Abstract
Gelatine hydrolysate is a type of gelatine that undergoes a controlled hydrolysis treatment to further break apart the gelatine or collagen molecules. In this study, gelatine hydrolysate was produced from commercial tilapia scale gelatine via controlled enzymatic hydrolysis. Commercial Alcalase 2.4 L, a protease enzyme was used to breakdown the peptide chains present in the gelatine. Optimization of hydrolysis conditions (temperature, time and enzyme to substrate ratio) was conducted by utilizing response surface methodology (RSM). Results showed that a hydrolysis temperature of 57.6 °C together with a hydrolysis time of 80 min and enzyme to substrate ratio of 1.20 % (v/w) were the optimum conditions to obtain the highest degree of hydrolysis (10.91 %). The freeze-dried gelatine hydrolysate was characterized with respect to chemical composition:approximate composition, viscosity and molecular weight. The gelatine hydrolysate produced contained a high content of protein (85.26 %); thus, it may serve as a potential protein source for human needs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Tilapia (Oreochromis spp.) is a freshwater species that has become the third most widely cultured fish, after carp and salmon (El-Sayed 2006). The production of tilapia has increased steadily and become an important source of fish supply in Malaysia (Jamilah and Harvinder 2002). It is classified as a durable, fast growing species compared to others and highly resistance to disease (Foh et al. 2011a). Its high protein content is comparable with meat sources and plays an important role in human supplementation (Ghorbani and Mirakabad 2010). The crude protein content of an adult tilapia is approximately 16–25 %, which is considerably high for freshwater species, while the fat content is very low (0.5–3.0 %) (Foh et al. 2011b).
Many studies have reported on gelatine production from fish sources. Over the past several decades, fine qualities of fish gelatine production have been widely studied from species as diverse as tilapia (Jamilah and Harvinder 2002; Muyonga et al. 2004), salmon (Arnesen and Gildberg 2007), cod (Arnesen and Gildberg 2006), and pollock (Zhou and Regenstein 2004). Another valuable product that can be derived from fish derivatives is hydrolysate. Hydrolysate, also known as gelatine hydrolysate or collagen hydrolysate, is a type of gelatine that undergoes a controlled hydrolysis treatment to further break apart the gelatine or collagen molecules. During the process, peptide chains are cleaved into smaller chains, which results in lower molecular weights, between 500 Da and 25 kDa. However, a wide variety of peptide chains will be generated depending on the enzyme’s specificity, environmental conditions and extent of the hydrolysis process (Guerard et al. 2002). Compared to gelatine, hydrolysate does not gel anymore but still has an active surface behaviour exactly like gelatine. In practical terms, hydrolysate can be manufactured into two ways; either derived from gelatine itself or directly from an animal’s pure collagen. However, pure collagen from animals is known for its high resistance; hence, special collagenases have to be used in large amounts, which will result in higher production costs. Therefore, in industry, gelatine is widely used as a raw material for production of gelatine hydrolysate.
Most of the gelatine hydrolysates investigated in previous studies were derived by enzymatic hydrolysis from yellowfin tuna (Guerard et al. 2001; Ovissipour et al. 2010), tilapia (Foh et al. 2011a; Yang et al. 2009), catfish (Aleman et al. 2011), salmon (See et al. 2011), persian sturgeon (Ovissipour et al. 2009), mackerel (Wua et al. 2003), cobia (Amiza et al. 2012), catla (Bhaskar et al. 2008), capelin (Shahidi et al. 1995) and cod (Slizyte et al. 2005a). Enzymatic treatment offers several advantages: the ease of controlling the reaction efficiently, minimal formation of by-products and the milder processing conditions required to run the hydrolysis (Alfonso Clemente 2000; Kristinsson and Rasco 2000a; Mannheim and Cheryan 1992). By employing enzymes to hydrolyze protein, one could improve the physiochemical, functional, sensory and also nutritional behaviour of the hydrolyzed proteins (Slizyte et al. 2005a). In addition, hydrolyzing protein can also improve its intestinal absorption (Kristinsson and Rasco 2000a). Research shows that extensive hydrolysis of proteins has a great potential to reduce immunological reactions, which is important for hyperallergic infants (Mahmoud 1994). Moreover, the products of hydrolysis, which are lower molecular weight peptides, can be easily absorbed in the gut, promoting better supplementation for humans and also animals (Slizyte et al. 2005b; Kristinsson and Rasco 2000a). In many countries, traditional and commercial preparations of fish protein hydrolysates are currently used as health foods, functional foods and nutraceuticals (Chalamaiah et al. 2012). Therefore, gelatine hydrolysate can be used as a supplement for clinical, infant and sports nutrition. Regarding the suitability of the enzymes in the protein hydrolyzation field, Alcalase, a microbial protease, is most preferred and is widely used. Microbial-derived enzymes are better suited as they offer a wide variety of available catalytic activities as well as greater pH and temperature stabilities (Diniz and Martin 1997). Previous research reported that Alcalase was the most efficient enzyme among the proteolytic enzymes studied in hydrolyzing protein, and was able to produce the highest degree of hydrolysis (DH) of hydrolysate (Ng and Mohd Khan 2012). The highest protein recovery and lowest lipid content were reported for hydrolysates prepared via hydrolysis with Alcalase compared with Papain and Neutrase (Adler-Nissen 1986). Moreover, it has been reported previously that hydrolysates from Alcalase had less bitterness compared to those prepared with Papain (Hoyle and Merritt 1994). Furthermore, Alcalase was also classified as the best choice for protein hydrolyzation based on enzyme cost per unit activity (Kristinsson and Rasco 2000b).
This study was carried out to investigate the effects of reaction parameters [temperature, time and enzyme to substrate (E:S) ratio] on degree of hydrolysis (DH) and to optimize the enzymatic hydrolysis condition for the highest degree of hydrolysis using response surface methodology (RSM).
Methodology
Chemicals and raw materials
The substrate used in this study was a commercial food grade gelatine from tilapia scales with a gel strength of 257 g. It was purchased from Halagel Sdn. Bhd. An endoproteinase from Bacillus licheniformis (Alcalase 2.4 L) with a declared activity of 2.4 AU/kg and density of 1.18 g/ml, was purchased directly from Novozymes. All reagents used were analytical grade.
Optimization of hydrolysis conditions by RSM
Response surface methodology (RSM) was utilised to obtain the optimum process parameters [hydrolysis temperature, hydrolysis time and enzyme to substrate (E:S) ratio] and maximum degree of hydrolysis (DH) for the enzymatic hydrolysis process. Hence, a number of experiments with various process parameters were carried out as an input in RSM.
Optimization of the hydrolysis conditions was accomplished by a central composite design (CCD) in RSM. CCD was efficiently applied to develop a second-order response model with only a small number of factors n; 2 ≤ n ≥ 6 (Ng et al. 2011). The experiment was designed using three independent factors and five levels. As an input, the three different independent variables were designated as hydrolysis temperature (A, °C), hydrolysis time (B, minutes) and enzyme to substrate ratio (C, % v/w); while, for output, only DH (Y, %) was chosen as response. The ranges for each variable are tabulated as coded levels in Table 1. Once the optimization was performed, an enzymatic hydrolysis experiment was carried out applying the optimized parameters from RSM. The hydrolysis was done in triplicate and the hydrolysates were tested and analysed.
CCD consists of three types of design points; two-level factorial or fractional factorial design points, axial points and centre points. Centre points have to be repeated four to six times to obtain a precise estimation of experimental error. Table 2 shows the 19 total runs rendered for the optimization process using RSM together with the actual levels for each variable and the response values. In this study, randomized experimental runs were carried out due to minimizing the effects of unexpected variability in the observed responses.
Enzymatic hydrolysis
Gelatine solution (5 % w/v) was prepared by dissolving some amount of pure gelatine substrate into the required amount of distilled water at an appropriate temperature range (50–60 °C). The reaction mixture was stirred by a four-blade impeller at a speed of 300 rpm. Once it was homogeneous, the temperature was adjusted to a certain level while the pH of the gelatine solution was increased to pH 8 by adding a small amount of 2 N NaOH. Then, the Alcalase 2.4 L enzyme was added into the homogenized gelatine solution at the desired concentrations. The hydrolysis process was conducted at the required period before being heated rapidly and maintained at a temperature of 90 °C for at least 10 min (Aleman et al. 2011) to deactivate the enzyme. The hydrolysates obtained were lyophilized using freeze-drying equipment (Martin Christ, Alpha 1-4 LSC, Germany) at temperature of −40 °C for almost 24 h. The freeze-dried hydrolysates were then crushed into smaller particles before being labelled and sealed in vacuum bags for storage in a dry place.
Degree of hydrolysis
Measurement of the degree of hydrolysis (DH) was carried out using the pH–stat method according to Adler-Nissen (1977). In this method, DH can be monitored continuously by maintaining the alkaline pH (pH 8) during the enzymatic hydrolysis process via direct addition of 2 N NaOH. From the total base consumed during the hydrolysis process, DH can be calculated directly from the Eq. (1) (Adler-Nissen 1986).
Where DH is the degree of hydrolysis, B is the base consumption (in ml), NB is the normality of the base (N), α is the average degree of dissociation of the α-NH groups, MP is the mass of protein (in g) and htot is the total number of peptide bonds in the protein substrate (11.1 meqv/g protein for gelatine). According to Kristinsson and Rasco (2000b), the degree of dissociation, α was calculated based on the Eq. (2).
The pK value was directly dependent on the process temperature, which varied between experiments. Therefore, the Eq. (3) was used to calculate the correct value of pK (Steinhardt and Beychok 1964).
Proximate analysis
The proximate analyses were carried out on the gelatine powder as a raw material and freeze-dried gelatine hydrolysate as a final product. Moisture and ash contents were determined according to Gelatine Manufacturer of Europe (GME) Monograph (2000). The oven method was used to determine moisture content, while the ash content was determined by charring the pre-dried sample in a crucible at 550 °C for about 17 h until white ash was formed. Finally, protein content was estimated by the differences. All analyses were carried out in triplicate and average values were taken.
Amino acid composition
Amino acid composition was determined after hydrolysis at 110 °C for 24 h in 6 N HCl. The amount amino acid composition was analysed by a high performance liquid chromatography (HPLC), equipped with Waters 410 Scanning Flourescence and AccQ Tag Column (3.9 × 150 mm). Amino acid composition was reported as g amino acid per 100 g protein.
Molecular weight
In order to characterize the hydrolysates based on their molecular weights, Tris–glycine sodium dodecyl sulphate polyacrylamide gel electrophoresis (Tris–Glycine SDS-PAGE) was performed on samples from the optimized enzymatic hydrolysis. The test was done using 17 % resolving gel and 5 % stacking gel according to Laemmli (1970) with some adjustments. The sample buffer was prepared from 0.5 M Tris HCl buffer pH 6.8, 10 % (w/v) glycerol, 0.5 % (w/v) SDS, bromophenol blue and 5 % β-mercaptoethanol. Samples were diluted until a concentration of 0.5 % was obtained. Three samples (S1, S2 and S3) of the same hydrolysate and one sample of gelatine (S0) were analysed for determination of their molecular weight. All samples prepared were denatured in 95 °C hot water for about 3 min. After electrophoresis, the gels were stained using GelCodeTM blue safe protein stain purchased from thermo scientific.
Viscosity
The viscosity of gelatine and gelatine hydrolysate was determined using modification method described by Cho et al. (2006) and Ninan et al. (2012). 5 % w/v gelatine solution was prepared by dissolving the dry gelatine powder in distilled water at a temperature of 60 °C. The viscosity of gelatine and gelatine hydrolysate was monitored continuously starting at 40 °C until it reached 60 °C using a computerized Brookfield digital viscometer (Model DV-II) together with spindle No. 1 at 60 rpm.
Statistical Analysis
All the results obtained from the experimentation were analysed using Design Expert 6.0.10 statistical software (Stat-Ease Inc., Minneapolis). Design-Expert fits linear, two-factor interaction (2FI), quadratic and cubic polynomials to the response. The program displays a measure of progress during the calculations.
Results and discussion
Optimization of enzymatic hydrolysis parameters
The experimental response, DH, was calculated based on the Eqs. (1–3), and inserted in the RSM to be analysed further. The response ranged from 5.43 to 11.50 %; hence, the ratio of the maximum to minimum is only 2.12. This indicates that no transformation is required (λ = 1.0), as the ratio was <10. In the Fit Summary section, certain data were shown based on the calculations carried out by the software to obtain the best model to predict the most accurate response.
Based on the Sequential Model Sum of Squares in Table 3, the best model was chosen based on the highest order polynomial criteria where the additional terms were significant and the model was not aliased. In this research, the software revealed that the linear and quadratic models were significant (P < 0.05). However, the quadratic model was chosen as it was a higher order polynomial compared to the linear model.
Lack of Fit in Table 4, the residual error was compared to the Pure Error from replicated design points. A high probability value showed insignificant lack of fit and it was desired. Therefore, the linear model definitely can be ruled out, because the Prob > F falls below 0.05. However, the quadratic model did not showed significant lack of fit, hence it was chosen. The result showed the quadratic model was fitted well with the DH data indicated that the generated or predicted model is able to be fitted with the data variations and significantly represents the actual relationships with the parameters of a reaction.
The Model Summary Statistics in Table 5 provides additional statistical measures that were useful in comparing the available models in the software. From the data obtained, the quadratic model was chosen due to the low standard deviation (Std. Dev.), high R-Squared (R2) values and a low PRESS. R-Squared is a correlation coefficient for the model while PRESS is a measure of how a particular model fits each design point. According to the R-Squared values, the closer the value to one, the better. In this studies showed that the highest R-Squared was obtained for the cubic model, followed by the quadratic model. However, the cubic model had been discarded earlier as it was aliased and could not be chosen; thus, the quadratic model was utilised.
Analysis of variance (ANOVA) was used to confirm the significant and adequacy of the quadratic model predicted, as shown in Table 6. The predicted quadratic model showed significant behaviour at a 95 % confidence level (P < 0.05). The significance of the predicted model was evaluated further for each parameter varied. From the result obtained, only X2, X3, X 21 and X 23 were significant (P < 0.05) while the rest (X1, X 22 and X1X2, X1X3 and X2X3) showed non-significant behaviour. However, F value of 17.58 implied the model was significant (P < 0.05). In addition, there was only a 0.01 % chance that a model F-Value this large could occur due to noise. Hence, the adequacy of the quadratic model was confirmed from ANOVA as the model Prob > F was <0.05.
Regression analysis was performed for the tree difference variables (X1, X2 and X3). From the regression analysis, the second-order polynomial model was predicted. It was consisted of 10 β-coefficients: three linear effects, three quadratic effects, three interaction effects and one constant.
Where Y represented the estimated dependent variable (DH) and X1, X2 and X3 were hydrolysis temperature, hydrolysis time and enzyme to substrate ratio respectively, while β0 was the constant term and βi, βii and βij were the linear, quadratic and interaction terms (i = 1−3 and j = 1−3), respectively. Finally, the second-order regression equation obtained from RSM was shown below.
Overall, the analysis of variance representing the second-order regression equation which exhibited the results fitted well to all the process parameters. Thus, the predicted model was statistically valid for enzymatic hydrolysis of tilapia skin gelatine. From the optimization through RSM, the optimal condition for enzymatic hydrolysis of tilapia skin gelatine was achieved at 57.6 °C of hydrolysis temperature (X1), 80 min of hydrolysis time (X2) and 1.20 % of enzyme to substrate ratio (% v/w) (X3). These optimal parameters gave a maximum value of yield in terms of DH, which was 10.91 %. Finally, by employing the optimal parameters (X1, X2 and X3), three sets of enzymatic hydrolysis experiments were conducted. The average results obtained from the three actual experimental gave a higher yield of DH (11.89 %).
A higher percentage of DH (19.9 %) was reported in a previous study for a controlled hydrolysis of catfish skin gelatine using high pressure (Aleman et al. 2011). The error in the yield for DH was calculated based on the predicted and actual value using the Eq. (4). The error calculated was acceptable with the value of 8.24 %.
where A is actual value and B is predicted value.
Effect of the hydrolysis parameter
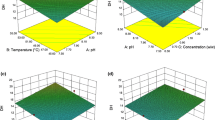
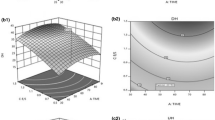
The effects of hydrolysis temperature, time and enzyme to substrate ratio, upon the degree of hydrolysis (DH) were determined by employing response surface methodology (RSM). The hydrolysis temperature (X1) and hydrolysis time (X2) were set to top re-arranged ranges while the enzyme to substrate ratio (X3) was set to a minimum value to achieve the maximum DH percentage. In order to obtain a clear view of the influences of these independent variables on the dependent variable, several three-dimensional views of response surface were plotted. The plots showed the effects of two independent variables toward DH by holding another independent variable at a constant value that was the optimal value.
All of the three-dimensional plots for the enzymatic hydrolysis of tilapia skin gelatine are presented in Figs. 1, 2 and 3. The plots are represented as a function of two factors and holding the other factor. Figure 1 shows the effect of hydrolysis time and temperature on DH by holding the enzyme to substrate ratio at an optimal value of 1.20 %. The result showed that DH increased as time and temperature increased. However, a decreasing trend was observed at temperatures above 57.6 °C. This finding can be related to the best range of temperature for the highest performance of Alcalase. Higher temperatures tend to deactivate the enzymes used, resulting in lower DH being achieved. This result was supported in a study on the hydrolysis of Catla visceral waste protein where the optimum temperature obtained was 55 °C (Bhaskar et al. 2008). In another study of hydrolysis on threadfin bream protein, 60 °C was determined as the optimum temperature (Normah et al. 2005). Figure 2 shows the effect of the enzyme to substrate ratio and hydrolysis temperature on DH by holding the value of the hydrolysis time constant at an optimum of 80 min. Here, it can be observed that the DH increased almost linearly with the increasing enzyme to substrate ratio. This finding showed that a higher enzyme concentration will eventually cleave more peptide bonds available in the substrate. The same results were reported in a previous hydrolysis study employing palm kernel expeller, where a higher concentration of Alcalase 2.4 L gave a higher value of DH (Ng and Mohd Khan 2012). In addition, a study on the hydrolysis of protein concentrate from sunflower wholemeal also supported the results (Ordonez et al. 2008). However, selection of enzyme concentration should considered the total concentration of peptide chains in the substrate and the degree of protein breakdown desired to avoid an excessive use of enzyme that will drive up the cost for industrial use. Furthermore, Fig. 3 shows both factors (enzyme to substrate ratio and hydrolysis time) were slightly linear relationships against the DH by holding the value of the hydrolysis temperature at an optimum of 57.6 °C.
Proximate composition
The composition according to the proximate analysis is tabulated in Table 7. The result showed that protein was the main constituent in both gelatine and hydrolysate (87.62 and 85.26 %, respectively). Followed by a fair amount of moisture content of gelatine and hydrolysate (12.30 and 12.35 %, respectively). The higher percentage of ash content in the hydrolysate (2.39 %) compared to the gelatine (0.08 %) indicated that it was contained greater amounts of salt. It was mainly due to the addition of sodium hydroxide and sulfuric acid during pH adjustment and pH control during the enzymatic treatment (Chen et al. 2012). Overall, the yield of hydrolysate was high in protein and low in inorganic minerals and it was a similar composition to that of the gelatine. This result was supported by Aleman et al. (2011), who obtained 88.11 % of protein composition and 11.52 % moisture content from catfish skin gelatine.
Amino acid composition
Table 8 shows an amino acid composition of commercial gelatine from tilapia scales and gelatine hydrolysate. After hydrolysis, the composition of total amino acid changed to be increased due to the optimum conditions during hydrolysis process. The higher amino acid contents which obtained from this study were similar to previous reports (Jongjareonrak et al. 2006; Sarabia et al. 2000 and Herpandi and Adzitey 2011). The results showed both gelatine and gelatine hydrolysate containing glycine (18.3/100 and 18.9/100 g respectively) as a main component, followed by proline, alanine and hydroxyproline.
Molecular weight
The molecular weight distribution was employed for selection of particular application based on their molecular sizes. Based on previous studies (Lim and Mohammad 2011; Gomez-Guillen et al. 2002) gelatine consisted mainly of α-chains followed by a fair number of β-chains, along with a low content of γ-chains. In this research, SDS-PAGE analysis was done on the gelatine and hydrolysate samples as mentioned previously. The electrophoretic patterns for both gelatine and hydrolysate samples are presented in Fig. 4 below. The result clearly showed that the sizes of gelatine molecules were within the range of 34–260 kDa. Whereas, the average molecular weight of three samples of the same hydrolysates was below 10 kDa. This result indicated that the hydrolysis process had successfully cleaved the peptide bonds, resulting in lower molecular weight and higher DH. In order to obtain protein hydrolysates of high nutritional value, the dietary protein in it should rich in low molecular weight species, with the amounts of free amino acids as low as possible (Vijayalakshmi et al. 1986).
Viscosity
Figure 5 shows the viscosity plots for gelatine and gelatine hydrolysate. The hydrolysate samples were obtained from triplicate runs employing the optimal parameters obtained from the optimization process. The average viscosity of the hydrolysates produced was 2.27–2.54 cP. The results showed higher viscosity for the gelatine, which was almost twice the viscosity of the hydrolysates. The viscosities showed decreasing trends as the temperature increased. In addition, the molecular weight of hydrolysates also influenced the viscosity of solution. Hydrolysates with low molecular weight tended to produce a solution of low viscosity that was easier to process even at relatively high concentration (Schrieber and Gareis 2007). Therefore, viscosity was an important measurement for the industry to monitor and control the hydrolysis process.
Conclusion
The degree of hydrolysis of gelatine hydrolysate from tilapia scales was significantly influenced by the hydrolysis conditions (hydrolysis time, hydrolysis temperature and enzyme to substrate ratio). Based on the model predicted, the optimum hydrolysis conditions were 57.6 °C for hydrolysis temperature, 80 min for hydrolysis time and 1.20 % (v/w) for the enzyme to substrate ratio. These optimum conditions gave a maximum value for yield, in terms of DH (10.91 %). The gelatine hydrolysates produced were rich in protein content and may serve as a better alternative source to fulfil human needs.
References
Adler-Nissen J (1977) Enzymatic hydrolysis of food proteins. Process Biochem 8:18–32
Adler-Nissen J (1986) Enzymatic hydrolysis of food proteins. Elsevier, Barking
Aleman A, Gimenez B, Gomez-Guillen MC, Montero P (2011) Enzymatic hydrolysis of fish gelatin under high pressure treatment. Int J Food Sci Technol 46:1129–1136
Amiza MA, Kong YL, Faazaz AL (2012) Effects of degree of hydrolysis on physicochemical properties of Cobia (Rachycentron canadum) frame hydrolysate. Int Food Res J 19(1):199–206
Arnesen JA, Gildberg A (2006) Extraction of muscle proteins and gelatine from cod head. Process Biochem 41:697–700
Arnesen JA, Gildberg A (2007) Extraction and characterisation of gelatine from Atlantic salmon (Salmo salar) skin. Bioresour Technol 98:53–57
Bhaskar N, Benila T, RaDHa C, Lalitha RG (2008) Optimization of enzymatic hydrolysis of visceral waste proteins of Catla (Catla catla) for preparing protein hydrolysate using a commercial protease. Bioresour Technol 99:335–343
Chalamaiah M, Dinesh Kumar B, Hemalatha R, Jyothirmayi T (2012) Fish protein hydrolysates: proximate composition, amino acid composition, antioxidant activities and applications: a review. Food Chem 135:3020–3038
Chen C, Yu-Jie C, Wei X (2012) Comparisons on the functional properties and antioxidant activity of spray-dried and freeze-dried egg white protein hydrolysate. Food Bioprocess Technol 5:2342–2352
Cho SH, Jahncke ML, Chin KB, Eun JB (2006) The effect of processing conditions on the properties of gelatin from skate (Raja kenojei) skins. Food Hydrocoll 20:810–816
Clemente Alfonso (2000) Enzymatic protein hydrolysates in human nutrition. Trends Food Sci Technol 11:254–262
Diniz FM, Martin AM (1997) Fish protein hydrolysates by enzymatic processing. Agro Food Ind Hi Tech 8:9–13
El-Sayed AM (2006) Tilapia culture. CABI publishing, Oxford, pp 1–24
Foh MBK, Amadou I, Kamara MT, Foh BM, Wenshui Xia (2011a) Effect of enzymatic hydrolysis on the nutritional and functional properties of Nile tilapia (Oreochromis niloticus) proteins. Am J Biochem Mol Biol 1(1):54–67
Foh MBK, Kamara MT, Amadou I, Foh BM, Wenshui Xia (2011b) Chemical and physicochemical properties of tilapia (Oreochromis Niloticus) fish protein hydrolysate and concentrate. Int J Biol Chem 5(1):21–36
Gelatine Manufacturer of Europe (GME) (2000) Monograph. Standardised methods for the testing of edible gelatin version 1
Ghorbani M, Mirakabad HZ (2010) Factor influencing on trout production in Khorasan Razavi Province. Trends Agric Econ 3:1–18
Gomez-Guillen MC, Turnay J, Fernandez-Diaz MD, Ulmo N, Lizarbe MA, Montero P (2002) Structural and physical properties extracted from different marine species: a comparative study. Food Hydrocoll 16:25–34
Guerard F, Dufosse L, De La Broise D, Binet A (2001) Enzymatic hydrolysis of proteins from yellowfin tuna (Thunnus albacares) wastes using Alcalase. J Mol Catal B Enzym 11:1051–1059
Guerard F, Guimas L, Binet A (2002) Production of tuna waste hydrolysates by a commercial neutral protease preparation. J Mol Catal B Enzym 19–20:489–498
Herpandi NH, Adzitey F (2011). Fish bone and scale as a potential source of halal gelatin. J Fish Aquat Sci, 6 (4):379–389
Hoyle NT, Merritt JH (1994) Quality of fish protein hydrolysate from Herring (Clupea harengus). J Food Sci 59:76–79
Jamilah B, Harvinder KG (2002) Properties of gelatins from skins of fish-black tilapia (Oreochromis mossambicus) and red tilapia (Oreochromis nilotica). Food Chem 77:81–84
Jongjareonrak A, Benjakul S, Vusessanguan W, Tanaka M (2006) Skin gelatin from bigeye snapper and brownstripe red snapper: chemical composition and effect of microbial transglutaminase on gel properties. Food Hydrocoll 20(8):1216–1222
Kristinsson HG, Rasco BA (2000a) Fish protein hydrolysates: production, biochemical and functional properties. Crit Rev Food Sci Nutr 40:43–81
Kristinsson HG, Rasco BA (2000b) Kinetics of the hydrolysis of Atlantic salmon (Salmo salar) muscle proteins by alkaline proteases and a visceral serine protease mixture. Process Biochem 36:131–139
Laemmli UK (1970) Cleavage of structural proteins during assembly of the head of bacteriophage T4. Nature 227:680–685
Lim YP, Mohammad AW (2011) Physicochemical properties of mammalian gelatin in relation to membrane process requirement. Food Bioprocess Technol 4:304–311
Mahmoud MI (1994) Physicochemical and functional properties of protein hydrolysates in nutritional products. Food Technol. 48:89–95
Mannheim A, Cheryan M (1992) Enzyme modified protein from corn gluten meal: preparation and functional properties. J Am Oil Chem Soc 69:1163–1169
Muyonga JH, Cole CGB, Duodu KG (2004) Extraction and physico-chemical characterisation of Nile perch (Lates niloticus) skin and bone gelatin. Food Hydrocoll 18:581–592
Ng KL, Mohd Khan A (2012) Enzymatic preparation of palm kernel expeller protein hydrolysate (PKEPH). Int Food Res J 19(2):721–725
Ng LY, Leo CP, Mohammad AW (2011) Optimizing the incorporation of silica nanoparticles in polysulfone/poly(vinyl alcohol) membranes with response surface methodology. J Appl Polym Sci 121(3):1804–1814
Ninan G, Joseph J, Aliyamveettil ZA (2012) A comparative study on the physical, chemical and functional properties of carp skin and mammalian gelatins. J Food Sci Technol 51:2085–2091
Normah I, Jamilah B, Saari N, Yaakob Che Man B (2005) Optimization of hydrolysis conditions for the production of threadfin bream (Nemipterus japonicus) hydrolysate by Alcalase®. J Muscle Foods 16:87–102
Ordonez C, Benitez C, Gonzalez JL (2008) Amino acid production from a sunflower wholemeal protein concentrate. Bioresour Technol 99:4749–4754
Ovissipour M, Abedian A, Motamedzadegan A, Rasco B, Safari R, Shahiri H (2009) The effect of enzymatic hydrolysis time and temperature on the properties of protein hydrolysates from Persian sturgeon (Acipenser persicus) viscera. Food Chem 115:238–242
Ovissipour M, Benjakul S, Safari R, Motamedzadegan A (2010) Fish protein hydrolysates production from yellowfin tuna (Th unnus albacares) head using Alcalase and Protamex. Int Aquat Res 2:87–95
Sarabia AI, Gómez-Guillén MC, Montero P (2000) The effect of added salts on the viscoelastic properties of fish skin gelatin. Food Chem 70:71–76
Schrieber R, Gareis H (2007) Gelatine Handbook Theory and Industrial Practice. Wiley, German
See SF, Hoo LL, Babji AS (2011) Optimization of enzymatic hydrolysis of Salmon (Salmo salar) skin by Alcalase. Int Food Res J 18(4):1359–1365
Shahidi F, Han XQ, Synowiecki J (1995) Production and characteristics of protein hydrolysates from capelin (Mallotus villosus). Food Chem 53:285–293
Slizyte R, Dauksas E, Falch E, Storro I, Rustad T (2005a) Characteristics of protein fractions generated from cod (Gadus morhua) by-products. Process Biochem 40:2021–2033
Slizyte R, Rustad T, Storro I (2005b) Enzymatic hydrolysis of cod (Gadus morhua) by-products Optimization of yield and properties of lipid and protein fractions. Process Biochem 40:3680–3692
Steinhardt H, Beychok S (1964) Interaction of protein with hydrogen ions and other small ions and molecules. In: Neurath H (ed) The Proteins. Academic Press, New York, vol 2. pp 139–304
Vijayalakshmi MA, Lemieux L, Amiot J (1986) High performance size exclusion liquid chromatography of small molecular weight peptides from protein hydrolysates using methanol as a mobile phase additive. J Liq Chromatogr 9:3559–3576
Wua HC, Chen HM, Shiau CY (2003) Free amino acids and peptides as related to antioxidant properties in protein hydrolysates of mackerel (Scomber austriasicus). Food Res Int 36:949–957
Yang J, Liang W, Chow C, Siebert KJ (2009) Process for the production of tilapia retorted skin gelatin hydrolysates withoptimized antioxidative properties. Process Biochem 44:1152–1157
Zhou P, Regenstein JM (2004) Optimization of extraction conditions for Pollock skin gelatin. J Food Sci 69:393–398
Acknowledgments
The authors would like to thank the Universiti Kebangsaan Malaysia for the Grant DIP-2012-01 and Post-graduate Scholarship for AGK Abdul-Aziz and the Ministry of Higher Education, Malaysia for MyBrain Scholarship.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Mohammad, A.W., Kumar, A.G. & Basha, R.K. Optimization of enzymatic hydrolysis of tilapia (Oreochromis Spp.) scale gelatine. Int Aquat Res 7, 27–39 (2015). https://doi.org/10.1007/s40071-014-0090-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40071-014-0090-6