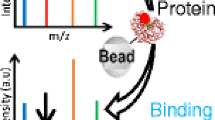
Abstract
The interaction between bioactive small molecule ligands and proteins is one of the important research areas in proteomics. Herein, a simple and rapid method is established to screen small ligands that bind to proteins. We designed an agarose slide to immobilize different proteins. The protein microarrays were allowed to interact with different small ligands, and after washing, the microarrays were screened by desorption electrospray ionization mass spectrometry (DESI MS). This method can be applied to screen specific protein binding ligands and was shown for seven proteins and 34 known ligands for these proteins. In addition, a high-throughput screening was achieved, with the analysis requiring approximately 4 s for one sample spot. We then applied this method to determine the binding between the important protein matrix metalloproteinase-9 (MMP-9) and 88 small compounds. The molecular docking results confirmed the MS results, demonstrating that this method is suitable for the rapid and accurate screening of ligands binding to proteins.

ᅟ
Similar content being viewed by others
Introduction
The interactions between small molecules and proteins constitute one of the most important parts of interconnected biological networks and are of vital importance for characterizing the expression and function of proteins related to discovering drug candidates for clinical diagnosis [1]. Therefore, the determination of the binding of small molecules to proteins has attracted considerable research attention in recent years [2–4]. Owing to large synthetic and natural product compound libraries becoming more easily accessible, the availability of efficient methods for the rapid screening of the binding of these compounds to proteins becomes imperative.
Many high-throughput screening (HTS) methods have been applied for the analysis of the binding of protein ligands, such as enzyme linked immunosorbent assays (ELISA) [5, 6] and yeast two-hybrid assays [7, 8]. However, these methods are not so convenient and often require expensive experimental setups. In the past, microarrays have been developed and used to study a variety of biological processes, such as protein–protein and protein–ligand interactions. Protein microarrays are designed using immobilized protein spots to screen molecular markers and pathway targets [9, 10]. Another type of microarray is the small-molecule microarray. In contrast to protein microarrays, in this microarray, small molecule ligands are immobilized on glass slides through covalent bonds [11, 12]. Small-molecule microarrays are well suited for the discovery of small compound ligands for proteins and other biological targets. The traditional detection method for microarrays is an optical method. Proteins or other antigens and antibodies are usually fluorescently labeled so that they can be identified by optical detection [13, 14]. However, the labeling of the proteins is time-consuming and sometimes difficult. Therefore, label-free approaches are emerging.
Mass spectrometry (MS) is a natural label-free analytical technique that can be used for measuring protein–ligand interactions in high-throughput screenings [15, 16]. Many studies have reported that traditional optical detection methods can be replaced by MS for the screening of protein or small molecule microarrays [17, 18]. This can avoid false positive results and directly provides molecular information about the analytes. Matrix-assisted laser desorption/ionization mass spectrometry (MALDI MS) allows the sensitive analysis of hundreds of different molecules in a single experiment. Therefore, it has been applied in the screening of protein microarrays to characterize proteins and analyze the binding between proteins and other molecules [19–21]. However, it is well known that the selection of matrix in MALDI experiments impacts the results. The selection of a suitable matrix and its preparation before the experiment is important. Therefore, a rapid and simple method for measuring protein–ligand binding is necessary.
Desorption electrospray ionization (DESI) is an ambient ionization technique for mass spectrometry [22, 23]. No matrix and complicated sample preparation process are needed in DESI-MS experiments. Therefore, DESI-MS has been broadly used for surface sampling and high-throughput analyses [24, 25]. The measurement of drugs binding to proteins by DESI-MS was carried out by our group recently [26, 27]. This method combined DESI-MS with centrifugal filtration to screen protein–ligand binding in ambient conditions. This method can rapidly provide molecular information about the binding ligand, demonstrating that DESI-MS can be used to screen protein–ligand binding. Therefore, if DESI-MS can be combined with protein microarrays, higher detection rates and sample capacities can be obtained. It is a high-throughput method, which makes the ligand screening more rapid and accurate.
In this study, we coupled DESI-MS and protein microarrays for the high-throughput detection of small molecule ligands binding to proteins. This method offers the following advantages: high-throughput detection because the protein microarrays have large sample capacities; label-free because DESI-MS can provide accurate and sensitive molecular information; and simple and rapid because both DESI-MS and protein microarrays are easy to operate without complicated preparation procedures. To have good protein immobilization performance, agarose modified microarrays were designed and optimized. This method can measure the specific binding of proteins and small ligands, such as avidin-biotin and α1-acidoglycoprotein (AGP)-propranolol. High-throughput screening of the protein microarrays can be achieved when the experimental parameters, such as the sample spacing and the movement of the platform, are optimized. Different proteins immobilized on agarose slides were screened against a large number of small molecules. The results show the different binding affinities of these ligands. We also applied this new method to screen ligands of the important protein matrix metalloproteinase-9 (MMP-9). Eighty-eight small molecular compounds were screened against MMP-9. Only 4 s were required to identify each sample spot. The results show that nicardipine, lacidipine, nimodipine, amlodipine, benidipine, nisoldipine, and nifedipine all have a binding capacity with MMP-9. However, the binding affinity involves the structures of the small molecules. Molecular docking experiments of these compounds to MMP-9 were performed, which confirmed the similar binding capacities of these compounds that bind to the S1 pocket of MMP-9, verifying the MS screening results.
Experimental
Reagents and Materials
Microscope glass slides were obtained from Biao Ltd. (Shanghai, China). MMP-9 and DNA topoisomerase I (Topo I) were purchased from SinoBio Biotech Ltd. (Shanghai, China). FITC-labeled human immune globulin G (IgG) and FITC labeled human avidin were purchased from the Shanghai Sangon Biotechnology Co. Ltd. (Shanghai, China). The other proteins, such as α1-acidoglycoprotein (AGP), human serum albumin (HSA), transferrin (Trf), hemoglobin (Hb), and lysozyme (LZM) were from Sigma-Aldrich (St. Louis, MO, USA). The commercially available drug compounds selected for this study were obtained from the National Institute for Food and Drug Control (Beijing, China). Bind-silane, agarose, and methanol were purchased from the Beijing Chemical Factory (Beijing, China). All of the reagents were of analytical reagent grade. Nitrogen (99.999%, ultra-high purity), purchased from the Qianxi Gas Co. Ltd. (Beijing, China), was used as a desolvation gas. The fluorescence images of the FITC-labeled proteins immobilized on protein microarrays were recorded by a bio-imaging system (UVP EC3; UVP Imaging System, Upland, CA, USA) at the wavelength of 365 nm.
Agarose Slide Fabrication
The microscope glass slides were immersed in “piranha” solution (3:1, v/v, 30% H2O2:98% H2SO4) for 2 h, followed sequentially by cleaning with water. The slides were immersed in a bind-silane/water solution (v/v, 4:300) for 1 h and then dried in air. A 1.3% agarose solution was put on the surfaces of the slides. After the slides were dried, they were cleaned with water. Then, the agarose slides were dried in air before use.
Protein Immobilization and Interaction of the Ligands
The proteins were dissolved in buffer (50 mM Tris, 150 mM NaCl, 10 mM CaCl2, 0.05% (w/v) Brij35, pH = 7.5). The protein solution, volume of 0.3 μL, was directly spotted onto the agarose slide. One protein sample was one spot. The diameter of each spot was 2 mm, and the distance between the centers of the two nearest spots was 4 mm. After an overnight reaction at 37°C, the slides were washed with PBST buffer (pH = 7.4, 10 mM, PBS 0.02% (v/v) Tween-20) three times; hen, the protein was immobilized on the slides. The protein microarray was fabricated with large protein spots binding on the slides. The ligands were dissolved in water or methanol with concentration of 1 mM. The ligand solutions were incubated with the protein microarray at 37°C for 1 h and washed with PBST buffer three times, and then washed three times with water. Then the microarrays were dried in the air before the DESI-MS analysis.
Desorption Electrospray Ionization Mass Spectrometry
MS experiments were performed on a Thermo LTQ linear ion trap mass spectrometer (Thermo Fisher Scientific, San Jose, CA, USA). A homemade DESI ion source prepared as previously reported [26] was coupled to the mass spectrometer. The operating parameters were as follows: spray voltage, 4 kV; nebulizing gas (nitrogen, 99.999%) pressure, 0.8 MPa; the DESI spray solvent was 1:1 (v/v) methanol/water with a pH of 7, and the solvent flow rate is 5 μL min−1; the instrument was set to a maximum ion trap injection time of 50 ms with two microscans per spectrum. The protein microarray was placed on an automatic sample moving stage. High-throughput screening was achieved when the DESI spray scanned across the surface of the protein microarray. The experiment was operated in positive ion mode, and the data were acquired via Xcalibur software.
Results and Discussion
Rationale for the Method
The protocol for the method is straightforward as shown in Figure 1. The target proteins were immobilized on the slide’s surface, forming the protein microarray with a large amount of sample spots. Different small ligands were incubated with the protein microarray to fully interact with the proteins. After washing of the microarrays, only the ligands that bound to the proteins remained on the slide’s surface. Then, DESI-MS was used to identify the molecular information of the ligands binding to proteins. This method combines the advantages of protein microarray and DESI-MS, with the former having a large sample capacity and the latter being label-free and suitable for high-throughput detection. Therefore, this method is simple and fast for rapid screening of ligands binding to proteins.
The Evaluation of the Protein Immobilization on the Agarose Slide
In the study, the surfaces of the glass slides were modified with different functional groups for interaction with the proteins, leading to the immobilization of the proteins. We tested the protein immobilization performance of aldehyde slides, agarose slides, and epoxy slides. The results show that the agarose slide has the best performance as shown in Supplementary Figure S-1. Then, the percentage of immobilized proteins on the agarose slides was evaluated. Supplementary Figure S-2 shows the amounts of FITC-labeled human IgG and FITC-labeled human avidin on the agarose slides before and after the agarose slides were washed. The brightness of the protein spot relates to the amount of protein. Therefore, we obtained the percentage of the immobilized proteins by the ratio of the protein spot brightness before the slide was washed to that after the slide was washed. The results show that the agarose slide has protein immobilization performance of 60% and 70% for FITC-labeled human IgG and FITC-labeled human avidin, respectively. This can meet the analytical demand. Therefore, we used agarose slides to fabricate the protein microarrays.
Method Verification
To determine whether the method could be used for the screening of small ligands that bind to the proteins, we selected the following two pairs of small molecules and proteins that are known to interact with each other: biotin-avidin and AGP-propranolol. Different proteins, including Topo I, avidin, HSA, IgG, Trf, pepsin, trypsin, AGP, and Hb were immobilized on the agarose slide. Figure 2 shows the screening of the microarray when it interacted with the small molecule biotin. As anticipated, the signal of biotin can only be observed on the spot containing avidin. However, when the microarray was interacted with propranolol, the signal of propranolol can only be observed on the spot containing AGP as shown in Figure 3. The results indicated that this method can be applied to the screening of small molecules that bind to targeted proteins. In addition, we also performed a control experiment for the protein microarray using an agarose slide without immobilized proteins. Figure 4 shows the signals of 9 small ligands after they interacted with the AGP protein microarray and agarose slide without AGP immobilization. The results indicated that the small ligands can be detected only when the AGP was immobilized on the agarose slide. Therefore, these results rule out the disturbance of the absorption by agarose.
The Optimization of the DESI MS Parameters
DESI operational parameters have an influence on the performance of the method. Therefore, the main operational parameters of DESI-MS were optimized. Supplementary Figure S-3 shows the optimization of the spray voltage, carrier gas pressure, spray solvent flow rate, and solvent pH for the analysis of propranolol binding to AGP. It shows that the signal intensity increases as the spray voltage and carrier gas pressure increase. We typically chose 4 kV for the spray voltage and 0.8 MPa for the gas pressure in the study. The spray solvent flow rate was set at 5 μL/min because when the flow rate is above 5 μL/min, the ionization efficiency decreases. In the experiment, the signal to noise ratio of the extracted ion chronogram increased when the spray solvent was in acidic conditions. But when the pH decreased, many other ion peaks were generated, and it was difficult to identify the analyte signals. Therefore, pH of 7 was selected in the study.
The Optimization of the Screening Rate
In the study, the protein microarray was placed on an automatic moving stage. The speed of movement can influence the screening rate. Therefore, we optimized the screening rate by obtaining the optimal moving rate for the stage. Figure 5 shows the extracted ion chronogram of propranolol screened against AGP by DESI-MS when the stage was moved at different speeds. The distance between the two protein spots is 4 mm. The diameter of one spot is approximately 2 mm. When the stage was moved at a rate of 0.4 mm/s, the signal peaks could be well distinguished. As the rate of movement increased, the screening time was more rapid. When the rate of movement increased to 1.1 mm/s, the signal peaks could also be identified. In the study, the optimal rate for the movement of the automatic stage was 1.1 mm/s. The signal peaks were overlapped at the scan rate of 1.4 mm/s, where it was difficult to distinguish the signal peaks of adjacent samples.
High-Throughput Screening of the Binding of Small Compounds to Proteins
We incubated the microarrays of AGP, LZM, Trf, IgG, and Topo I with 34 small compounds to test the application of the HTS experiment. The 34 compounds were classified as four types: beta blockers, alkaloid, angiotensin receptor blockers, or calcium channel blockers. The HTS of the protein microarrays against these compounds was performed by DESI-MS. The results show that the intensities of the ligand candidates are different on different protein spots as shown in Figure 6. Different proteins exhibited different binding affinities. For the beta blocker compounds, AGP had a higher binding affinity to propranolol than the other proteins. However, for the angiotensin receptor blocker compounds, most proteins exhibited low signal intensities and binding affinities. In the HTS experiment, only 4 s were required for the screening of one sample spot. Therefore, this method is simple and rapid for ligand–protein screening.
High-throughput screening of the following four types of ligands: (a) beta blockers (1–10: propranolol, esmolol, bisoprolol, labetalol, celiprolol, carteolol, metoprolol, sotalol, atenolol, and bevantolol); (b) alkaloid (1–6: berberine, oxymatrine, jateorhizine, camptothecin, quercetin, and palmatine); (c) angiotensin receptor blockers (1–9: olmesartan, candesartan potassium, losartan potassium, valsartan, irbesartan, telmisartan, pravastatin, fluvastatin sodium, and lovastatin); (d) calcium channel blockers (1–9: verapamil, nicardipine, benidipine, felodipine, nisoldipine, nimodipine, lacidipine, nifedipine, amlodipine) against Topo I, LZM, AGP, IgG, and Trf
Screening of the Binding of Small Ligands to MMP-9
Matrix metalloproteinases (MMPs) are a family of zinc-dependent endopeptidases. In humans, this family comprises 23 different MMPs. MMPs are involved in pathological processes, including cardiovascular diseases, autoimmune diseases, and cancer. Therefore, MMPs are believed to be potential drug targets, and MMP inhibition is considered to be useful for the treatment of these troublesome diseases. Over the past 30 years, many MMP inhibitors (MMPIs) have been investigated in clinical trials. Unfortunately, most of these trails failed mainly because of their poor selectivity [28, 29].
Recently, growing evidence indicates that among MMPs, MMP-9 is a major player in the pathophysiology of these related diseases. Hence, specific inhibitors that only inhibit MMP-9 might be more promising [30–33]. However, the lack of an effective screening method makes it difficult to search for new selective MMP-9 inhibitors. In order to solve this dilemma, herein we tried to apply our HTS method to the screening of specific MMP-9 inhibitors. We hope that this new approach can contribute to drug discovery.
Table 1 shows the screening results of the binding between MMP-9 and 88 ligand compounds. The results of each compound were obtained by the detection of three sample spots. Among the ligand compounds, we found that the calcium channel blockers (nicardipine, benidipine, nisoldipine, felodipine, nimodipine, lacidipine, nifedipine, and amlodipine) had relatively higher signal intensities. These calcium channel blockers are dihydropyridine compounds. The mass spectra results suggested that they might interact with MMP-9. In addition, molecular docking experiments were carried out to compare the binding interactions between MMP-9 and these compounds. The molecular docking experiments confirmed the mass spectra results. More details can be seen in the Supporting Information. Therefore, this method can provide novel scaffolds for specific MMP-9 inhibitors, which may rationalize both the affinity and the selectivity, exert therapeutic effects, and overcome undesired side effects. The results encourage us to extend this methodology toward the research and development of new drugs in a broader domain.
Conclusion
In this study, a new method for screening protein ligands was proposed. A protein microarray was applied in the DESI-MS platform, which makes studying the interactions between proteins and ligands easier and more convenient. We used agarose slides to immobilize the proteins because of their large sample capacity and their ability to meet the requirement for high-throughput detection. The new method was applied to screen ligand compounds for their binding to target proteins. We found that some calcium channel blockers had high signal intensities for the screening of MMP-9 ligands. The molecular docking experiments confirmed the MS results, demonstrating that the method was rapid and accurate. Compared with conventional methods, this is a label-free and high-throughput method. It is suitable for studying the binding of various types of synthetic and natural product compounds and biological macromolecules.
References
Kuhn, M., Szklarczyk, D., Franceschini, A., Campillos, M., von Mering, C., Jensen, L.J., Beyer, A., Bork, P.: STITCH 2: an interaction network database for small molecules and proteins. Nucleic Acids Res. 38, D552–D556 (2010)
Ong, S.E., Schenone, M., Margolin, A.A., Li, X., Do, K., Doud, M.K., Mani, D.R., Kuai, L., Wang, X., Wood, J.L., Tolliday, N.J., Koehler, A.N., Marcaurelle, L.A., Golub, T.R., Gould, R.J., Schreiber, S.L., Carr, S.A.: Identifying the proteins to which small-molecule probes and drugs bind in cells. Proc. Natl. Acad. Sci. U. S. A. 106, 4617–4622 (2009)
DeArmond, P.D., Xu, Y., Strickland, E.C., Daniels, K.G., Fitzgerald, M.C.: Thermodynamic analysis of protein–ligand interactions in complex biological mixtures using a shotgun proteomics approach. J. Proteome Res. 10, 4948–4958 (2011)
Burgett, A.W.G., Poulsen, T.B., Wangkanont, K., Anderson, D.R., Kikuchi, C., Shimada, K., Okubo, S., Fortner, K.C., Mimaki, Y., Kuroda, M., Murphy, J.P., Schwalb, D.J., Petrella, E.C., Cornella-Taracido, I., Schirle, M., Tallarico, J.A., Shair, M.D.: Natural products reveal cancer cell dependence on oxysterol-binding proteins. Nat. Chem. Biol. 7, 639–647 (2011)
Pang, W., Wang, R.R., Gao, Y.D., Yang, L.M., Sun, Y., Huang, J.F., Tien, P., Zheng, Y.T.: A novel enzyme-linked immunosorbent assay for screening HIV-1 fusion inhibitors targeting HIV-1 Gp41 core structure. J. Biomol. Screen. 16, 221–229 (2011)
Capule, C.C., Yang, J.: Enzyme-linked immunosorbent assay-based method to quantify the association of small molecules with aggregated amyloid peptides. Anal. Chem. 84, 1786–1791 (2012)
Le Breton, M., Meyniel-Schicklin, L., Deloire, A., Coutard, B., Canard, B., de Lamballerie, X., Andre, P., Rabourdin-Combe, C., Lotteau, V., Davoust, N.: Flavivirus NS3 and NS5 proteins interaction network: a high-throughput yeast two-hybrid screen. BMC Microbiol. 11(234), 1–10 (2011)
Rajagopala, S.V., Uetz, P.: Analysis of protein–protein interactions using high-throughput yeast two-hybrid screens. Methods Mol. Biol. (Clifton, N.J.) 781, 1–29 (2011)
Zhang, Y., Lou, J., Jenko, K.L., Marks, J.D., Varnum, S.M.: Simultaneous and sensitive detection of six serotypes of botulinum neurotoxin using enzyme-linked immunosorbent assay-based protein antibody microarrays. Anal. Biochem. 430, 185–192 (2012)
MacBeath, G., Schreiber, S.L.: Printing proteins as microarrays for high-throughput function determination. Science 289, 1760–1763 (2000)
Wu, H., Ge, J., Uttamchandani, M., Yao, S.Q.: Small molecule microarrays: the first decade and beyond. Chem. Commun. 47, 5664–5670 (2011)
Kanoh, N., Kumashiro, S., Simizu, S., Kondoh, Y., Hatakeyama, S., Tashiro, H., Osada, H.: Immobilization of natural products on glass slides by using a photoaffinity reaction and the detection of protein-small-molecule interactions. Angew. Chem. Int. Ed. 42, 5584–5587 (2003)
Hergenrother, P.J., Depew, K.M., Schreiber, S.L.: Small-molecule microarrays: Covalent attachment and screening of alcohol-containing small molecules on glass slides. J. Am. Chem. Soc. 122, 7849–7850 (2000)
Berrade, L., Garcia, A.E., Camarero, J.A.: Protein microarrays: novel developments and applications. Pharm. Res. 28, 1480–1499 (2011)
Jonker, N., Kool, J., Irth, H., Niessen, W.M.A.: Recent developments in protein–ligand affinity mass spectrometry. Anal. Bioanal. Chem. 399, 2669–2681 (2011)
Pacholarz, K.J., Garlish, R.A., Taylor, R.J., Barran, P.E.: Mass spectrometry based tools to investigate protein–ligand interactions for drug discovery. Chem. Soc. Rev. 41, 4335–4355 (2012)
Ouyang, Z., Takats, Z., Blake, T.A., Gologan, B., Guymon, A.J., Wiseman, J.M., Oliver, J.C., Davisson, V.J., Cooks, R.G.: Preparing protein microarrays by soft-landing of mass-selected ions. Science 301, 1351–1354 (2003)
Roth, M.J., Kim, J., Maresh, E.M., Plymire, D.A., Corbett, J.R., Zhang, J., Patrie, S.M.: Thin-layer matrix sublimation with vapor-sorption induced co-crystallization for sensitive and reproducible SAMDI-TOF MS analysis of protein biosensors. J. Am. Soc. Mass Spectrom. 23, 1661–1669 (2012)
Spera, R., Labaer, J., Nicolini, C.: MALDI-TOF characterization of NAPPA-generated proteins. J. Mass Spectrom. 46, 960–965 (2011)
Beloqui, A., Calvo, J., Serna, S., Yan, S., Wilson, I.B.H., Martin-Lomas, M., Christian Reichardt, N.: Analysis of microarrays by MALDI-TOF MS. Angew. Chem. Int. Ed. 52, 7477–7481 (2013)
Becker, C.F.W., Wacker, R., Bouschen, W., Seidel, R., Kolaric, B., Lang, P., Schroeder, H., Muller, O., Niemeyer, C.M., Spengler, B., Goody, R.S., Engelhard, M.: Direct readout of protein-protein interactions by mass spectrometry from protein-DNA microarrays. Angew. Chem. Int. Ed. 44, 7635–7639 (2005)
Vismeh, R., Waldon, D.J., Teffera, Y., Zhao, Z.: Localization and quantification of drugs in animal tissues by use of desorption electrospray ionization mass spectrometry imaging. Anal. Chem. 84, 5439–5445 (2012)
Ferreira, C.R., Eberlin, L.S., Hallett, J.E., Cooks, R.G.: Single oocyte and single embryo lipid analysis by desorption electrospray ionization mass spectrometry. J. Mass Spectrom. 47, 29–33 (2012)
Da Costa, C., Reynolds, J.C., Whitmarsh, S., Lynch, T., Creaser, C.S.: The quantitative surface analysis of an antioxidant additive in a lubricant oil matrix by desorption electrospray ionization mass spectrometry. Rapid Commun. Mass Spectrom. 27, 2420–2424 (2013)
Nielen, M.W.F., Hooijerink, H., Zomer, P., Mol, J.G.J.: Desorption electrospray ionization mass spectrometry in the analysis of chemical food contaminants in food. Trac-Trend Anal. Chem. 30, 165–180 (2011)
Yao, C.X., Na, N., Huang, L.Y., He, D.C., Ouyang, J.: High-throughput detection of drugs binding to proteins using desorption electrospray ionization mass spectrometry. Anal. Chim. Acta 794, 60–66 (2013)
Lu, X., Ning, B.M., He, D.C., Huang, L.Y., Yue, X.J., Zhang, Q.M., Huang, H.W., Liu, Y., He, L., Ouyang, J.: High throughput screening of high-affinity ligands for proteins with anion-binding sites using desorption electrospray ionization (DESI) mass spectrometry. J. Am. Soc. Mass Spectrom. 25, 454–463 (2014)
Cuniasse, P., Devel, L., Makaritis, A., Beau, F., Georgiadis, D., Matziari, M., Yiotakis, A., Dive, V.: Future challenges facing the development of specific active-site-directed synthetic inhibitors of MMPs. Biochimie 87, 393–402 (2005)
Murphy, G., Nagase, H.: Progress in matrix metalloproteinase research. Mol. Asp. Med. 29, 290–308 (2008)
Mao, X., Yin, W., Liu, M., Ye, M., Liu, P., Liu, J., Lian, Q., Xu, S., Pi, R.: Osthole, a natural coumarin, improves neurobehavioral functions and reduces infarct volume and matrix metalloproteinase-9 activity after transient focal cerebral ischemia in rats. Brain Res. 1385, 275–280 (2011)
Fontana, V., Silva, P.S., Gerlach, R.F., Tanus-Santos, J.E.: Circulating matrix metalloproteinases and their inhibitors in hypertension. Clin. Chim. Acta 413, 656–662 (2012)
Tauro, M., Laghezza, A., Loiodice, F., Agamennone, M., Campestre, C., Tortorella, P.: Arylamino methylene bisphosphonate derivatives as bone seeking matrix metalloproteinase inhibitors. Bioorg. Med. Chem. 21, 6456–6465 (2013)
Yeh, J.L., Hsu, J.H., Liang, J.C., Chen, I.J., Liou, S.F.: Lercanidipine and labedipinedilol--A attenuate lipopolysaccharide/interferon-γ-induced inflammation in rat vascular smooth muscle cells through inhibition of HMGB1 release and MMP-2, 9 activities. Atherosclerosis 226, 364–372 (2013)
Acknowledgments
The authors gratefully acknowledge the support from the National Nature Science Foundation of China (21175014, 21475011, 21422503), National Grant of Basic Research Program of China (2011CB915504), and the Fundamental Research Funds for the Central Universities.
Author information
Authors and Affiliations
Corresponding author
Additional information
Chenxi Yao and Tao Wang contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOC 2577 kb)
Rights and permissions
About this article
Cite this article
Yao, C., Wang, T., Zhang, B. et al. Screening of the Binding of Small Molecules to Proteins by Desorption Electrospray Ionization Mass Spectrometry Combined with Protein Microarray. J. Am. Soc. Mass Spectrom. 26, 1950–1958 (2015). https://doi.org/10.1007/s13361-015-1221-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13361-015-1221-z