Abstract
The world tomato production is threatened by the invasive tomato borer Tuta absoluta. Difficulties in managing this pest were imposed mainly by the development of resistance in strains treated with conventional chemical insecticides. Resistance problems were even reported to insecticides of natural origin, leading to search for other control alternatives. P2 is a spontaneous mutant of the entomopathogenic fungus Beauveria bassiana. It was previously selected from a local strain (P1) and was characterized as hyper-producer of extracellular proteases. Here, the insecticidal potential of P1 and P2 strains was evaluated against T. absoluta larvae under laboratory conditions. Both strains were effective but P2 showed stronger effect than P1; median lethal concentration of P2 is tenfold lower than that of P1. Enzymatic assay analysis showed that extracellular enzymes are differently expressed by the two strains, especially proteases and chitinases which are known as cuticle degrading enzymes. The major expressed subtilisin-like protease (SBP) was upregulated at the transcriptional level in P2 strain. Proteomic analysis revealed four SBP isoforms which are highly over-expressed in this strain compared to P1. Post-translational regulation, most probably phosphorylation, was further suggested to control the SBP protease expression in B. bassiana P1 and P2 strains. The enzymatic profile in the two strains might explain their different insecticidal potential against the tomato borer. This is the first report showing such efficiency of Beauveria strains against this dangerous pest. Particularly, P2 strain showed high virulence reaching almost total larval mortality within 5 days post-application. It thus should be recommended as a new tool for the biocontrol of T. absoluta.
Similar content being viewed by others
1 Introduction
The tomato leaf miner, or tomato borer, Tuta absoluta (Meyrick) (Lepidoptera: Gelechiidae) is considered as one of the most damaging tomato pest. It originated in South America and has recently spread to Europe and North African countries. It leads to serious losses, affecting up to 100 % of tomato crops (Campos et al. 2014; Pratissoli and Parra 2000). Larvae, which are the harmful stage, can feed on all parts of the plants (leaf, stem or fruit) and form mines within (Fig. 1). Several chemical insecticides such as abamectin, cartap and permethrin have been widely used against T. absoluta but later were abandoned because of widespread resistance (Siqueira et al. 2000). Other considerable problems were associated with the use of these conventional insecticides, such as environmental, ecological and human health effects. Furthermore, organically produced tomatoes have been another factor leading to the use of bioinsecticides, mainly spinosad. However, reports of resistance counterpoint the potential for its further use (Campos et al. 2014). Another promising biocontrol strategy is the use of indigenous natural enemies. Various predators (i.e. Nesidiocoris tenuis, Mimulus pygmaeus) and parasitoids (i.e. Trichogramma achaea, Neochrysocharis formosa, Bracon sp.) species spontaneously attack T. absoluta in tomato crops worldwide. Some of these are already commercialized and have been employed successfully in integrated pest management strategies, i.e. the parasitoid T. achaea which is used by periodic inundative releases, individually and in association with mirid predators, i.e. M. pygmaeus and N. tenuis (Zappala et al. 2013). Bacillus thuringiensis is also efficient in controlling T. absoluta when pulverized in greenhouse and open-field tomato plants (González-Cabrera et al. 2011).
T. absoluta, a dangerous insect pest to tomato plants and fruit. Tomato leaf and fruit damages. Larvae feed on the leaf mesophyll, ripe and unripe fruit creating mines and holes (a). Signs of damage on the tomato plant. Clear patches appear on the leaves affecting the photosynthetic capacity of plants and later become necrotic resulting in a subsequent reduction in the crop yield (b)
Entomopathogenic fungi are under intensive study since they are known to be non-toxic for animals and humans and to be safer for the environment than chemical products. They have been formulated for application in agricultural insect pest management systems with successful results.
The most common commercial mycoinsecticides and mycoacaricides on the market are based on Beauveria bassiana (33.9 %), Metarhizium anisopliae (33.9 %), Isaria fumosorosea (5.8 %) and Beauveria brongniartii (4.1 %) (Faria and Wraight 2007). These fungi infect the host insect by direct penetration of the insect cuticle which consists mainly of proteins and chitin, by producing extracellular enzymes directed to hydrolyse these cuticular components. Proteases and chitinases, which are the primary virulence determinants, were the most studied enzymes and their role in virulence is well documented (Fan et al. 2007; St. Leger et al. 1996). Furthermore, fungal virulence may attribute other factors and molecules including the hydrophobicity of conidia and the secretion of toxins (Ortiz-Urquiza et al. 2010). Few studies have investigated on the pathogenicity of Beauveria strains towards T. absoluta. Laboratory experiments showed that different isolates of this fungus, pulverized on eggs and larvae of the tomato borer, were more virulent to eggs (Pires et al. 2010). Higher virulence was found when the first instar larvae were treated with M. anisopliae, compared to B. bassiana strains. However, no total efficiency was found using any strain (Inanli et al. 2012; Pires et al. 2010). Better results were obtained when T. absoluta larvae were fed with leaves treated with B. bassiana (Giustolin et al. 2001; Shalaby et al. 2013), reaching 100 % of total larval mortality (Klieber and Reineke 2015).
In this study, we evaluated two Tunisian strains of B. bassiana for their pathogenicity towards T. absoluta larvae. These strains, P1 and P2, were previously isolated and studied for their extracellular proteases production (Borgi and Gargouri 2014). Higher yields were found in P2, and the major expressed protease (subtilisin-like protease [SBP]), which is a serine subtilisin-like, was purified and the gene encoding this enzyme was sequenced (Borgi and Gargouri 2014). Here, the secretomes from both strains was compared via two-dimensional electrophoresis (2-DE) coupled to mass spectrometry analysis. These strains were also used for the assays of cuticle degrading enzymes.
2 Materials and methods
2.1 Chemical reagents
All reagents and chemicals were obtained from either Sigma-Aldrich (USA/Steinheim, Germany), Biorad (München, Germany) or Amersham-Biosciences (Amersham, UK).
2.2 Fungal strains
B. bassiana P1 and P2 are laboratory strains, identified by sequencing the 18S ribosomal ribonucleic acid (rRNA) genes and comparing sequences with GenBank database via Blast (https://blast.ncbi.nlm.nih.gov/Blast.cgi). Identification was confirmed by the “Centraal Bureau voor Schimmelculture” of Holland (Borgi and Gargouri 2014). The P1 wild-type strain was deposited in the National Collection of Microorganisms of the Center of Biotechnology of Sfax (Tunisia) under the reference CTM10549 P1.
B. bassiana strains (P1 and P2) were cultured on Luria Bertani solid medium (5 g NaCl, 10 g bacto-peptone, 5 g yeast extract, 17 g agar and 1 l distilled water) at 30 °C for 12 days before being used in experiments. Aerial conidia were harvested in distilled water supplemented with 0.1 % Tween 80, filtered and counted using a haemocytometer. The filtrates were centrifuged, and the final concentrations were adjusted to 109 conidia/ml in the sterile water supplemented with glycerol 10 %.
2.3 Bioassays with T. absoluta larvae
A laboratory colony of T. absoluta was established from a Tunisian tomato field. This colony was maintained on tomato plants which were placed in pots and held in rearing plastic cages (50 cm × 50 cm × 50 cm) under laboratory conditions (25 ± 3 °C, 60 ± 5 % relative humidity with a natural photoperiod of ~16 h light/8 h dark). Emerged adults from pupae were fed on 10 % honey solution and tomato terminal buds and fresh leaves. After oviposition, T. absoluta larvae were used in the bioassays.
Ten conidial suspensions of Beauveria strains P1 and P2 (102 to 109 conidia/ml) were prepared by serial dilution and used for infection. Each suspension was spread onto larvae (second to fourth larval instars), resulting in 10 μl of conidial suspension/larvae. Control larvae were treated with sterile H2O. For each experimental condition, 30 larvae were used. Each 10 larvae were transferred in individual Petri dishes containing 10 % honey solution and fresh leaves and incubated in an incubator at 26 ± 0.5 °C and 72 ± 5 % relative humidity with a natural photoperiod of ~16 h light/8 h dark. The number of dead larvae was recorded daily. All experiments were repeated three times.
2.4 Enzyme production and assays
Hydrolytic enzymes were produced on modified Mandels liquid medium (Borgi and Gargouri 2014), supplemented with 1 % of skimmed milk. Cultures were inoculated with 107 conidia/ml of P1 or P2 strain and incubated at 30 °C and 150 rpm. After different incubation times, cultures media were centrifuged at 6000 g for 10 min at room temperature and the supernatants were used for the assays as follows.
Protease activity was measured by the method of Kembhavi et al. (1993) using casein as substrate. One unit of protease activity was defined as the amount of enzyme required to liberate 1 μg tyrosine/ml in 1 min at pH 8 and 60 °C.
Chitinase activity assay was performed as previously described (Kim and Yang 2003). One unit of chitinase activity was defined as the amount of enzyme that released sugars equivalent to 1 mol of N-acetylglucosamine per hour at pH 5 and 50 °C.
β-Glucosidase was assayed with p-nitrophenyl-β-d-glucopyranoside (pNPG; Sigma) according to Saibi and Gargouri (2011), with one unit of activity being the amount of enzyme required to produce 1 μmol of p-nitrophenol per minute at pH 5 and 60 °C.
β-galactosidase activity was monitored by measuring the increase in glucose concentrations over time using lactose as substrate at pH 6 and 60 °C (Nguyen et al. 2006). The glucose formed in the reaction was determined as described by Kunst et al. (1988). One unit of activity corresponds to the amount of enzyme that liberates 1 μmol of glucose per minute in the reaction conditions.
2.5 Two-dimensional electrophoresis
Six-day cultures were used to analyse extracellular proteins from P1 and P2 strains. Two hundred microgrammes of the extracellular supernatants (filtered through a 0.45-μm filter) was loaded on isoelectro focussing strips with a non-linear gradient of ampholytes ranging from pH 3 to 10 using the isoelectro focussing cell system. After the IEF process, the strips were run in SDS-PAGE according to Laemmli (1970). Gels were stained with colloidal Coomassie blue G250. Gels were scanned using the GS-800 imaging densitometer (Bio-Rad) and analysed using the PDQuest software (version 8.0.1; Bio-Rad). Proteins making difference between P1 and P2 in 2-D profiles were manually excised. This experiment was repeated three times.
2.6 Protein identification by mass spectrometry
Spots were destained in 25 mM ammonium bicarbonate (NH4HCO3), 50 % (v/v) acetonitrile (ACN; VWR Chemicals) and shrunk in ACN for 10 min. After ACN removal, gel pieces were dried at room temperature. Proteins were digested by incubating each gel slice with 10 ng/μl of trypsin (T6567, Sigma-Aldrich) in 40 mM NH4HCO3, 10 % (v/v) ACN; rehydrated at 4 °C for 10 min; and finally incubated overnight at 37 °C. The resulting peptides were extracted from the gel in three steps: a first incubation in 40 mM NH4HCO3, 10 % (v/v) ACN for 15 min at room temperature and two incubations in 47.5 % (v/v) ACN and 5 % (v/v) formic acid (Sigma) for 15 min at room temperature. The three collected extractions were pooled with the initial digestion supernatant, dried in a vacuum centrifuge (SpeedVac; Eppendorf) and resuspended with 25 μl of 0.1 % (v/v) formic acid before performing the nano-LC-MS/MS analysis.
Peptide mixtures were analysed by online capillary nano-HPLC (LC Packings, Amsterdam, The Netherlands) coupled to a nano-spray LCQ Deca XP ion trap mass spectrometer (ThermoFinnigan, San Jose, CA, USA). Ten microlitres of each peptide extract were loaded on a 300 μm ID × 5 mm PepMap C18 precolumn (LC Packings, Dionex, USA) at a flow rate of 20 μl/min. After 5 min desalting, peptides were online separated on a 75 μm internal diameter × 15 cm C18 PepMapTM column (LC Packings, Amsterdam, The Netherlands) with a 5–40 % linear gradient of solvent B in 48 min (solvent A was 0.1 % (v/v) formic acid in 5 % (v/v) ACN, and solvent B was 0.1 % (v/v) formic acid in 80 % (v/v) ACN). The separation flow rate was set at 200 nl/min. The mass spectrometer operated in positive ion mode at a 1.8-kV needle voltage and a 4-V capillary voltage. Data acquisition was performed in a data-dependent mode alternating in a single run, an MS scan survey over the range m/z 300–1700 and three MS/MS scans with collision induced dissociation (CID) as activation mode. MS/MS spectra were acquired using a 2-m/z unit ion isolation window, a 35 % relative collision energy and a 0.5 min dynamic exclusion duration.
Mascot, MS Amanda and Sequest algorithms through Proteome Discoverer 1.4 Software (Thermo Fisher Scientific Inc., USA) were used for protein identification against the UniProt B. bassiana database (http://www.uniprot.org/taxonomy/176275 ; 22,152 entries). Two missed enzyme cleavages were allowed. Mass tolerances in MS and MS/MS were set to 2 and 1 Da, respectively. Oxidation of methionine, deamidation of asparagine and glutamine and acetylation of lysine and protein N-terminus were searched as dynamic modifications. Carbamidomethylation on cysteine was searched as static modification. Peptide validation was performed using Target Decoy PSM Validator, and only “high confidence” peptides were retained corresponding to a 1 % false positive rate at peptide level. The mass spectrometry proteomics data have been deposited to the ProteomeXchange Consortium (Vizcaín et al. 2014) via the PRIDE partner repository (http://www.ebi.ac.uk/pride/help/archive/about) with the dataset identifier PXD003287. Identification results are presented in supplementary data 1.
2.7 Statistical analysis
SPSS version 16.0 for Windows was used for all statistical analysis (SPSS Inc. 2008, USA). Cumulative larval mortality was corrected for natural mortality using Abbott’s formula (Abbott 1925). Larval mortality and enzyme production data were statistically analysed using repeated measure ANOVA and the general linear model (GLM). Means were separated by Tukey’s HSD test (p = 0.05). The median lethal concentration and time values were calculated using the Probit analysis.
3 Results and discussion
3.1 Evaluation of P1 and P2 strains for their virulence against T. absoluta
The tomato leafminer, T. absoluta, is a major pest insect which attacks solanaceous crops. Chemical control failed to overcome this pest because of resistance development, the mine-feeding behaviour of larvae and deficient spraying technology (Siqueira et al. 2000). In the search for other pest management, mycoinsecticides are an important tool among the new alternatives to be considered.
In this study, two strains P1 and P2 of B. bassiana were evaluated for their virulence towards T. absoluta larvae, under laboratory conditions. Larvae were chosen for treatments since it is the damaging stage affecting all parts of plant especially fruits. Bioassays were performed by topical application which is the conventional method used in biological control applications.
Different concentrations of both strains conidia were tested on the daily larval mortality of T. absoluta (Table 1). Larval mortality caused by each strain increased proportionally with conidia concentration and time post-applications. Repeated measures ANOVA analysis showed a significant difference among treatments (at all concentrations) over the whole period of bioassay (F = 74.9; df = 1.51; p < 0.001). At each concentration, there was a statistically significant difference between P1 and P2 treatments. For example, at 102 conidia/ml, F = 15.9; df = 1.4; p = 0.016; At 109 conidia/ml, F = 32.8; df = 1.4; p = 0.005 (Table 1). The concentration/time–mortality responses for each strain were fitted to regression lines, and the respective median lethal concentration and time values were obtained using the Probit analysis (Table 2). For all the regression lines, the chi-squared values were non-significant (p > 0.05), indicating a good fit of the regressions. At day 5 post-applications, the median lethal concentration of P1 was 10 times higher than that of P2 (1.22 × 105 and 1.21 × 104 conidia/ml, respectively). Moreover, at a concentration of 105 conidia/ml, the median lethal times were 5.8 days and only 1.03 day for P1 and P2, respectively (Table 2). The lower median lethal concentration and time found with P2 conidia treatment indicated the higher virulence of this strain towards T. absoluta larvae than P1. This is the first study showing such efficiency of B. bassiana in controlling T. absoluta, reaching 96.7 % of larval mortality using topical application of P2 strain (109 conidia/ml).
Pires et al. (2010) evaluated the entomopathogenic action of five B. bassiana isolates against eggs and larvae of T. absoluta. The tested isolates were pathogenic to eggs and larvae with a higher effect on eggs. However, the total mortality of the first instar larvae treated by the most virulent isolate of Beauveria (107 conidia/ml) reached no more than 37 % by the end of the period of larval development. The same report showed higher larval mortality using M. anisopliae conidia at the same concentration, reaching 56 %. Later, Inanli et al. (2012) found B. bassiana and M. anisopliae strains pathogenic on the first larval stage of T. absoluta with mortality of 12.5 and 91.67 %, respectively, 9 days after application. Higher efficiency was shown by Shalaby et al. (2013): total larval mortality reached about 70 % when T. absoluta larvae were fed with leaves treated with B. bassiana.
In general, 5 to 10 days are required to kill an insect pest after application of a mycopesticide (Quesada-Moraga and Vey 2004). Increasing the speed of kill is the aim of recent researches to pursue the development of entomopathogenic fungi as commercial mycopesticides (Quesada-Moraga and Vey 2004). We showed that the death of 50.5 % of T. absoluta larvae treated with 105 conidia/ml of P2 was recorded after only 1 day. Higher concentration of conidia (8.108 conidia/ml) allowed increasing the larval mortality to 86.3 and 92.5 %, 1 and 2 days post-application. P1 strain exhibited lower virulence than P2; 91.3 % of larval mortality could be reached 5 days post-application of 109 conidia/ml (Table 1).
3.2 Regulation of enzymes associated with virulence
In this study, topical application method was used to mimic the natural mode of infection which does not require any specialized way of invasion. B. bassiana acts by direct penetration anywhere on the insect cuticle after the germination of conidia and the release of cuticle-degrading enzymes. Subtilisin-like serine proteases and chitinases are classified among these enzymes because they are involved in the penetration and digestion of insect cuticles; therefore, they are strongly associated with virulence (Fang et al. 2005; Fan et al. 2007; Joshi et al. 1995; Li et al. 2010). Besides, β-glucosidase, β-galactosidase, and N-acetylglucosaminidase activities from a strain of B. bassiana showed a specific activity against locusts (Quesada-Moraga and Vey 2004). For this reason, the study of secreted enzymes is an important field in research on filamentous fungi.
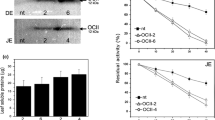
To find enzymatic correlation with the higher virulence of P2, we compared the production of extracellular protease, chitinase, β-glucosidase and β-galactosidase. As previously shown, P2 exhibited a protease activity, nine times more than P1, by day 10 (Borgi and Gargouri 2014). A high significant difference was shown over the whole period of production (F = 688.5; df = 1.4; p < 0.001) (Fig. 2a). Likewise, this strain over-produced at a lesser but significant extent a chitinase activity, approximately fourfold higher than P1 (F = 858.1; df = 1.4; p < 0.001) (Fig. 2b). β-Galactosidase was produced at the same yield (F = 1.3; df = 1.4; p = 0.304) in both strains, while P2 showed lower β-glucosidase activity than P1 (F = 36.3; df = 1.4; p < 001) (Fig. 2c, d). The over-expression of protease and chitinase is well demonstrated to be associated with increased virulence of M. anisopliae and B. bassiana strains (Fang et al. 2005; Fan et al. 2007; St Leger et al. 1996). Obviously, the increased pathogenesis of P2 strain to T. absoluta larvae is correlated with the increased levels of these enzymes.
In a previous report, we isolated the gene encoding a subtilisin-like protease (SBP) produced by P1 and P2 strains (Borgi and Gargouri 2014). Its sequence is available in GenBank with the accession no. KF562267.1. Subtilisins are among the most important determinants of entomopathogens virulence by degrading host cuticles, providing nutrition and inhibiting antimicrobial peptides (Bagga et al. 2004). These enzymes are well represented in the genomes of entomopathogenic fungi due to lineage-specific duplications (Gao et al. 2011; Li et al. 2010). It has been shown that SBP is highly homologous to subtilisins having a cuticle degrading capacity (Borgi and Gargouri 2014). Therefore, it was important to confirm if the higher virulence of P2 is correlated with a different SBP regulation compared to P1. The hyper-production of total extracellular protease in P2 might suggest an increased genic dose of the sbp protease gene in this strain. Southern blot analysis with genomic DNA digested with HindIII showed that sbp is a single-copy gene in both strains B. bassiana P1 and P2 (data not shown). This result implies that there is no duplication of the sbp gene in the P2 genome. Likewise, Fang et al. (2002) showed that a cuticle-degrading endoprotease (exhibiting 99 % of homology with SBP) was present as singly copy in the genome of B. bassiana. Instead, a transcriptional upregulation was suggested to be responsible of the protease hyper-production in P2 as the sbp transcript level in this strain was strongly increased versus P1 (data not shown).
3.3 Comparative extracellular proteomes of P1 and P2 via 2-DE/mass spectrometry analysis
The 2-DE coupled to LC-MS/MS was used to investigate all proteins differentially expressed in P1 and P2 secretomes. This study is useful to probe hyper-production of virulence-associated proteins, particularly to elucidate the SBP regulation at the translational level. The total extracellular proteins of P1 and P2 strains were fractionated using the 2-DE and 15 among the differently expressed ones were identified via mass spectrometry (Fig. 3). The comparison of the 2-DE maps revealed striking similarities. Almost all identified proteins were expressed in both strains but showed differences in abundance. The majority of these proteins are hydrolytic enzymes. The most abundant enzyme was the SBP identified in five different spots which correspond to the same molecular weight of around 30 kDa and pI varying from 3 to 10 (noted SBP 1 to SBP 5). It could be hypothesized that post-translational modification would be responsible for this electrophoretic behaviour. The most probable modification is phosphorylation as it greatly modifies the charge and faintly the weight of proteins. Only one of these isozymes, SBP 3, was equally expressed in both strains. However, the others were either exclusive to P2 (SBP 1 and SBP 4) or accumulated to significantly higher amounts in this strain compared to P1 (SBP 2 and SBP 5) (Fig. 3). These results suggested that the expression of SBP in Beauveria strains is under a double control: transcriptional and post-translational. Previous reports showed the occurrence of multiple subtilisin isoforms with different pI values and unique N-terminal sequences in the related entomopathogenic fungi M anisopliae (St Leger et al. 1994) and Verticillium chlamydosporium (Segers et al. 1999). It has been suggested that isoforms having the same molecular weight are post-translational variants or they represent gene families. Bye and Charnley (2008) found significant differences in substrate specificity and regulation of subtilisin-like proteases between isoforms of the same isolate and between different isolates of Lecanicillium spp. These isoforms, having pI values beween 6.48 and >9.47, included those sharing the same N-terminal sequence, which may have been derived from different transcripts from a single gene or from related genes.
Two-dimensional electrophoresis analysis. Extracellular proteins of P1 (on the left) and P2 strains (on the right). Numbers and arrows indicate the position of identified proteins. 1 β-glucosidase; 2 arabinofuranosidase; 3 tripeptidyl peptidase; 4 carboxypeptidase Y; 5, 6 and 7 major allergen Mal f1; 8 carboxypeptidase Y; 9 α-amylase; and 10 chitinase. Arrows from 11 to 15 denote SBP protease isoforms, named SBP 1 to SBP 5, respectively. Proteins from 1 to 8 and from 9 to 15 are upregulated in P1 and P2, respectively, except for 13 which is expressed at the same level in both strains. The pH range is indicated below the gel. Molecular weight marker in kilodalton is loaded on the left. This experiment was repeated three times
Recently, it has been demonstrated that the Cdc14 phosphatase has a hub role in asexual development multiple stress responses and virulence in B. bassiana, by regulating protein expression and many phosphorylation events (Wang et al. 2013, 2016). Phosphoproteomic analysis revealed 14 phosphorylation motifs from all the identified phosphorylation sites as potential substrates of various protein kinases targeted by Cdc14 (Wang et al. 2016). We found that 9 of these motifs exist in 14 phosphorylation sites predicted in the SBP protease sequence (GenBank accession no. KF562267.1), including 1 site SphxxE, 2 RxxTph, 1 SphP, 1 TphP, 3 RxSph, 1 RxxSph, 1 DSphxxE, 3 SphxP and 1 site TphxxP (Sph, Tph and x denote a phosphorylated serine, a phosphorylated threonine and a random amino acid residue, respectively). However, neither expression regulation nor phosphorylation of Pr1 proteases has been detected as events associated with Cdc14 in the study of Wang et al. (2016). This could be due to the time chosen by these authors (3-day-old culture samples) which was so early to identify all differently regulated proteins in fungi, especially post-translational modifications such phosphorylation of extracellular proteins, as suggested by the authors.
Figure 3 shows that a chitinase, a carboxypeptidase Y (spot 8) and an α-amylase were equally present in higher quantities in P2 strain. In P1 strain, four enzymes were found upregulated which are a β-glucosidase, an arabinofuranosidase, a carboxypeptidase Y (spot 4) and a tripeptidyl peptidase. Two isoforms of the major allergen Mal f1 were exclusive to P1 and another was over-expressed compared to P2.
A previous study showed by comparative transcriptome analysis that growth in culture containing insect cuticles does not mimic the environment experienced on the insect surfaces. Secreted proteins, particularly proteases, were highly more induced on insect cuticle surfaces (Gao et al. 2011). As the experiments of this study were performed in vitro, further experimentation is required to demonstrate the production, accumulation and localization of individual SBP protease isoforms and chitinase in insects during infection processes.
4 Conclusion
Difficulty in managing the tomato borer led to the spread of this dangerous specie in most of the countries, threatening the current world tomato production. Mycoinsecticides are among the best choices to control this pest. Here, we showed a high insecticidal potential of B. bassiana P1 and P2 strains towards T. absoluta larvae and significant better efficiency of P2 than P1. This allows the recommendation of P2 for the application in the management of the tomato borer. P2 is a mutant hyper-producer of extracellular proteases and chitinase, compared to the wild-type strain P1. We have identified four isoforms of the SBP, which were over-expressed in P2. Differently expressed isoforms were suggested to be the result of different transcriptional and translational regulation in both strains, and this might explain the higher virulence of P2 towards T. absoluta. These results showed that the profile of fungal extracellular enzymes, especially proteases, could be considered as a fingerprint reflecting pathogenicity towards insects; enzymatic assays may contribute to the screening of the most virulent strains.
References
Abbott WS (1925) A method of computing the effectiveness of an insecticide. J Econ Entomol. doi:10.1093/jee/18.2.265a
Bagga S, Hu G, Screen SE, St Leger RJ (2004) Reconstructing the diversification of subtilisins in the pathogenic fungus Metarhizium anisopliae. Gene. doi:10.1016/j.gene.2003.09.031
Borgi I, Gargouri A (2014) Investigations on a hyper-proteolytic mutant of Beauveria bassiana: broad substrate specificity and high biotechnological potential of a serine protease. FEMS Microbiol Lett. doi:10.1111/1574-6968.12339
Bye NJ, Charnley AK (2008) Regulation of cuticle-degrading subtilisin proteases from the entomopathogenic fungi, Lecanicillium spp: implications for host specificity. Arch Microbiol. doi:10.1007/s00203-007-0296-8
Campos MR, Rodrigues ARS, Silva WM, Silva TBM, Silva VRF, Guedes RNC, Siqueira HAA (2014) Spinosad and the tomato borer Tuta absoluta: a bioinsecticide, an invasive pest threat, and high insecticide resistance. PLoS One. doi:10.1371/journal.pone.0103235
Fan Y, Fang W, Guo S, Pei X, Zhang Y, Xiao Y, D L, Jin K, Bidochka MJ, Pei Y (2007) Increased insect virulence in Beauveria bassiana strains overexpressing an engineered chitinase. Appl Environ Microbiol. doi:10.1128/AEM.01974-06
Fang W, Zhang Y, Yang X, Wang Z, Pei Y (2002) Cloning and characterization of cuticle degrading enzyme CDEP-1 from Beauveria bassiana. Yi Chuan Xue Bao 29:278–282
Fang W, Leng B, Xiao Y, Jin K, J M, Fan Y, Feng J, Yang X, Zhang Y, Pei Y (2005) Cloning of Beauveria bassiana chitinase gene Bbchit1 and its application to improve fungal strain virulence. Appl Environ Microbiol. doi:10.1128/AEM.71.1.363-370.2005
Faria M, Wraight SP (2007) Mycoinsecticides and mycoacaricides: a comprehensive list with worldwide coverage and international classification of formulation types. Biol Control. doi:10.1016/j.biocontrol.2007.08.001
Gao Q, Jin K, Ying S-H, Zhang Y, Xiao G, Shang Y, Duan Z, Hu X, Xie X-Q, Zhou G, Peng G, Luo Z, Huang W, Wang B, Fang W, Wang S, Zhong Y, Ma L-J, St Leger RJ, Zhao G-P, Pei Y, Feng M-G, Xia Y, Wang C (2011) Genome sequencing and comparative transcriptomics of the model entomopathogenic fungi Metarhizium anisopliae and M. acridum. PLoS Genet. doi:10.1371/journal.pgen.1001264
Giustolin TA, Vendramim JD, Alves SB, Vieira SA (2001) Pathogenicity of Beauveria bassiana (Bals.) Vuill. to Tuta absoluta (Meyrick) (Lepidoptera: Gelechiidae) reared on two genotypes of tomato. Neotrop Entomol. doi:10.1590/S1519-566X2001000300013
González-Cabrera J, Mollá O, Montón H, Urbaneja A (2011) Efficacy of Bacillus thuringiensis (Berliner) in controlling the tomato borer, Tuta absoluta (Meyrick) (Lepidoptera: Gelechiidae). BioControl. doi:10.1007/s10526-010-9310-1
Inanli C, Yoldas Z, Birgücü AK (2012) Effects of entomopathogenic fungi, Beauveria bassiana (Bals.) and Metarhizium anisopliae (Metsch.) on larvae and egg stages of Tuta absoluta (Meyrick) (Lepidoptera: Gelechiidae). Ege Üniversitesi Ziraat Fakültesi Dergisi 49: 239–242
Joshi L, St Leger RJ, Bidochka MJ (1995) Cloning of a cuticle degrading protease from the entomopathogenic fungus, Beauveria bassiana. FEMS Microbiol Lett. doi:10.1111/j.1574-6968.1995.tb07360.x
Kembhavi AA, Kulharni A, Pant AA (1993) Salt-tolerant and thermostable alkaline protease from Bacillus subtilis NCIM no 64. Appl Biochem Biotechnol. doi:10.1007/BF02916414
Kim K-J, Yang Y-J (2003) Purification and characterization of chitinase from Streptomyces sp. M-20. J Biochem Mol Biol. doi:10.5483/BMBRep.2003.36.2.185
Klieber J, Reineke A (2015) The entomopathogen Beauveria bassiana has epiphytic and endophytic activity against the tomato leaf miner Tuta absoluta. J Appl Entomol. doi:10.1111/jen.12287
Kunst A, Draeger B, Ziegernhorn J (1988) Colorimetric methods with glucose oxidase and peroxidase. In: Bergmeyer HU, Bergmeyer J, Grassl M (eds) Methods of enzymatic analysis, 3rd edn. VCH Publishers, Weinheim, Ger, pp. 178–185
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. doi:10.1038/227680a0
Li J, Yu L, Yang J, Dong L, Tian B, Yu Z, Liang L, Zhang Y, Wang X, Zhang K (2010) New insights into the evolution of subtilisin-like serine protease genes in Pezizomycotina. BMC Evol Biol. doi:10.1186/1471-2148-10-68
Nguyen T-H, Splechtna B, Steinböck M, Kneifel W, Lettner HP, Kulbe KD, Haltrich D (2006) Purification and characterization of two novel β-galactosidases from Lactobacillus reuteri. J Agric Food Chem. doi:10.1021/jf053126u
Ortiz-Urquiza A, Riveiro-Miranda L, Santiago-Álvarez C, Quesada-Moraga E (2010) Insect-toxic secreted proteins and virulence of the entomopathogenic fungus Beauveria bassiana. J Invertebr Pathol. doi:10.1016/j.jip.2010.07.003
Pires LM, Marques JE, Oliveira VJ, Ahmed SB (2010) Selection of isolates of entomopathogenic fungi for controlling Tuta absoluta (Meyrick) (Lepidoptera: Gelechiidae) and their compatibility with insecticides used in tomato crop. Neotrop Entomol. doi:10.1590/S1519-566X2010000600020
Pratissoli D, Parra JRP (2000) Fertility life table of Trichogramma pretiosum (Hym., Trichogrammatidae) in eggs of Tuta absoluta and Phthorimaea operculella (Lep., Gelechiidae) at different temperatures. J Appl Ent. doi:10.1046/j.1439-0418.2000.00477.x
Quesada-Moraga E, Vey A (2004) Bassiacridin, a protein toxic for locusts secreted by the entomopathogenic fungus Beauveria bassiana. Mycol Res. doi:10.1017/S0953756204009724
Saibi W, Gargouri A (2011) Purification and biochemical characterization of an atypical β-glucosidase from Stachybotrys microspora. J Mol Catal B. doi:10.1016/j.molcatb.2011.05.007
Segers R, Butt TM, Carder JH, Keen JN, Kerry BR, Peberdy JF (1999) The subtilisins of fungal pathogens of insects, nematodes and plants: distribution and variation. Mycol Res. doi:10.1017/S0953756298007345
Shalaby HH, Faragalla FH, El-Saadany HM, Ibrahim AA (2013) Efficacy of three entomopathogenic agents for control the tomato borer, Tuta absoluta (Meyrick) (Lepidoptera: Gelechiidae. Nat Sci 11:63
Siqueira HQA, Guedes RNC, Picanco MC (2000) Insecticide resistance in populations of Tuta absoluta. Agric For Entomol. doi:10.1046/j.1461-9563.2000.00062.x
SPSS Version 16.0 for Windows 2008. SPSS Inc., Chicago
St Leger RJ, Bidochka MJ, Roberts DW (1994) Isoforms of the cuticle degrading Prl protease and production of a metalloproteinase by Metarhizium anisopliae. Arch Biochem Biophys. doi:10.1006/abbi.1994.1350
St Leger RJ, Joshi L, Bidochka MJ, Roberts DW (1996) Construction of an improved mycoinsecticide overexpressing a toxic protease. Proc Natl Acad Sci U S A. doi:10.1073/pnas.93.13.6349
Vizcaín JA, Deutsc EW, Wan R, Csorda A, Reisinge F, Río D, Diane JA, Su Z, Farra T, Bandeir N, Biz PA, Xenarios I, Eisenacher M, Mayer G, Gatto L, Campos A, Chalkley RJ, Kraus HJ, Albar JP, Martinez-Bartolomé S, Apweiler R, Omenn GS, Martens L, Jones AR, Hermjakob H (2014) ProteomeXchange provides globally coordinated proteomics data submission and dissemination. Nature Biotechnol. doi:10.1038/nbt.2839
Wang J, Liu J, Hu Y, Ying S-H, Feng M-G (2013) Cytokinesis-required Cdc14 is a signaling hub of asexual development and multi-stress tolerance in Beauveria bassiana. Sci Rep. doi:10.1038/srep03086
Wang Z-K, Wang J, Liu J, Ying S-H, Peng X-J, Feng M-G (2016) Proteomic and phosphoproteomic insights into a signaling hub role for Cdc14 in asexual development and multiple stress responses in Beauveria bassiana. PLoS One. doi:10.1371/journal.pone.0153007
Zappala L, Biondi A, Alma A, Al-Jboory IJ, Arno J, Bayram A, Chailleux A, El-Arnaouty A, Gerling D, Guenaoui Y, Shaltiel-Harpaz L, Siscaro G, Stavrinides M, Tavella L, Aznar, RV, Urbaneja A, Desneux N (2013) Natural enemies of the South American moth, Tuta absoluta, in Europe, North Africa and Middle East, and their potential use in pest control strategies. J Pest Sci. doi: 10.1007/s10340-013-0531-9
Acknowledgments
This work was undertaken and funded in the frame of our contract with the Ministry of Higher Education and Scientific Research, Tunisia.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors that they have no conflict of interest.
Electronic supplementary material
Data 1
Overview of identified homologous proteins in spots of interest. Identification was performed via nanoLC-MS/MS analysis using UniProt Beauvaria bassiana database. Spots number, spot label on figure 3; Spot number PRIDE, spot label on the PRIDE database; Proteins / spot, number of proteins identified in the corresponding spot; Accession, accession number of protein in the UniProt Beauvaria bassiana database; Description, name of protein; Coverage, Percentage of the protein sequence covered by identified peptides; # Proteins, Number of identified proteins in the protein group of a master protein; # Unique Peptides, Number of peptide sequences unique to a protein group; # Peptides, Number of distinct peptide sequences in the protein group; # PSMs, Total number of identified peptide sequences (PSM = peptide spectrum matches) for the protein, including those redundantly identified; # AAs, Sequence length of the protein; MW [kDa], Calculated molecular weight of the protein; calc. pI, Theoretically calculated isoelectric point; Peptides sequences, Lowercase letters are modified amino acids, (c) : Carbamidomethylation on cysteine, (m) : oxidation on methionine, (n, q) :Deamidation on asparagine or glutamine, (k) : acetylation on lysine; Score from Sequest, Sum of all peptide Xcorr values above the specified score threshold (1.6); Coverage from Sequest, Percentage of the protein sequence covered by identified peptides; # Peptides from Sequest, Number of distinct peptide sequences in the protein group; # PSM from Sequest, Total number of identified peptide sequences (PSM = peptide spectrum matches) for the protein, including those redundantly identified; Score from Mascot, Cumulative protein score based on summing the ion scores of the unique peptides identified for that protein; Coverage from Mascot, Percentage of the protein sequence covered by identified peptides; # Peptides from Mascot, Number of distinct peptide sequences in the protein group; # PSM from Mascot, Total number of identified peptide sequences (PSM = peptide spectrum matches) for the protein, including those redundantly identified; Score from MS Amanda, Cumulative protein score based on summing the ion scores of the unique peptides identified for that protein; Coverage from MS Amanda, Percentage of the protein sequence covered by identified peptides; # Peptides from MS Amanda, Number of distinct peptide sequences in the protein group; # PSM from MS Amanda, Total number of identified peptide sequences (PSM = peptide spectrum matches) for the protein, including those redundantly identified. (XLSX 21 kb)
About this article
Cite this article
Borgi, I., Dupuy, JW., Blibech, I. et al. Hyper-proteolytic mutant of Beauveria bassiana, a new biological control agent against the tomato borer. Agron. Sustain. Dev. 36, 60 (2016). https://doi.org/10.1007/s13593-016-0394-6
Accepted:
Published:
DOI: https://doi.org/10.1007/s13593-016-0394-6