Abstract
Background
IGF-1 plays a role in the growth of multiple tumor types, including pancreatic cancer. IGF-1 also serves as a growth factor for muscle. The impact of therapeutic targeting of IGF-1 on muscle mass is unknown.
Methods
We evaluated muscle mass at L3 in patients enrolled in a randomized phase II study of MK-0646 (M), a monoclonal antibody directed against the IGF-1 protein, in patients with metastatic pancreatic cancer (MPC). Two different doses of M were tested, 5 and 10 mg/kg. We used the Slice-o-matic (ver 4.3) software to segregate CT images into muscle and fat components and measured muscle area (cm2) at baseline and after 2 and 4 months of treatment. Patients received either gemcitabine with erlotinib (G + E), G + E + M, or G + M. Differences between the groups were compared using t tests.
Results
Fifty-three patients had both baseline and 2-month imaging available for analysis. Of these, 42 received M with their chemo, and 11 had G + E only. After 2 months of treatment, both groups demonstrated decrease in muscle mass. G + E patients lost 5.6 % of muscle mass; M patients lost 9.1 and 8.6 % after treatment with 5 and 10 mg/kg, respectively (p = 0.53). Patients demonstrating a response lost less muscle (median 4.6 %) than those with stable disease (9.6 %) and progressive disease (8.9 %, p = 0.14). Muscle retention from baseline to 2-month imaging, defined as loss of <6 cm2 of muscle, correlated with better survival than those patients demonstrating a muscle loss (HR 0.51, p = 0.03).
Conclusions
MPC patients can be expected to lose muscle mass even while having clinical benefit (PR or SD) from chemotherapy. Muscle loss correlated with a risk of study drop-out and death. There was a non-significant trend toward greater muscle mass loss in patients on anti-IGF-1R therapy. However, it is unclear if this loss translates into functional differences between patients.
Similar content being viewed by others
1 Introduction
Metastatic pancreatic cancer remains one of the most morbid of diseases, with a 5-year survival rate of less than 5 % and a median survival of approximately 1 year in the highest quality studies [1]. The family of insulin-like growth factor receptors (IGF-1R, IGF-2R) may play a role in the growth of these tumors. Binding of the IGF ligands (IGF-1 and IGF-2) to IGF-1R allows autophosphorylation of the receptor, which in turn allows docking of the insulin receptor substrates (IRS 1-4)[2]. These in turn may activate both the MEK and PI-3 kinase/AKT pathways in pancreatic cancer cells which lead to proliferation of tumor. Conversely, inhibition of IGF-1 and IGF-2 binding to IGF-1R with a monoclonal antibody inhibits growth of a variety of tumor types, including pancreatic cancer [3]. For this reason, we began a therapeutic trial of one such inhibitor, MK-0646; the results of which have been presented in abstract form [4].
Aside from its effect on cancer cells, there is a possibility that IGF-receptor inhibition may influence muscle mass [5]. Transgenic mice that overexpress IGF develop muscle mass hypertrophy [6]. IGF-1 is believed to mediate exercise-induced muscle hypertrophy and injury-triggered regeneration in skeletal muscle, [7, 8], and may increase skeletal muscle proliferation, cause differentiation of myoblasts, increase muscle protein synthesis, and decrease protein degradation [9].
We therefore hypothesized that exposure to MK-0646 as part of our therapeutic treatment protocol may promote muscle loss. We analyzed the change in muscle mass among patients treated with and without the IGF-receptor antagonist to determine if this is the case. We also determined whether different radiologic response to chemotherapy correlates with the degree of muscle loss, and whether muscle mass loss correlates with survival.
2 Methods
2.1 Inclusion criteria
Patients were recruited from December 2008 through June 2011. Individuals were considered eligible if they carried a diagnosis of stage IV pancreatic adenocarcinoma and had at least one measurable lesion. They could not have had prior treatment for metastatic disease and could not have had prior exposure to IGF-1R antagonists. Prior treatment with adjuvant therapy was allowed if at least 6 months had elapsed since the end of their initial treatment. All patients were at least 18 years of age, had an Eastern Cooperative Oncology Group (ECOG) performance status (PS) 0–1 (with one exception for a patient with ECOG 2 PS), and adequate hematologic, renal, and liver function. This study was approved by the MD Anderson Institutional Review Board.
2.2 Treatment arms
In the phase I portion of the study, all patients received gemcitabine at 1,000 mg/m2 on days 1, 8, and 15 of a 28-day cycle. In arm A, gemcitabine was combined with MK-0646 (weekly without interruption) at either 5 mg/kg (level 1) or 10 mg/kg (level 2) doses. In arm B of the phase I portion of the study, the same doses were used, and erlotinib was added to both drugs at a dose of 100 mg daily.
In the phase II portion of the study, patients were assigned to one of three treatment arms. These included the following: (a) gemcitabine 1,000 mg/m2 + MK-0646 10 mg/kg, (b) gemcitabine + MK-0646 + erlotinib 100 mg daily, and (c) gemcitabine plus erlotinib as a control arm.
Dose reductions for relevant toxicities were specified in the protocol. Patients were permitted to remain on study until either unacceptable toxicity or disease progression was observed. We routinely performed baseline computerized tomography (CT) scans, followed by additional scans after every 8 weeks (56 days ± 7) of treatment.
To analyze the change in muscle, we included patients treated on study who had baseline and 2-month CT scans as noted above. We excluded patients who only had CT scans after 1 month of therapy, and we excluded patients who crossed over to another arm during the first 2 months of therapy. For the purposes of this study, patients were divided into two treatment groups: those who received MK-0646 for at least the first 2 months on study (“MK” group) and those who received therapy without MK (“no MK” group). We furthered divided the MK group into those who received 10 mg and those who received 5 mg for some analyses. We also analyzed patients by response group: those with partial response (PR), stable disease (SD), or progressive disease (PD).
During treatment, glucocorticoids were prescribed for nausea on the day of chemotherapy administration. There were, otherwise, no specific requirements for steroid use, though physicians were free to prescribe them as they deemed necessary. No centralized recording of steroid use is available.
2.3 Muscle and adipose tissue measures
Changes in skeletal muscle were determined using routinely acquired CT scans for patients on the study. We calculated skeletal muscle cross-sectional area (cm2) at the third lumbar vertebra (L3) as determined by a study author (DF or MK). A single slice at the mid-point of L3 was used, preferably a 2.5-mm slice if available, and a 5-mm slice if a 2.5-mm slice was not available. The L3 is a landmark that has been extensively used and validated in studies assessing body composition in cancer patients [10, 11]. Skeletal muscle surface areas were determined using Slice-o-Matic softwareTM, version 4.3 (Tomovision, Montreal, QC, Canada) using pre-established thresholds of Hounsfield units (HU) of −29 to +150 [12]. We additionally evaluated adipose tissue cross-sectional area (subcutaneous, visceral, and intramuscular) in the L3 region as described previously [13]. Changes in muscle and adipose tissue surface areas over time were determined for each patient from the baseline study to the first CT post treatment, done after 2 months. We looked at muscle loss as a continuous variable and defined “meaningful loss” as greater than a 6 cm2 difference from the baseline to the second scan. We chose this cut point as it is equivalent to 1 kg of skeletal muscle and is associated with physical function (i.e., muscle strength). Additionally, this cut point has been used to investigate significant changes in muscle mass throughout cancer disease trajectory [10, 14, 15].
The CT analysis was done by two authors (DF, MK) who had practiced using the software under the guidance of two experienced investigators with a track record of publication in using this software (CP, JL). As part of this training, three of the authors (DF, MK, JL) each interpreted a series of five images demonstrating satisfactory inter-observer agreement.
In addition to a radiologic response, we assessed patient characteristics, including age, sex, race, performance status, weight, and statin use at the time of initial presentation, as possible factors associated with changes in muscle area. Baseline laboratory data included CA 19-9 level, IGF-1 level, albumin, hemoglobin, and neutrophil to lymphocyte (N:L) ratio.
2.4 Statistical analysis
Our primary purpose was to compare changes in muscle area from baseline to after 2 months of treatment with gemcitabine with MK-0646, with or without erlotinib (MK group), as compared to standard treatment with gemcitabine plus erlotinib (non-MK group) in patients with metastatic pancreatic cancer. We also determined changes in adipose tissue from baseline to 2 months. We used Wilcoxon signed rank tests to determine whether there was a significant change in muscle area measures from baseline for each treatment group (MK 10, MK 5, and non-MK) and for each response group (PR, SD, and PD). We used the Kruskal-Wallis test to compare the percent change in muscle mass across the three treatment groups and across the three response groups. We compared all baseline patient characteristics across the three treatment groups using chi-square tests or Fisher’s exact tests for categorical variables and the Kruskal-Wallis test for continuous variables.
We also determined if muscle loss was associated with overall survival and time to drop out among the different treatment and response groups. We analyzed survival as a function of “meaningful” muscle area loss, defined as loss of at least 6 cm2 of muscle area between the baseline and 2-month CT scans. We performed a Kaplan-Meier analysis to compare survival between MK- and non-MK-treated patients, and by the presence or absence of meaningful muscle area loss. We constructed Cox proportional hazard models for survival by meaningful muscle area loss, treatment groups, response groups, and baseline patients’ characteristics to determine their relationship with overall survival. Using variables associated with survival with a p < 0.10 in univariate analysis, we constructed a multivariable Cox proportional hazards model to determine the independent effect of muscle loss when adjusting for other factors associated with survival.
All analyses for this study were completed using Stata IC version 11.2 (Statcorp, College Station, Texas). All analyses considered a significance level of p < 0.05.
3 Results
3.1 Patients
A total of 72 patients were enrolled into the study. Of these, 56 had scans before and after 2 months of treatment. We excluded patients who had crossed treatment groups during the first 2 months (n = 2) or had a privacy lock on their medical record (n = 1). Therefore, 53 patients were included in the analysis: 11 patients in the no MK group (gemcitabine/erlotinib only), 20 patients in the MK 5 mg/kg group, and 22 patients in the MK 10 mg/kg group.
Patient characteristics are listed in Table 1. There were no significant differences in age, sex, race, performance status, statin use, weight, or body surface area at baseline among the treatment groups. There were also no differences in N:L ratio (as a marker of inflammation), CA 19-9, albumin, hemoglobin, or IGF-1 serum level at baseline.
3.2 Muscle and adipose tissue changes
Mean muscle area at baseline was not statistically different between treatment groups. We measured mean values of 147.1 cm2 in the MK 10 group, 140.8 cm2 in the MK 5 group, and 127.7 cm2 in the non-MK group, p = 0.27. Each group lost a significant amount of muscle area after the 2 months of therapy. The MK 10 group lost 8.6 % of muscle area; the MK 5 group lost 9.1 % of muscle area, compared to a 5.4 % loss in the non-MK group (Table 2). Despite the trend toward greater muscle area loss in the MK-treated patients, there was no significant difference in muscle area change between the three groups (p = 0.53), or between the MK and non-MK groups (p = 0.26, Fig. 1a).
Changes in measures of body composition by response and treatment groups. a Muscle mass. Treatment group was not significantly associated with the change in muscle mass (p = 0.53). There was no difference in muscle mass loss between the response groups (p = 0.14) or when comparing PR to SD (p = 0.16), but the PD group lost more muscle mass than the PR group (p = 0.04). b Subcutaneous (SC) fat. Treatment group was not associated with change in SC fat loss (p = 0.14). Response group was not associated with SC fat loss (p = 0.06). There was no difference in SC fat loss between PR and SD groups (p = 0.15), but SC fat loss was significantly greater in the PD group compared to the PR group (p = 0.02). c Visceral fat. Treatment group was not associated with visceral fat loss (p = 0.36). Response group was associated with visceral fat loss (p = 0.04), with higher visceral fat loss in the SD group compared to the PR group (p = 0.02) and higher visceral fat loss in the PD group compared to the PR group (p = 0.04). d Intramuscular (IM) fat. There was no association of IM fat loss with the treatment group (p = 0.53) or response group (p = 0.19). MK MK-0646, PR partial response, SD stable disease, PD progressive disease
Patients whose disease responded to therapy (Table 3, Fig. 2) lost 4.3 % of muscle, as compared to patients with stable or progressive disease (9.6 and 8.9 % of muscle, respectively). Despite the trend, these differences did not reach statistical significance. There was no visible pattern of subcutaneous, visceral, or intramuscular fat loss between MK- and non-MK-treated patients (Fig. 1). There were no differences between the MK 5 and 10 mg/kg groups.
When broken down by response group (Fig. 1), there was an increase in muscle loss in the MK-treated patients with stable disease and progressive disease, though none of these differences reached statistical significance. Only two patients in the non-MK group achieved a partial response, making comparison with the MK-treated responders difficult.
Only 19 patients have 4-month muscle data available (including all patients with PR), again making comparison at this time point difficult. This data is presented in Table 4. Given the limited numbers, it is difficult to draw conclusions or perform proper statistical analysis from the 4-month data. We do observe that the two responders who did not receive MK preserved their muscle mass between months 2 and 4, while the responding patients receiving MK on average lost a small amount of muscle during this time period.
3.3 Survival analysis
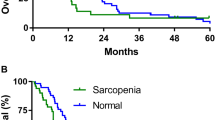
Median time on study was longer in the non-MK group; however, median overall survival was not significantly longer in the MK group (264 vs. 174 days; p = 0.09; Fig. 2). Unsurprisingly, survival was longer in those patients who responded to therapy (partial response 510 days, stable disease 171 days, progressive disease 148 days). When we grouped patients by meaningful muscle area loss, 34 patients who met the definition lost a mean of 19.1 cm2 (±10.9 cm2). The 19 patients classified as stable or improved muscle area had a mean loss of only 1.38 cm2 (±4.2 cm2). Overall survival was significantly associated with meaningful muscle loss, in both univariate and multivariable analysis (Table 5, Fig. 3). Additionally, female gender, higher serum albumin, treatment with MK, and radiologic response each predicted for survival.
4 Discussion
In our initial assessment of this therapy, patients treated with MK-0646 demonstrated a trend toward increased muscle mass loss over 2 months of therapy. This trend did not reach statistical significance, which is not surprising given the small number of patients in this study, particularly in the control group.
A number of trends were observed that lend credence to the notion of IGF-1R inhibition as contributory to muscle loss. Each of the MK dose levels (5 and 10 mg/kg) had an increased (albeit not significant) loss of muscle as compared to the non-MK-treated patients. This held true when divided into those patients with stable disease and those with progressive disease (again, not meeting criteria for statistical significance). The pattern did not hold for those patients who responded to treatment; however, only two patients in the non-MK group responded, limiting our ability to assess this group.
It is clear from our analysis that muscle loss does correlate with survival. However, as the survival of the MK patients (with increased muscle loss) was better than that of the non-MK patients, it would seem that the muscle mass difference was outweighed by benefit of the drug.
We found notable the fact that even those patients with partial responses lost muscle mass. This observation leads us to conclude that even patients who appear to be responding to treatment should be pushed toward isometric exercise and nutritional intervention in the hope of retaining muscle mass. In this retrospective review, we were unable to assess the impact of muscle mass on quality of life or individual symptoms. However, this would be a fruitful subject for further research.
Limitations to our study included a small sample size. As our study has recently resumed enrollment, subsequent analyses might yield more power to demonstrate differences in treatment groups. We are particularly limited by the relatively small control group (11 patients) relative to the treatment group (42 patients). Unfortunately, no new patients will be added to this arm of the study. Additionally, patients who fared particularly poorly might not have received follow-up imaging, skewing our results toward healthier patients who may have lost less muscle than those who dropped out early. Unfortunately, we have no way to control for this.
In conclusion, we observed that patients treated with anti-IGF-1 therapy did not lose significantly more muscle than the non-MK-treated patients, though there was a trend in this direction.
References
Conroy T et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364:1817–25.
Asano T et al. Insulin receptor substrate is a mediator of phosphoinositide 3-kinase activation in quiescent pancreatic cancer cells. Cancer Res. 2005;65:9164–8.
Maloney EK et al. An anti-insulin-like growth factor I receptor antibody that is a potent inhibitor of cancer cell proliferation. Cancer Res. 2003;63:5073–83.
Javle MM, V.G., Shroff RT, Bhosale P, Overman MJ, Weatherly J, Wolff RA, Abbruzzese JL, Phase I/II study of MK-0646, the humanized monoclonal IGF-1R antibody in combination with gemcitabine or gemcitabine plus erlotinib (E) for advanced pancreatic cancer. J Clin Oncol, 2010. 28: p. 4039.
Hameed M, Harridge SD, Goldspink G. Sarcopenia and hypertrophy: a role for insulin-like growth factor-1 in aged muscle? Exerc Sport Sci Rev. 2002;30:15–9.
Musaro A et al. Localized Igf-1 transgene expression sustains hypertrophy and regeneration in senescent skeletal muscle. Nat Genet. 2001;27:195–200.
Mourkioti F, Rosenthal N. IGF-1, inflammation and stem cells: interactions during muscle regeneration. Trends Immunol. 2005;26:535–42.
Adams GR. Role of insulin-like growth factor-I in the regulation of skeletal muscle adaptation to increased loading. Exerc Sport Sci Rev. 1998;26:31–60.
Ge X, Zhang Y, Jiang H. Signaling pathways mediating the effects of insulin-like growth factor-I in bovine muscle satellite cells. Mol Cell Endocrinol. 2013;372:23–9.
Shen W et al. Total body skeletal muscle and adipose tissue volumes: estimation from a single abdominal cross-sectional image. J Appl Physiol. 2004;97:2333–8.
Prado CM, Birdsell LA, Baracos VE. The emerging role of computerized tomography in assessing cancer cachexia. Curr Opin Support Palliat Care. 2009;3:269–75.
Mitsiopoulos N et al. Cadaver validation of skeletal muscle measurement by magnetic resonance imaging and computerized tomography. J Appl Physiol. 1998;85:115–22.
Mourtzakis M et al. A practical and precise approach to quantification of body composition in cancer patients using computed tomography images acquired during routine care. Appl Physiol Nutr Metab. 2008;33:997–1006.
Frontera WR et al. Strength conditioning in older men: skeletal muscle hypertrophy and improved function. J Appl Physiol. 1988;64:1038–44.
Prado CM et al. Central tenet of cancer cachexia therapy: do patients with advanced cancer have exploitable anabolic potential? Am J Clin Nutr. 2013;98:1012–9.
Acknowledgement
This work was partly funded by a philanthropic donation from the Crain-Maling foundation. The authors each certify that they comply with the ethical guidelines for authorship and publishing of the Journal of Cachexia, Sarcopenia, and Muscle. 2010; 1:7–8 (von Haehling S, Morley JE, Coats AJ, and Anker SD).
Conflict of interest
David R. Fogelman, Holly Holmes, Khalil Mohammed, Matthew H.G. Katz, Carla Prado, Jessica Lieffers, Naveen Garg, Gauri R Varadhachary, Rachna Shroff, Michael J Overman, Robert A Wolff, and Milind Javle each have declared that they have no conflict of interest relevant to this work.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Fogelman, D.R., Holmes, H., Mohammed, K. et al. Does IGFR1 inhibition result in increased muscle mass loss in patients undergoing treatment for pancreatic cancer?. J Cachexia Sarcopenia Muscle 5, 307–313 (2014). https://doi.org/10.1007/s13539-014-0145-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13539-014-0145-y