Abstract
Purpose
Patients with pancreatic cancer often have cancer cachexia at diagnosis. Recent studies suggested that loss of skeletal muscle mass was related to cancer cachexia, which hindered continuance of chemotherapy and could be one of prognostic factors in pancreatic cancer, however the association remains unclear in patients receiving gemcitabine and nab-paclitaxel (GnP).
Methods
We retrospectively studied 138 patients with unresectable pancreatic cancer receiving first-line GnP at the University of Tokyo from January 2015 to September 2020. We calculated body composition in CT images before chemotherapy and at initial evaluation, and evaluated the association of both body composition before chemotherapy and its changes at initial evaluation.
Results
Compared by skeletal muscle mass index (SMI) change rate between pre-chemotherapy and initial evaluation, there were statistically significantly differences in the median OS: 16.3 months (95%CI 12.3–22.7) and 10.3 months (95%CI 8.3–18.1) between SMI change rate ≥ -3.5% and < -3.5% groups (P = 0.01). By multivariate analysis for OS, CA19-9 (HR 3.34, 95%CI 2.00–5.57, P < 0.01), PLR (HR 1.68, 95%CI 1.01–2.78, P = 0.04), mGPS (HR 2.32, 95%CI 1.47–3.65, P < 0.01) and relative dose intensity (HR 2.21, 95%CI 1.42–3.46, P < 0.01) were significantly poor prognostic factors. SMI change rate (HR 1.47, 95%CI 0.95–2.28, P = 0.08) showed a trend to poor prognosis. Sarcopenia before chemotherapy was not significantly associated with PFS or OS.
Conclusion
Early skeletal muscle mass decline was associated with poor OS. Further investigation is warranted whether the maintenance of skeletal muscle mass by nutritional support would improve prognosis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The incidence of pancreatic cancer (PC) is increasing worldwide [1]. Despite surgery being the only curative treatment, 80–85% of patients present with an advanced stage [2, 3]. Immunotherapy has been investigated as one of treatment options, but systemic cytotoxic chemotherapy is still the standard of care for locally advanced or metastatic PC, including gemcitabine plus nab-paclitaxel (GnP) [4], and FOLFIRINOX (5-fluorouracil, leucovorin, irinotecan, and oxaliplatin) [5]. Despite the improvement of survival by those intense combination regimens, they are associated with adverse effects (AEs) and require appropriate patient selection.
Patients with PC, especially elderly patients, are often underweight and undernourished at diagnosis, with 50% reported to have cancer cachexia at diagnosis [6, 7]. Cancer cachexia is defined as a multifactorial syndrome defined by an ongoing loss of skeletal muscle mass that cannot be fully reversed by conventional nutritional support and leads to progressive functional impairment [8]. Recent studies suggested that loss of skeletal muscle mass was associated with cancer cachexia, which hindered continuance of chemotherapy, and can be one of prognostic factors of survival in PC [9,10,11,12,13]. However, it remains unclear whether sarcopenia at diagnosis or decline in skeletal muscle mass during chemotherapy is more prognostic of survival in PC, with various regimens such as FOLFIRINOX [14, 15] and GnP [16] being evaluated.
In this retrospective study, we investigated the association of both body composition before chemotherapy and its changes at initial evaluation of chemotherapy in patients receiving first-line GnP for unresectable PC.
Methods
Patients
Data on patients with unresectable PC who started GnP as first-line chemotherapy at the Department of Gastroenterology, the University of Tokyo from January 2015 to September 2020 were retrospectively studied. The analysis was based on follow-up information, which was received until April 2022. This study was approved by the ethics committee of the University of Tokyo Hospital.
All patients were histologically or cytologically diagnosed as pancreatic ductal adenocarcinoma and were diagnosed as locally advanced or metastatic diseases on CT. Chemotherapy was administered on days 1, 8 and 15 of a 28-day cycle, combined gemcitabine at 1000 mg/m2 and nab-paclitaxel at 125 mg/m2 [17].
Data collection
We extracted data, including age, sex, height, weight, Eastern Cooperative Oncology Group performance status (ECOG PS), laboratory data (white blood cell with differential, albumin, C-reactive protein, carcinoembryonic antigen and carbohydrate antigen 19–9 [CA19-9]) from our prospectively maintained pancreatic cancer database and electric medical records in our hospital.
Body Mass Index (BMI) was calculated by dividing the weight (kg) by the square of the height (m), and the cutoff value was set at 22, the standard value in Japan. Neutrophil/lymphocyte ratio (NLR), Platelet/lymphocyte ratio (PLR) and modified Glasgow Prognostic Score (mGPS) were calculated from the above-mentioned data. The cutoff value of NLR and PLR was set by creating receiver operating characteristic (ROC) curve with a dichotomous variable divided by median overall survival (353.5 days) as the dependent variable.
In addition, we evaluated relative dose intensity (RDI) up to first 2 cycles, early tumor shrinkage (ETS) and presence of dose reduction at 1st cycle to analyze prognostic factors. RDI was calculated by dividing the actual dose by the standard dose of gemcitabine and nab-paclitaxel up to first 2 cycles, the cutoff value was set at the median. The standard dose was set at 125 mg/m2 for nab-paclitaxel and 1000 mg/m2 for gemcitabine, based on the results of the phase 3 study with metastatic pancreatic cancer [4, 17]. ETS was calculated from the maximum tumor diameter before chemotherapy and at initial evaluation according to RECIST 1.1, the cutoff value was set at 20% [18,19,20].
Body composition assessment
We calculated the skeletal muscle mass area (cm2), subcutaneous fat area (cm2) and visceral fat area (cm2) at the level of the third lumbar vertebra in CT images before chemotherapy introduction and at initial evaluation by using SliceOmatic medical imaging software (Tomovision, Canada) [21]. The ranges of tissue Hounsfield unit (HU) thresholds were within -29 to 150 HU for skeletal muscle mass area, -190 to -30 HU for subcutaneous fat area, and -150 to -50 HU for visceral fat area, as shown Fig. 1 [22]. Skeletal muscle area, subcutaneous fat area, and visceral fat area were normalized for height in meters squared (m2) and reported as skeletal muscle mass index (SMI) (cm2/m2), subcutaneous adipose tissue index (SATI) (cm2/m2), and visceral adipose tissue index (VATI) (cm2/m2). Visceral-to-subcutaneous fat area ratio (VSR) was calculated by dividing visceral fat area by subcutaneous fat area to assess for the presence of visceral obesity. SMI change rate (%) was calculated by subtracting SMI before chemotherapy from SMI at initial evaluation, and dividing by SMI before chemotherapy, and standardizing at 60 days.
Assessment of body composition. The image illustrates the different proportions of skeletal muscle area (red), subcutaneous fat area (turquoise), and visceral fat area (yellow). Skeletal muscle area (cm2), subcutaneous fat area (cm2), and visceral fat area (cm2) at the level of the third lumbar vertebra in CT scan were quantified by using SliceOmatic medical imaging software. Skeletal muscle area highlighted red was quantified within -29 to 150 HU, subcutaneous fat area highlighted turquoise was quantified within -190 to -30 HU, and visceral fat area highlighted yellow was quantified within -150 to -50 HU
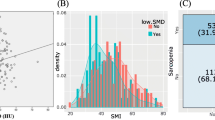
Sarcopenia was defined as male SMI < 42 cm2/m2 and female SMI < 38 cm2/m2 based on the criteria proposed by the Hepatology Society of Japan [23, 24]. The cutoff values for VSR and SMI change rate were set by creating the ROC curve with a dichotomous variable divided by median overall survival as the dependent variable.
Statistical analysis
We investigated the association of sarcopenia and changes in body composition during chemotherapy with progression free survival (PFS), overall survival (OS), AEs, and tumor response including response rate (RR) and disease control rate (DCR).
Both PFS and OS were calculated starting from the CT date of initial evaluation. PFS and OS were estimated using Kaplan–Meier method and survival curves were compared using log-rank test. Comparisons between two groups were evaluated using the Mann–Whitney U test for continuous variables and using the Fisher’s exact test for categorical data. AEs were evaluated according to CTCAE ver 4.0. Hazard ratios (HRs) with 95% confidence intervals (CIs) for OS and PFS were estimated by a Cox proportional hazards model to determine the independent prognostic factors. Factors with p-values < 0.20 in the univariable analyses were evaluated in the multivariable analyses. All tests were 2-sided, and p-value < 0.05 was considered statistically significant. Statistical analyses were performed using JMP version 16 software (SAS Institute Inc., Cary, NC).
Results
Patient characteristics
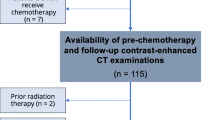
Between January 2015 and September 2020, 152 patients started GnP as first-line chemotherapy, and pre-chemotherapy and initial evaluation CT scans were available in 138 patients. Fourteen patients who did not receive follow up CT evaluation were excluded from the analysis (Fig. 2). The median interval between pre-chemotherapy and initial evaluation CT scan was 61.5 days (interquartile range (IQR), 54–70). The median follow-up period was 13.1 months (IQR, 8.9–22.5). Baseline characteristics at chemotherapy introduction are summarized in Table 1. Median age was 67.5 years old (IQR, 59.7–74) and 80 patients (58.0%) were male. Distant metastasis was present in 97 patients (70.3%); liver in 62 (44.9%), lung in 20 (14.5%), lymph nodes in 38 (27.5%) and peritoneal dissemination in 21 (15.2%). ECOG PS was 0 in 76 patients (55.1%). The median SMI, VATI, SATI, VSR were 40.9cm2/m2 (IQR, 35.8–46.9), 31.6 cm2/m2 (IQR, 14.2–48.2), 33.7cm2/m2 (IQR, 21.8–47.1) and 0.91 (IQR, 0.49–1.39), respectively (Table 2). Sixty-one patients (44.2%) were diagnosed as sarcopenia. The median PFS was 6.5 months (95%CI 5.1–8.2) and the median OS was 15.2 months (95%CI 11.2–19.0).
SMI change rate and clinical outcomes
By creating ROC curve with a dichotomous variable divided by the median OS as the dependent variable, the cutoff value for SMI change rate was set at -3.5% (Fig. 3). The median OS in the total cohort was 16.3 months (95%CI 12.3–22.7) and 10.3 months (95%CI 8.3–18.1) in SMI change rate ≥ -3.5% and < -3.5% groups (P = 0.01, Fig. 4A).
Overall survival according to the SMI change rate. Solid lines indicate SMI change rate < -3.5% and broken lines indicate SMI change rate ≥ -3.5%. A. Overall survival of the total cohort. The median overall survival was 10.3 months (95%CI, 8.3–18.1) for SMI change rate < -3.5% and 16.3 months (95%CI, 12.3–22.7) for SMI change rate ≥ -3.5% (P = 0.01). B. Overall survival in non-elderly (< 75 years old) patients. The median overall survival was 11.8 months (95%CI, 8.2–19.0) for SMI change rate < -3.5% and 15.8 months (95%CI, 11.2–22.7) for SMI change rate ≥ -3.5% (P = 0.07). C. Overall survival in elderly (≥ 75 years old) patients. The median overall survival was 9.5 months (95%CI, 5.5–30.2) for SMI change rate < -3.5% and 16.5 months (95%CI, 10.2–40.4) for SMI change rate ≥ -3.5% (P = 0.11)
Patient characteristics divided by SMI change rate are shown in Table 1. The rates of male sex and biliary drainage were significantly higher in SMI change rate < -3.5% group. Body composition before chemotherapy and at the initial evaluation is shown in Table 2. The median SMI before chemotherapy was higher in SMI change rate < -3.5% group: 39.8 and 43.8 cm2/m2 (P = 0.02), but the difference was not significant at the initial evaluation. The rate of sarcopenia at the initial evaluation was significantly higher in SMI change rate < -3.5% group: 40.5% and 59.3% (P = 0.03).
There were no significant differences in objective response (P = 0.55): RR was 23.8% and 16.7% and DCR was 89.3% and 81.5% in SMI change rate ≥ -3.5% and < -3.5% groups (Table 3). The median PFS by SMI change rate in the total cohort was not significantly different: 7.2 months (95%CI 5.3–9.1) and 5.4 months (95%CI 3.7–8.7) in SMI change rate ≥ -3.5% and < -3.5% groups, respectively (P = 0.24, Fig. 5A).
Progression free survival according to the SMI change rate. Solid lines indicate SMI change rate < -3.5% and broken lines indicate SMI change rate ≥ -3.5%. A. Progression free survival of the total cohort. The median PFS was 5.4 months (95%CI, 3.7–8.7) for SMI change rate < -3.5% and 7.2 months (95%CI, 5.3–9.1) for SMI change rate ≥ -3.5% (p = 0.24). B. Progression free survival in non-elderly (< 75 years old) patients. The median progression free survival was 5.1 months (95%CI, 3.5–8.7) for SMI change rate < -3.5% and 7.2 months (95%CI, 5.1–9.8) for SMI change rate ≥ -3.5% (P = 0.16). C. Progression free survival in elderly (≥ 75 years old) patients. The median progression free survival was 6.3 months (95%CI, 3.5-NA) for SMI change rate < -3.5% and 8.0 months (95%CI, 4.0–9.6) for SMI change rate ≥ -3.5% (P = 0.66). CI; confidence interval, SMI; Skeletal muscle mass index
In terms of safety, the incidences of AEs were comparable between two groups, other than all grades neutropenia (Table 4). However, SMI change rate < -3.5% group had experienced more discontinuations at initial evaluation (P = 0.02), and fewer total cycles of chemotherapy (P = 0.01) compared to SMI change rate ≥ -3.5% group.
Prognostic factors for PFS and OS
The results of univariable and multivariable analyses of PFS and OS are shown in Tables 5A, B. In the multivariable analysis, CA19-9 (HR 2.12, 95% CI 1.34–3.36, P < 0.01) and mGPS (HR 1.58, 95% CI 1.02–2.44, P = 0.03) were significant prognostic factors for PFS. Meanwhile, CA19-9 (HR 3.34, 95% CI 2.00–5.57, P < 0.01), PLR (HR 1.68, 95%CI 1.01–2.78, P = 0.04), mGPS (HR 2.32, 95%CI 1.47–3.65, P < 0.01) and RDI up to 2 cycles (HR 2.21, 95%CI 1.42–3.46, P < 0.01) were significantly prognostic factors for OS. SMI change rate (HR 1.47, 95%CI 0.95–2.28, P = 0.08) and ETS (HR 1.53, 95%CI 0.94–2.49, P = 0.08) was also associated with OS, though statistically not significant. Neither sarcopenia before chemotherapy nor sarcopenia at initial evaluation was significantly associated with PFS or OS.
Exploratory analyses of body composition by age
Twenty-nine patients (21.0%) were ≥ 75 years old in our cohort. There were no significant differences in RR (19.3% vs. 27.6%, P = 0.34), the median PFS (6.3 vs. 7.1 months, P = 0.79) and the median OS (14.1 vs. 16.3 months, P = 0.77) between non-elderly (< 75 years old) and elderly (≥ 75 years old) patients. When body composition was compared between non-elderly and elderly patients (Table 6), VATI both before chemotherapy and at initial evaluation was significantly higher in elderly patients. There were no significant differences in sarcopenia (44.0% and 44.8%, P = 0.93) or SMI change rates (-2.4% and -1.8%, P = 0.23) in non-elderly and elderly patients. The median PFS was 5.1 and 7.2 months in SMI change rate < -3.5% and SMI change rate ≥ -3.5% groups in non-elderly patients (P = 0.16, Fig. 5B), while it was 6.3 and 8.0 months in SMI change rate < -3.5% and SMI change rate ≥ -3.5% groups in elderly patients (P = 0.66, Fig. 5C). SMI change rate was associated with OS, though not statistically significant. While the median OS was 11.8 and 15.8 months in SMI change rate < -3.5% and SMI change rate ≥ -3.5% groups in non-elderly patients (P = 0.07, Fig. 4B), it was 9.5 and 16.5 months for SMI change rate < -3.5% and SMI change rate ≥ -3.5% groups in elderly patients (P = 0.11, Fig. 4C).
Discussion
In this retrospective study, we found that early skeletal muscle mass decline was associated with shorter OS in patients receiving first-line GnP for unresectable PC. On the other hands, sarcopenia before chemotherapy was not associated with OS. Our study results suggested early decline of SMI after introduction of chemotherapy rather than the value of SMI before chemotherapy might be prognostic of survival in patients with unresectable PC.
Sarcopenia as one of prognostic factors in patients with cancer is increasingly reported in various cancers. Recent studies suggested the role of sarcopenia in patients receiving palliative chemotherapy for PC. In our cohort, sarcopenia was observed in 44.2% at the time of diagnosis, which was similar to that of previous reports [7, 16]. While some studies suggested association of sarcopenia at diagnosis with prognosis [12, 14, 25], others reported change in body composition was associated with survival [10,11,12, 26]. Sarcopenia before chemotherapy was not associated with PFS or OS in our cohort, by using the criteria developed by the Hepatology Society of Japan based on the AWGS criteria (male SMI < 42 cm2/m2 and female SMI < 38 cm2/m2) [23]. However, SMI change up to initial evaluation was associated with OS, suggesting body composition change can be predictive of prognosis in patients receiving palliative chemotherapy for PC. Interestingly, our definition of SMI decline > -3.5% was not associated with tumor response or PFS. The reason for discrepancy between PFS and OS is unclear but the similar outcomes were also observed in elderly patients receiving GnP chemotherapy [16].
In terms of safety, it was suggested that SMI change was not significantly associated with either AEs, other than all grades neutropenia, or RDI up to first 2 cycles of chemotherapy. Since reduced RDI was associated with poor survival, the maintenance of RDI is as important as the control of severe AEs, as previous studies reported the association of RDI with efficacy of FOLFIRINOX for PC [27, 28]. In our study, though 2-cycle RDI was comparable, discontinuation of chemotherapy after initial evaluation (24.1% and 9.5%) and discontinuation due to poor condition (13.0% and 7.1%) were more often encountered in SMI change rate ≥ -3.5% group compared to SMI change rate < -3.5% group. As a result, the number of cycles was higher in SMI change rate ≥ -3.5% group. Thus, sarcopenia during chemotherapy can lead to cessation of chemotherapy due to the deteriorated patient condition and non-chemotherapeutic support to prevent sarcopenia might improve clinical outcomes of palliative chemotherapy in PC.
Nutritional support has been increasingly investigated in the field of oncology. Anamorelin, an oral ghrelin-like agent, reportedly improved body weight and anorexia-related symptoms in cancer patients [29] and we also reported that insufficient protein intake was a poor prognostic factor in patients with unresectable PC receiving chemotherapy [30]. Nutritional interventions such as nutritional supplements [31] or pancreatic exocrine replacement treatment [32, 33] might also affect body composition. Thus, we should further investigate whether those nutritional interventions would improve sarcopenia during chemotherapy and lead to the improved prognosis or not.
Age itself can affect body composition and its impact on chemotherapy might differ by age. However, in our exploratory analyses, the associations of SMI change were comparable between elderly and non-elderly patients. The median OS tended to be longer in cases with SMI decline ≥ -3.5%, regardless of age. Meanwhile, a previous study of pancreatic cancer receiving GnP chemotherapy reported that sarcopenia at diagnosis was associated with poor OS only in elderly (> 70 years old) patients [16]. We previously reported comorbidity, rather than age, was an important prognostic factors in gemcitabine-based chemotherapy [34]. Recently, the importance of cognitive assessment is also reported in elderly patients [35, 36]. The relation of age, comorbidity and body composition can be multifactorial and more comprehensive evaluation in a large prospective cohort is warranted.
Our study had several limitations. Firstly, this was a retrospective study at a single academic center and the selection bias was inevitable. For example, the rate of sarcopenia at diagnosis of PC was similar between elderly and non-elderly patients. Elderly patients who could receive GnP might be a selected population in a good clinical condition. Thus, our study results need to be validated in the external cohort. Secondly, definition of sarcopenia using CT scan have not been established. The AWGS 2019 definition uses grip strength, physical function (walking speed, 5 times stand up, short physical performance battery) and skeletal muscle mass measurement by dual energy X-ray absorptiometry or bioelectrical impedance analysis to determine sarcopenia [37]. We applied the criteria for sarcopenia by the Hepatology Society of Japan since this was retrospective study. Definition of sarcopenia in cases with malignancy including PC receiving palliative chemotherapy needs further investigation.
In conclusion, short-term decline of skeletal muscle mass was associated with poor OS in patients receiving GnP for unresectable PC. Further investigation is warranted whether the maintenance of skeletal muscle mass by nutritional support or medications would improve prognosis or not.
Data availability
The datasets generated during and/or analysed during the current study are not publicly available but are available from the corresponding author on reasonable request.
Abbreviations
- AEs:
-
Adverse effects
- BMI:
-
Body mass index
- CA19-9:
-
Carbohydrate antigen 19–9
- CCI:
-
Charlson comorbidity index
- CI:
-
Confidence interval
- DCR:
-
Disease control rate
- ECOG PS:
-
Eastern Cooperative Oncology Group performance status
- ETS:
-
Early tumor shrinkage
- GnP:
-
Gemcitabine and nab-paclitaxel
- HR:
-
Hazard ratio
- HU:
-
Hounsfield unit
- IQR:
-
Interquartile range
- mGPS:
-
Modified Glasgow Prognostic Score
- NLR:
-
Neutrophil/lymphocyte ratio
- OS:
-
Overall survival
- PC:
-
Pancreatic cancer
- PFS:
-
Progression free survival
- PLR:
-
Platelet/lymphocyte ratio
- RDI:
-
Relative dose intensity
- ROC:
-
Receiver operating characteristic
- RR:
-
Response rate
- SATI:
-
Subcutaneous adipose tissue index
- SMI:
-
Skeletal muscle mass index
- VATI:
-
Visceral adipose tissue index
- VSR:
-
Visceral-to-subcutaneous fat area ratio
References
Siegel RL, Miller KD, Fuchs HE, Jemal A (2021) Cancer Statistics, 2021. CA Cancer J Clin 71(1):7–33. https://doi.org/10.3322/caac.21654
Mizrahi JD, Surana R, Valle JW, Shroff RT (2020) Pancreatic cancer. Lancet 395(10242):2008–2020. https://doi.org/10.1016/s0140-6736(20)30974-0
Siegel RL, Miller KD, Jemal A (2020) Cancer statistics, 2020. CA Cancer J Clin 70(1):7–30. https://doi.org/10.3322/caac.21590
Von Hoff DD, Ervin T, Arena FP, Chiorean EG, Infante J, Moore M et al (2013) Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med 369(18):1691–1703. https://doi.org/10.1056/NEJMoa1304369
Conroy T, Desseigne F, Ychou M, Bouché O, Guimbaud R, Bécouarn Y et al (2011) FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med 364(19):1817–1825. https://doi.org/10.1056/NEJMoa1011923
Mitsunaga S, Kasamatsu E, Machii K (2020) Incidence and frequency of cancer cachexia during chemotherapy for advanced pancreatic ductal adenocarcinoma. Support Care Cancer 28(11):5271–5279. https://doi.org/10.1007/s00520-020-05346-8
Takeda T, Sasaki T, Suzumori C, Mie T, Furukawa T, Yamada Y et al (2021) The impact of cachexia and sarcopenia in elderly pancreatic cancer patients receiving palliative chemotherapy. Int J Clin Oncol 26(7):1293–1303. https://doi.org/10.1007/s10147-021-01912-0
Fearon K, Strasser F, Anker SD, Bosaeus I, Bruera E, Fainsinger RL et al (2011) Definition and classification of cancer cachexia: an international consensus. Lancet Oncol 12(5):489–495. https://doi.org/10.1016/s1470-2045(10)70218-7
Martin L, Birdsell L, Macdonald N, Reiman T, Clandinin MT, McCargar LJ et al (2013) Cancer cachexia in the age of obesity: skeletal muscle depletion is a powerful prognostic factor, independent of body mass index. J Clin Oncol 31(12):1539–1547. https://doi.org/10.1200/jco.2012.45.2722
Salinas-Miranda E, Deniffel D, Dong X, Healy GM, Khalvati F, O’Kane GM et al (2021) Prognostic value of early changes in CT-measured body composition in patients receiving chemotherapy for unresectable pancreatic cancer. Eur Radiol 31(11):8662–8670. https://doi.org/10.1007/s00330-021-07899-6
Nakano O, Kawai H, Kobayashi T, Kohisa J, Ikarashi S, Hayashi K et al (2021) Rapid decline in visceral adipose tissue over 1 month is associated with poor prognosis in patients with unresectable pancreatic cancer. Cancer Med 10(13):4291–4301. https://doi.org/10.1002/cam4.3964
Choi Y, Oh DY, Kim TY, Lee KH, Han SW, Im SA et al (2015) Skeletal Muscle Depletion Predicts the Prognosis of Patients with Advanced Pancreatic Cancer Undergoing Palliative Chemotherapy, Independent of Body Mass Index. PLoS One 10(10):e0139749. https://doi.org/10.1371/journal.pone.0139749
Griffin OM, Duggan SN, Ryan R, McDermott R, Geoghegan J, Conlon KC (2019) Characterising the impact of body composition change during neoadjuvant chemotherapy for pancreatic cancer. Pancreatology 19(6):850–857. https://doi.org/10.1016/j.pan.2019.07.039
Kurita Y, Kobayashi N, Tokuhisa M, Goto A, Kubota K, Endo I et al (2019) Sarcopenia is a reliable prognostic factor in patients with advanced pancreatic cancer receiving FOLFIRINOX chemotherapy. Pancreatology 19(1):127–135. https://doi.org/10.1016/j.pan.2018.11.001
Uemura S, Iwashita T, Ichikawa H, Iwasa Y, Mita N, Shiraki M et al (2021) The impact of sarcopenia and decrease in skeletal muscle mass in patients with advanced pancreatic cancer during FOLFIRINOX therapy. Br J Nutr 125(10):1140–1147. https://doi.org/10.1017/S0007114520003463
Asama H, Ueno M, Kobayashi S, Fukushima T, Kawano K, Sano Y et al (2022) Sarcopenia: prognostic value for unresectable pancreatic ductal adenocarcinoma patients treated with gemcitabine plus Nab-Paclitaxel. Pancreas 51(2):148–152. https://doi.org/10.1097/mpa.0000000000001985
Ueno H, Ikeda M, Ueno M, Mizuno N, Ioka T, Omuro Y et al (2016) Phase I/II study of nab-paclitaxel plus gemcitabine for chemotherapy-naive Japanese patients with metastatic pancreatic cancer. Cancer Chemother Pharmacol 77(3):595–603. https://doi.org/10.1007/s00280-016-2972-3
Cremolini C, Loupakis F, Antoniotti C, Lonardi S, Masi G, Salvatore L et al (2015) Early tumor shrinkage and depth of response predict long-term outcome in metastatic colorectal cancer patients treated with first-line chemotherapy plus bevacizumab: results from phase III TRIBE trial by the Gruppo Oncologico del Nord Ovest. Ann Oncol 26(6):1188–1194. https://doi.org/10.1093/annonc/mdv112
Heinemann V, Stintzing S, Modest DP, Giessen-Jung C, Michl M, Mansmann UR (2015) Early tumour shrinkage (ETS) and depth of response (DpR) in the treatment of patients with metastatic colorectal cancer (mCRC). Eur J Cancer 51(14):1927–1936. https://doi.org/10.1016/j.ejca.2015.06.116
Vivaldi C, Fornaro L, Cappelli C, Pecora I, Catanese S, Salani F et al (2019) Early Tumor Shrinkage and Depth of Response Evaluation in Metastatic Pancreatic Cancer Treated with First Line Chemotherapy: An Observational Retrospective Cohort Study. Cancers (Basel) 11(7). https://doi.org/10.3390/cancers11070939
Fujiwara N, Nakagawa H, Kudo Y, Tateishi R, Taguri M, Watadani T et al (2015) Sarcopenia, intramuscular fat deposition, and visceral adiposity independently predict the outcomes of hepatocellular carcinoma. J Hepatol 63(1):131–140. https://doi.org/10.1016/j.jhep.2015.02.031
Mitsiopoulos N, Baumgartner RN, Heymsfield SB, Lyons W, Gallagher D, Ross R (1998) Cadaver validation of skeletal muscle measurement by magnetic resonance imaging and computerized tomography. J Appl Physiol (1985) 85(1):115–22. https://doi.org/10.1152/jappl.1998.85.1.115
Nishikawa H, Shiraki M, Hiramatsu A, Moriya K, Hino K, Nishiguchi S (2016) Japan Society of Hepatology guidelines for sarcopenia in liver disease (1st edition): Recommendation from the working group for creation of sarcopenia assessment criteria. Hepatol Res 46(10):951–963. https://doi.org/10.1111/hepr.12774
Chen LK, Liu LK, Woo J, Assantachai P, Auyeung TW, Bahyah KS et al (2014) Sarcopenia in Asia: consensus report of the Asian Working Group for Sarcopenia. J Am Med Dir Assoc 15(2):95–101. https://doi.org/10.1016/j.jamda.2013.11.025
Naumann P, Eberlein J, Farnia B, Hackert T, Debus J, Combs SE (2019) Continued Weight Loss and Sarcopenia Predict Poor Outcomes in Locally Advanced Pancreatic Cancer Treated with Chemoradiation. Cancers (Basel) 11(5). https://doi.org/10.3390/cancers11050709
Basile D, Parnofiello A, Vitale MG, Cortiula F, Gerratana L, Fanotto V et al (2019) The IMPACT study: early loss of skeletal muscle mass in advanced pancreatic cancer patients. J Cachexia Sarcopenia Muscle 10(2):368–377. https://doi.org/10.1002/jcsm.12368
Lee JC, Kim JW, Ahn S, Kim HW, Lee J, Kim YH et al (2017) Optimal dose reduction of FOLFIRINOX for preserving tumour response in advanced pancreatic cancer: Using cumulative relative dose intensity. Eur J Cancer 76:125–133. https://doi.org/10.1016/j.ejca.2017.02.010
Vary A, Lebellec L, Di Fiore F, Penel N, Cheymol C, Rad E et al (2021) FOLFIRINOX relative dose intensity and disease control in advanced pancreatic adenocarcinoma. Ther Adv Med Oncol 13:17588359211029824. https://doi.org/10.1177/17588359211029825
Hamauchi S, Furuse J, Takano T, Munemoto Y, Furuya K, Baba H et al (2019) A multicenter, open-label, single-arm study of anamorelin (ONO-7643) in advanced gastrointestinal cancer patients with cancer cachexia. Cancer 125(23):4294–4302. https://doi.org/10.1002/cncr.32406
Hasegawa Y, Ijichi H, Saito K, Ishigaki K, Takami M, Sekine R et al (2021) Protein intake after the initiation of chemotherapy is an independent prognostic factor for overall survival in patients with unresectable pancreatic cancer: A prospective cohort study. Clin Nutr 40(7):4792–4798. https://doi.org/10.1016/j.clnu.2021.06.011
Kim SH, Lee SM, Jeung HC, Lee IJ, Park JS, Song M et al (2019) The Effect of Nutrition Intervention with Oral Nutritional Supplements on Pancreatic and Bile Duct Cancer Patients Undergoing Chemotherapy. Nutrients 11(5). https://doi.org/10.3390/nu11051145
Saito T, Hirano K, Isayama H, Nakai Y, Saito K, Umefune G et al (2017) The Role of Pancreatic Enzyme Replacement Therapy in Unresectable Pancreatic Cancer: A Prospective Cohort Study. Pancreas 46(3):341–346. https://doi.org/10.1097/mpa.0000000000000767
Saito T, Nakai Y, Isayama H, Hirano K, Ishigaki K, Hakuta R et al (2018) A Multicenter Open-Label Randomized Controlled Trial of Pancreatic Enzyme Replacement Therapy in Unresectable Pancreatic Cancer. Pancreas 47(7):800–806. https://doi.org/10.1097/mpa.0000000000001079
Nakai Y, Isayama H, Sasaki T, Sasahira N, Tsujino T, Kogure H et al (2011) Comorbidity, not age, is prognostic in patients with advanced pancreatic cancer receiving gemcitabine-based chemotherapy. Crit Rev Oncol Hematol 78(3):252–259. https://doi.org/10.1016/j.critrevonc.2010.05.007
Wildiers H, Heeren P, Puts M, Topinkova E, Janssen-Heijnen ML, Extermann M et al (2014) International Society of Geriatric Oncology consensus on geriatric assessment in older patients with cancer. J Clin Oncol 32(24):2595–2603. https://doi.org/10.1200/jco.2013.54.8347
Hamaker ME, Te Molder M, Thielen N, van Munster BC, Schiphorst AH, van Huis LH (2018) The effect of a geriatric evaluation on treatment decisions and outcome for older cancer patients - A systematic review. J Geriatr Oncol 9(5):430–440. https://doi.org/10.1016/j.jgo.2018.03.014
Chen LK, Woo J, Assantachai P, Auyeung TW, Chou MY, Iijima K et al (2020) Asian Working Group for Sarcopenia: 2019 Consensus Update on Sarcopenia Diagnosis and Treatment. J Am Med Dir Assoc 21(3):300–7.e2. https://doi.org/10.1016/j.jamda.2019.12.012
Acknowledgements
We would like to thank all patients and doctors for their participation in this study.
Funding
Open access funding provided by The University of Tokyo.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by Yukari Suzuki, Kei Saito and Yousuke Nakai. The first draft of the manuscript was written by Yukari Suzuki and reviewed by Kei Saito and Yousuke Nakai. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval
This study was performed in line with the principals of the declaration of Helsinki. The study was approved by the ethics committee of the University of Tokyo Hospital.
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Consent for publication
Consent to publish was received from all individual participants included in the study.
Conflict of interest
Youske Nakai has received research grants by Taiho Pharmaceutical. No funding agency had input into the design and conduct of this study.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Suzuki, Y., Saito, K., Nakai, Y. et al. Early skeletal muscle mass decline is a prognostic factor in patients receiving gemcitabine plus nab-paclitaxel for unresectable pancreatic cancer: a retrospective observational study. Support Care Cancer 31, 197 (2023). https://doi.org/10.1007/s00520-023-07659-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00520-023-07659-w