fish ecology and populations dynamic studies (e.g., migrations, reproduction and growth studies of farmed, escaped, and wild fish), as well as to evaluate their applicability on sustainable coastal aquaculture and fisheries management.
References
Alasalvar C, Taylor KDA, Zubcov E, Shahidi F, Alexis M (2002) Differentiation of cultured and wild sea bass (Dicentrarchus labrax): total lipid content fatty acid and trace mineral composition. Food Chem 79:145–150
Arechavala-Lopez P, Uglem I, Fernandez-Jover D, Bayle-Sempere JT, Sanchez-Jerez P (2011) Immediate post-escape behaviour of farmed seabass (Dicentrarchus labrax L.) in the Mediterranean Sea. J Appl Ichthyol 27(6):1375–1378
Arechavala-Lopez P, Uglem I, Fernandez-Jover D, Bayle-Sempere JT, Sanchez-Jerez P (2012a) Post-escape dispersion of farmed seabream (Sparus aurata L.) and recaptures by local fisheries in the Western Mediterranean Sea. Fish Res 121:126–135
Arechavala-Lopez P, Sanchez-Jerez P, Bayle-Sempere JT, Sfakianakis DG, Somarakis S (2012b) Discriminating farmed gilthead sea bream Sparus aurata and European sea bass Dicentrarchus labrax from wild stocks through scales and otoliths. J Fish Biol 80(6):2159–2175
Arechavala-Lopez P, Sanchez-Jerez P, Bayle-Sempere JT, Uglem I, Mladineo I (2013a) Reared fish, farmed escapees and wild fish stocks—a triangle of pathogen transmission of concern to Mediterranean aquaculture management. Aquac Environ Interact 3(2):153–161
Arechavala-Lopez P, Fernandez-Jover D, Black KD, Ladoukakis E, Bayle-Sempere JT, Sanchez-Jerez P, Dempster T (2013b) Differentiating the wild or farmed origin of Mediterranean fish: a review of tools for sea bream and sea bass. Rev Aquac 5(3):137–157
Arechavala-Lopez P, Izquierdo-Gomez D, Sanchez-Jerez P, Bayle-Sempere JT (2014) Simulating escapes of farmed sea bass from Mediterranean open sea-cages: low recaptures by local fishermen. J Appl Ichthyol 30(1):185–188
Arechavala-Lopez P, Valero-Rodriguez JM, Peñalver-García J, Izquierdo-Gomez D, Sanchez-Jerez P (2015) Linking coastal aquaculture of meagre (Argyrosomus regius) and Western Mediterranean coastal fisheries through escapes incidents. Fish Manag Ecol 22(4):317–325
Bell JG, Preston T, Henderson RJ, Strachan F, Bron JE, Cooper K, Morrison DJ (2007) Discrimination of wild and cultured European sea bass (Dicentrarchus labrax) using chemical and isotopic analyses. J Agric Food Chem 55:5934–5941
Campana SE (1999) Chemistry and composition of fish otoliths: pathways, mechanisms and applications. Mar Ecol Prog Ser 188:263–297
Campana SE, Gagne JA (1995) Cod stock discrimination using ICP-MS elemental assays of otoliths. In: Secor DH, Dean JM, Campana SE (eds) Recent developments in fish otolith research. University of South Carolina Press, Columbia, pp 671–691
Campana SE, Neilson JD (1985) Microstructures of fish otoliths. Can J Fish Aquat Sci 42:1014–1032
Campana SE, Thorrold SR (2001) Otoliths, increments, and elements: keys to a comprehensive understanding of fish populations? Can J Fish Aquat Sci 58(1):30–38
Campana SE, Fowler AJ, Jones CM (1994) Otolith elemental fingerprinting for stock identification of Atlantic cod (Gadus morhua) using Laser Ablation ICP MS. Can J Fish Aquat Sci 51:1942–1950
Campana SE, Chouinard GA, Hanson JM, Frechet A, Brattey J (2000) Otolith elemental fingerprints as biological tracers of fish stocks. Fish Res 46(1):343–357
Campana SE, Valentin A, Sévigny JM, Power D (2007) Tracking seasonal migrations of redfish (Sebastes spp.) in and around the Gulf of St. Lawrence using otolith elemental fingerprints. Can J Fish Aquat Sci 64(1):6–18
Carpene E, Martin B, Dalla Libera L (1998) Biochemical differences in lateral muscle of wild and farmed gilthead sea bream (Sparus aurata L.). Fish Physiol Biochem 19:229–238
Dempster T, Moe H, Fredheim A, Jensen Ø, Sanchez-Jerez P (2007) Escapes of marine fish from sea-cage aquaculture in the Mediterranean Sea: status and prevention. CIESM Workshop Monogr 32:55–60
Dimitriou E, Katselis G, Moutopoulos DK, Akovitiotis C, Koutsikopoulos C (2007) Possible influence of reared gilthead sea bream (Sparus aurata L.) on wild stocks in the area of the Messolonghi lagoon (Ionian Sea, Greece). Aquac Res 38:398–408
Elsdon TS, Gillanders BM (2004) Fish otolith chemistry influenced by exposure to multiple environmental variables. J Exp Mar Biol Ecol 313(2):269–284
FAO (2016) Fisheries and aquaculture software. FishStatJ—software for fishery statistical time series. In: FAO Fisheries and Aquaculture Department (online), Rome
Fernandes D, Porte C, Bebianno MJ (2007) Chemical residues and biochemical responses in wild and cultured European sea bass (Dicentrarchus labrax L.). Environ Res 103:247–256
Ferreira M, Caetano M, Antunes P, Costa J, Gil O, Bandarra N, Pousão-Ferreira P, Vale C, Reis-Henriquesa MA (2010) Assessment of contaminants and biomarkers of exposure in wild and farmed sea bass. Ecotoxicol Environ Saf 73:579–588
Fiske P, Lund RA, Hansen P (2005) Identifying fish farm escapees. In: Cadrin SX, Friedland KD, Waldman JR (eds) Stock identification methods: applications in fishery science. Elsevier, Amsterdam, pp 659–680
Fuentes A, Fernández-Segovia I, Serra JA, Barat JM (2010) Comparison of wild and cultured sea bass (Dicentrarchus labrax) quality. Food Chem 119:1514–1518
García-Rodriguez M, Fernández AM, Esteban A (2006) Characterisation, analysis and catch rates of the small-scale fisheries of the Alicante Gulf (SE Spain) over a 10 years’ time series. Fish Res 77:226–238
Geffen AJ, Pearce N, Perkins W (1998) Metal concentrations in fish otoliths in relation to body composition after laboratory exposure to mercury and lead. Mar Ecol Prog Ser 165:235–245
Gillanders BM (2002) Temporal and spatial variability in elemental composition of otoliths: implications for determining stock identity and connectivity of populations. Can J Fish Aquat Sci 59(4):669–679
Gillanders BM (2005) Using elemental chemistry of fish otoliths to determine connectivity between estuarine and coastal habitats. Estuar Coast Shelf Sci 64(1):47–57
Gillanders BM, Joyce TC (2005) Distinguishing aquaculture and wild yellowtail kingfish via natural elemental signatures in otoliths. Mar Fresh Res 56(5):693–704
Gillanders BM, Kingsford MJ (2000) Elemental fingerprints of otoliths of fish may distinguish estuarine ‘nursery’ habitats. Mar Ecol Prog Ser 201:273–286
Gillanders BM, Sanchez-Jerez P, Bayle-Sempere J, Ramos-Esplá A (2001) Trace elements in otoliths of the two-banded bream from a coastal region in the south-west Mediterranean: are there differences among locations? J Fish Biol 59(2):350–363
Higgins RM, Danilowicz BS, Balbuena JA, Daníelsdóttir AK, Geffen AJ, Meijer WG, Modin J, Montero FE, Pampoulie C, Perdiguero-Alonso D, Schreiber A, Stefánsson MÖ, Wilson B (2010) Multi-disciplinary fingerprints reveal the harvest location of cod Gadus morhua in the northeast Atlantic. Mar Ecol Prog Ser 404:197–206
Hindar K, L’Abéelund JH (1992) Identification of hatchery-reared and naturally produced Atlantic salmon, Salmo salar L., juveniles based on examination of otoliths. Aquac Res 23(2):235–241
Limburg KE, Walther BD, Lu Z, Jackman G, Mohan J, Walther Y, Nissling A, Weber PK, Schmitt AK (2015) In search of the dead zone: use of otoliths for tracking fish exposure to hypoxia. J Mar Syst 141:167–178
Marohn L, Prigge E, Zumholz K, Klügel A, Anders H, Hanel R (2009) Dietary effects on multi-element composition of European eel (Anguilla anguilla) otoliths. Mar Biol 156(5):927–933
Minganti V, Drava G, De Pellegrini R, Siccardi C (2010) Trace elements in farmed and wild gilthead sea bream, Sparus aurata. Mar Pollut Bull 60:2022–2025
Mnari A, Bouhlel I, Chouba L, Hammami M, El Cafsi M, Chaouch A (2010) Total lipid content, fatty acid and mineral compositions of muscles and liver in wild and farmed sea bass (Dicentrarchus labrax). Afr J Food Sci 4:522–530
Monti G, De Napoli L, Mainolfi P, Barone R, Guida M, Marino G, Amoresano A (2005) Monitoring food quality by microfluidic electrophoresis, gas chromatography, and mass spectrometry techniques: effects of aquaculture on the sea bass (Dicentrarchus labrax). Anal Chem 77:2587–2594
Morrison J, Preston T, Bron JE, Henderson RJ, Cooper K, Strachan F, Bell JG (2007) Authenticating production origin of gilthead sea bream (Sparus aurata) by chemical and isotopic fingerprinting. Lipids 42:537–545
Orban E, Di Lena G, Nevigato T, Casini I, Santaroni G, Marzetti A, Caproni R (2002) Quality characteristics of sea bass intensively reared and from lagoon as affected by growth conditions and the aquatic environment. Food Chem Toxicol 67:542–546
Perrier C, Daverat F, Evanno G, Pécheyran C, Bagliniere JL, Roussel JM (2011) Coupling genetic and otolith trace element analyses to identify river-born fish with hatchery pedigrees in stocked Atlantic salmon (Salmo salar) populations. Can J Fish Aquat Sci 68(6):977–987
Rooker JR, Secor DH, Zdanowicz VS, De Metrio G, Relini LO (2003) Identification of Atlantic bluefin tuna (Thunnus thynnus) stocks from putative nurseries using otolith chemistry. Fish Oceanogr 12(2):75–84
Sanchez-Jerez P, Gillander BM, Kingsford MJ (2002) Spatial variability of trace elements in fish otoliths: comparison with dietary items and habitat constituents in sea grass meadows. J Fish Biol 61:801–821
Santaella M, Martínez Graciá C, Periago MJ (2007) Wild and farmed sea bass (Dicentrarchus labrax) comparison: chemical composition and variations in the fatty acid profile after cooking. Anal Vet Murcia 23:105–119
Šegvić-Bubić T, Talijančić I, Grubišić L, Izquierdo-Gomez D, Katavić I (2014) Morphological and molecular differentiation of wild and farmed gilthead sea bream Sparus aurata: implications for management. Aquac Environ Interact 6:43–54
Sturgeon RE, Willie SN, Yang L, Greenberg R, Spatz RO, Chen Z, Scriver C, Clancy V, Lama JW, Thorroldd S (2005) Certification of a fish otolith reference material in support of quality assurance for trace element analysis. J Anal At Spectrosc 20(10):1067–1071
Thorrold SR, Jones CM, Campana SE, McLaren JW, Lam JWH (1998) Trace element signatures in otoliths record natal river of juvenile American shad (Alosa sapidissima). Limnol Oceanogr 43:1826–1835
Thresher RE (1999) Elemental composition of otoliths as a stock delineator in fishes. Fish Res 43(1):165–204
Toledo-Guedes K, Sanchez-Jerez P, Brito A (2014a) Influence of a massive aquaculture escape event on artisanal fisheries. Fish Manag Ecol 21(2):113–121
Toledo-Guedes K, Sanchez-Jerez P, Benjumea ME, Brito A (2014b) Farming-up coastal fish assemblages through a massive aquaculture escape event. Mar Environ Res 98:86–95
Veinott G, Porter R (2005) Using otolith microchemistry to distinguish Atlantic salmon (Salmo salar) parr from different natal streams. Fish Res 71(3):349–355
Zitek A, Sturm M, Waidbacher H, Prohaska T (2010) Discrimination of wild and hatchery trout by natural chronological patterns of elements and isotopes in otoliths using LA-ICP-MS. Fish Manag Ecol 17(5):435–445
Acknowledgments
The authors thank the marine research centre CIMAR (Excmo. Ayto. de Santa Pola and University of Alicante, Spain) for sharing their facilities. This study was financed by the EU-proyect ‘PreventEscape’ (7th Framework European Commission, num. 226885; http://www.preventescape.eu/).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Arechavala-Lopez, P., Milošević-González, M. & Sanchez-Jerez, P. Using trace elements in otoliths to discriminate between wild and farmed European sea bass (Dicentrarchus labrax L.) and Gilthead sea bream (Sparus aurata L.). Int Aquat Res 8, 263–273 (2016). https://doi.org/10.1007/s40071-016-0142-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40071-016-0142-1