Abstract
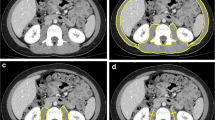
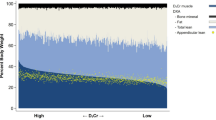
Age-related decreases in muscle mass and function, known as sarcopenia, have been shown to be related to functional limitation, frailty, and an increase in morbidity and mortality. While the most accurate method to assess muscle mass is biopsy, this is impractical clinically. There are numerous methods to assess skeletal muscle mass including dual-energy X-ray absorptiometry (DEXA) and bioelectrical impedance analysis (BIA) which are low cost and accessible. There are also more specific standards for assessing muscle mass or cross-sectional muscle area including magnetic resonance imaging (MRI) and computerized tomography (CT). Each method has its own advantages and limitations in clinical practice. Other emerging methods include peripheral quantitative CT and ultrasound. The ideal test would be valid and reliable, low cost, and practical combined with simple measurements of isometric strength to define sarcopenia and predict future health events.

Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Puthucheary ZA, Rawal J, McPhail M, et al. Acute skeletal muscle wasting in critical illness. JAMA. 2013;310(15):1591–600.
Dvir D, Cohen J, Singer P. Computerized energy balance and complications in critically ill patients: an observational study. Clin Nutr. 2006;25(1):37–44.
Villet S, Chiolero RL, Bollmann MD, et al. Negative impact of hypocaloric feeding and energy balance on clinical outcome in ICU patients. Clin Nutr. 2005;24(4):502–9.
Al-Majid S, Gray DP. A biobehavioral model for the study of exercise interventions in cancer-related fatigue. Biol Res Nurs. 2009;10(4):381–91.
Finnerty CC, Jeschke MG, Qian WJ, et al. Determination of burn patient outcome by large-scale quantitative discovery proteomics. Crit Care Med. 2013;41(6):1421–34.
Hart DW, Wolf SE, Chinkes DL, et al. Determinants of skeletal muscle catabolism after severe burn. Ann Surg. 2000;232(4):455–65.
Morley JE, Argiles JM, Evans WJ, et al. Nutritional recommendations for the management of sarcopenia. J Am Med Dir Assoc. 2010;11(6):391–6. Provides Society for Sarcopenia, Cachexia, and Wasting Disease recommendations for prevention and management of sarcopenia, advocating for exercise with both adequate protein and energy intake.
Cuenca AG, Cuenca AL, Winfield RD, et al. Novel role for tumor-induced expansion of myeloid-derived cells in cancer cachexia. J Immunol. 2014;192(12):6111–9.
Schefold JC, Bierbrauer J, Weber-Carstens S. Intensive care unit-acquired weakness (ICUAW) and muscle wasting in critically ill patients with severe sepsis and septic shock. J Cachexia Sarcopenia Muscle. 2010;1(2):147–57.
Delano MJ, Moldawer LL. The origins of cachexia in acute and chronic inflammatory diseases. Nutr Clin Pract. 2006;21(1):68–81.
WHO Consultation on Obesity. Obesity: preventing and managing the global epidemic. Report of a WHO consultation. World Health Organ Tech Rep Ser. 2000;894:i–xii, 1–253.
Martino JL, Stapleton RD, Wang M, et al. Extreme obesity and outcomes in critically ill patients. Chest. 2011;140(5):1198–206.
Pickkers P, de Keizer N, Dusseljee J, Weerheijm D, van der Hoeven JG, Peek N. Body mass index is associated with hospital mortality in critically ill patients: an observational cohort study. Crit Care Med. 2013;41(8):1878–83. A large observational database supports the obesity paradox in critically ill patients—an inverse association between obesity and hospital mortality.
Akinnusi ME, Pineda LA, El Solh AA. Effect of obesity on intensive care morbidity and mortality: a meta-analysis. Crit Care Med. 2008;36(1):151–8.
Hutagalung R, Marques J, Kobylka K, et al. The obesity paradox in surgical intensive care unit patients. Intensive Care Med. 2011;37(11):1793–9.
Arabi YM, Dara SI, Tamim HM, et al. Clinical characteristics, sepsis interventions and outcomes in the obese patients with septic shock: an international multicenter cohort study. Crit Care. 2013;17(2):R72.
Hauner H. Secretory factors from human adipose tissue and their functional role. Proc Nutr Soc. 2005;64(2):163–9.
Bornstein SR, Licinio J, Tauchnitz R, et al. Plasma leptin levels are increased in survivors of acute sepsis: associated loss of diurnal rhythm, in cortisol and leptin secretion. J Clin Endocrinol Metab. 1998;83(1):280–3.
Serhan CN, Chiang N, Van Dyke TE. Resolving inflammation: dual anti-inflammatory and pro-resolution lipid mediators. Nat Rev Immunol. 2008;8(5):349–61. A biochemical study showing resolution of inflammation to be an active biochemical set of programs to return inflamed tissues to baseline.
Wendel M, Paul R, Heller AR. Lipoproteins in inflammation and sepsis. II. Clinical aspects. Intensive Care Med. 2007;33(1):25–35.
Kalantar-Zadeh K, Streja E, Molnar MZ, et al. Mortality prediction by surrogates of body composition: an examination of the obesity paradox in hemodialysis patients using composite ranking score analysis. Am J Epidemiol. 2012;175(8):793–803.
Adamo ML, Farrar RP. Resistance training, and IGF involvement in the maintenance of muscle mass during the aging process. Ageing Res Rev. 2006;5(3):310–31.
Weijs PJ, Vansant GA. Validity of predictive equations for resting energy expenditure in Belgian normal weight to morbid obese women. Clin Nutr. 2010;29(3):347–51.
Thoresen L, Frykholm G, Lydersen S, et al. Nutritional status, cachexia and survival in patients with advanced colorectal carcinoma. Different assessment criteria for nutritional status provide unequal results. Clin Nutr. 2013;32(1):65–72.
Prado CM, Lieffers JR, McCargar LJ, et al. Prevalence and clinical implications of sarcopenic obesity in patients with solid tumours of the respiratory and gastrointestinal tracts: a population-based study. Lancet Oncol. 2008;9(7):629–35.
Fielding RA, Vellas B, Evans WJ, et al. Sarcopenia: an undiagnosed condition in older adults. Current consensus definition: prevalence, etiology, and consequences. International working group on sarcopenia. J Am Med Dir Assoc. 2011;12(4):249–56.
Delmonico MJ, Harris TB, Lee JS, et al. Alternative definitions of sarcopenia, lower extremity performance, and functional impairment with aging in older men and women. J Am Geriatr Soc. 2007;55(5):769–74.
Bartali B, Frongillo EA, Bandinelli S, et al. Low nutrient intake is an essential component of frailty in older persons. J Gerontol A Biol Sci Med Sci. 2006;61(6):589–93.
Mangione KK, Miller AH, Naughton IV. Cochrane review: improving physical function and performance with progressive resistance strength training in older adults. Phys Ther. 2010;90(12):1711–5.
Campbell WW. Synergistic use of higher-protein diets or nutritional supplements with resistance training to counter sarcopenia. Nutr Rev. 2007;65(9):416–22.
Houston DK, Nicklas BJ, Ding J, et al. Dietary protein intake is associated with lean mass change in older, community-dwelling adults: the Health, Aging, and Body Composition (Health ABC) Study. Am J Clin Nutr. 2008;87(1):150–5.
Paddon-Jones D, Short KR, Campbell WW, Volpi E, Wolfe RR. Role of dietary protein in the sarcopenia of aging. Am J Clin Nutr. 2008;87(5):1562S–6.
Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56(3):M146–56.
Hubbard RE, Woodhouse KW. Frailty, inflammation and the elderly. Biogerontology. 2010;11(5):635–41.
Hubbard RE, O’Mahony MS, Calver BL, Woodhouse KW. Nutrition, inflammation, and leptin levels in aging and frailty. J Am Geriatr Soc. 2008;56(2):279–84.
Villareal DT, Smith GI, Sinacore DR, Shah K, Mittendorfer B. Regular multicomponent exercise increases physical fitness and muscle protein anabolism in frail, obese, older adults. Obesity (Silver Spring). 2011;19(2):312–8.
Manning EM, Shenkin A. Nutritional assessment in the critically ill. Crit Care Clin. 1995;11(3):603–34.
Chi-Fishman G, Hicks JE, Cintas HM, Sonies BC, Gerber LH. Ultrasound imaging distinguishes between normal and weak muscle. Arch Phys Med Rehabil. 2004;85(6):980–6.
Freilich RJ, Kirsner RL, Byrne E. Isometric strength and thickness relationships in human quadriceps muscle. Neuromuscul Disord. 1995;5(5):415–22.
Mueller N, Murthy S, Tainter CR, et al. Can sarcopenia quantified by ultrasound of the rectus femoris predict adverse outcome of surgical intensive care patients as well as frailty? A prospective, observational cohort study. Ann Surg. 2015.
Grimm A, Teschner U, Porzelius C, et al. Muscle ultrasound for assessment of illness neuromyopathy in severe sepsis. Crit Care. 2013;17(5):R227.
Takai Y, Katsumata Y, Kawakami Y, Kanehisa H, Fukunaga T. Ultrasound method for estimating the cross-sectional area of the psoas major muscle. Med Sci Sports Exerc. 2011;43(10):2000–4.
Miyatani M, Kanehisa H, Ito M, Kawakami Y, Fukunaga T. The accuracy of volume estimates using muscle thickness measurements in different muscle groups. Eur J Appl Physiol. 2004;91(2-3):264–72.
Sanada K, Kearns CF, Midorikawa T, Abe T. Prediction and validation of total and regional skeletal muscle mass by ultrasound in Japanese adults. Eur J Appl Physiol. 2006;96(1):24–31.
Berger A. Bone mineral density scans. BMJ. 2002;325(7362):484.
Visser M, Fuerst T, Lang T, Salamone L, Harris TB. Validity of fan-beam dual-energy X-ray absorptiometry for measuring fat-free mass and leg muscle mass. Health, Aging, and Body Composition Study—Dual-Energy X-ray Absorptiometry and Body Composition Working Group. J Appl Physiol (1985). 1999;87(4):1513–20.
Wang ZM, Visser M, Ma R, et al. Skeletal muscle mass: evaluation of neutron activation and dual-energy X-ray absorptiometry methods. J Appl Physiol (1985). 1996;80(3):824–31.
Rothney MP, Brychta RJ, Schaefer EV, Chen KY, Skarulis MC. Body composition measured by dual-energy X-ray absorptiometry half-body scans in obese adults. Obesity (Silver Spring). 2009;17(6):1281–6.
Janssen I, Baumgartner RN, Ross R, Rosenberg IH, Roubenoff R. Skeletal muscle cutpoints associated with elevated physical disability risk in older men and women. Am J Epidemiol. 2004;159(4):413–21.
Levine JA, Abboud L, Barry M, Reed JE, Sheedy PF, Jensen MD. Measuring leg muscle and fat mass in humans: comparison of CT and dual-energy X-ray absorptiometry. J Appl Physiol (1985). 2000;88(2):452–6.
Reid KF, Naumova EN, Carabello RJ, Phillips EM, Fielding RA. Lower extremity muscle mass predicts functional performance in mobility-limited elders. J Nutr Health Aging. 2008;12(7):493–8.
Kyle UG, Bosaeus I, De Lorenzo AD, et al. Bioelectrical impedance analysis—part I: review of principles and methods. Clin Nutr. 2004;23(5):1226–43.
Lukaski HC, Bolonchuk WW, Hall CB, Siders WA. Validation of tetrapolar bioelectrical impedance method to assess human body composition. J Appl Physiol (1985). 1986;60(4):1327–32.
Máttar JA. Application of total body bioimpedance to the critically ill patient. Brazilian Group for Bioimpedance Study. New Horiz. 1996;4(4):493–503.
Sheean PM, Peterson SJ, Gomez Perez S, et al. The prevalence of sarcopenia in patients with respiratory failure classified as normally nourished using computed tomography and subjective global assessment. JPEN J Parenter Enteral Nutr. 2014;38(7):873–9.
Hanna JS. Sarcopenia and critical illness: a deadly combination in the elderly. JPEN J Parenter Enteral Nutr. 2015;39(3):273–81.
Denison HJ, Cooper C, Sayer AA, Robinson SM. Prevention and optimal management of sarcopenia: a review of combined exercise and nutrition interventions to improve muscle outcomes in older people. Clin Interv Aging. 2015;10:859–69.
Deutz NE, Bauer JM, Barazzoni R, et al. Protein intake and exercise for optimal muscle function with aging: recommendations from the ESPEN Expert Group. Clin Nutr. 2014;33(6):929–36.
McClave SA, Taylor BE, Martindale RG, et al. Guidelines for the provision and assessment of nutrition support therapy in the adult critically ill patient: Society of Critical Care Medicine (SCCM) and American Society for Parenteral and Enteral Nutrition (A.S.P.E.N.). J Parenter Enteral Nutr. 2016;40(2):159–211.
Berger A. Magnetic resonance imaging. BMJ. 2002;324(7328):35.
Ross R, Pedwell H, Rissanen J. Effects of energy restriction and exercise on skeletal muscle and adipose tissue in women as measured by magnetic resonance imaging. Am J Clin Nutr. 1995;61(6):1179–85.
Ross R, Rissanen J, Pedwell H, Clifford J, Shragge P. Influence of diet and exercise on skeletal muscle and visceral adipose tissue in men. J Appl Physiol (1985). 1996;81(6):2445–55.
Kim J, Wang Z, Heymsfield SB, Baumgartner RN, Gallagher D. Total-body skeletal muscle mass: estimation by a new dual-energy X-ray absorptiometry method. Am J Clin Nutr. 2002;76(2):378–83.
Visser M, Goodpaster BH, Kritchevsky SB, et al. Muscle mass, muscle strength, and muscle fat infiltration as predictors of incident mobility limitations in well-functioning older persons. J Gerontol A Biol Sci Med Sci. 2005;60(3):324–33.
Reeves ND, Maganaris CN, Narici MV. Ultrasonographic assessment of human skeletal muscle size. Eur J Appl Physiol. 2004;91(1):116–8.
Pahor M, Manini T, Cesari M. Sarcopenia: clinical evaluation, biological markers and other evaluation tools. J Nutr Health Aging. 2009;13(8):724–8. Reviews the most common methods to assess sarcopenia.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Panna A. Codner declares that she has no conflict of interest.
Kristin Shields declares that she has no conflict of interest.
Matthew Kappus declares that he has no conflict of interest.
Bryan Collier has received honoraria for lectures from the Nestlé Nutrition Institute and Abbott Nutrition.
Martin Rosenthal declares that he has no conflict of interest.
Robert G. Martindale declares that he has no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Gastroenterology, Critical Care, and Lifestyle Medicine
Rights and permissions
About this article
Cite this article
Codner, P.A., Shields, K., Kappus, M. et al. Comparative Measures of Lean Body Tissues in the Clinical Setting. Curr Nutr Rep 5, 191–198 (2016). https://doi.org/10.1007/s13668-016-0169-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13668-016-0169-3