Abstract
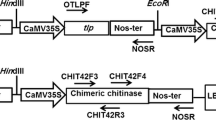
Sclerotinia stem rot caused by Sclerotinia sclerotiorum is one of the major fungal diseases of Brassica napus L. To develop resistance against this fungal disease, the defensin gene from Raphanus sativus and chimeric chit42 from Trichoderma atroviride with a C-terminal fused chitin-binding domain from Serratia marcescens were co-expressed in canola via Agrobacterium-mediated transformation. Twenty transformants were confirmed to carry the two transgenes as detected by polymerase chain reaction (PCR), with 4.8 % transformation efficiency. The chitinase activity of PCR-positive transgenic plants were measured in the presence of colloidal chitin, and five transgenic lines showing the highest chitinase activity were selected for checking the copy number of the transgenes through Southern blot hybridisation. Two plants carried a single copy of the transgenes, while the remainder carried either two or three copies of the transgenes. The antifungal activity of two transgenic lines that carried a single copy of the transgenes (T4 and T10) was studied by a radial diffusion assay. It was observed that the constitutive expression of these transgenes in the T4 and T10 transgenic lines suppressed the growth of S. sclerotiorum by 49 % and 47 %, respectively. The two transgenic lines were then let to self-pollinate to produce the T2 generation. Greenhouse bioassays were performed on the transgenic T2 young leaves by challenging with S. sclerotiorum and the results revealed that the expression of defensin and chimeric chitinase from a heterologous source in canola demonstrated enhanced resistance against sclerotinia stem rot disease.







Similar content being viewed by others
References
Alvarez ML, Guelman S, Halford NG, Lustig S, Reggiardo MI, Ryabushkina N, Schewry P, Stein J, Vallejos RH (2000) Silencing of HMW glutenins in transgenic wheat expressing extra HMW subunits. Theor Appl Genet 100:319–327
Ayers AR, Ebel J, Valent B, Albersheim P (1976) Host–pathogen interactions: X. Fractionation and biological activity of an elicitor isolated from the mycelial walls of Phytophthora megasperma var. sojae. Plant Physiol 57:760–765
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein–dye binding. Anal Biochem 72:248–254
Broekaert WF, Terras FRG, Cammue BPA, Osborn RW (1995) Plant defensins: novel antimicrobial peptides as components of the host defense system. Plant Physiol 108:1353–1358
Chhikara S, Chaudhury D, Dhankher OP, Jaiwal PK (2012) Combined expression of a barley class II chitinase and type I ribosome inactivating protein in transgenic Brassica juncea provides protection against Alternaria brassicae. Plant Cell Tissue Organ Cult 108:83–89
Cho HS, Cao J, Ren JP, Earle ED (2001) Control of Lepidopteran insect pests in transgenic Chinese cabbage (Brassica rapa ssp. pekinensis) transformed with a synthetic Bacillus thuringiensis cry1C gene. Plant Cell Rep 20:1–7
Das B, Goswami L, Ray S, Ghosh S, Bhattacharyya S, Das S, Majumder AL (2006) Agrobacterium-mediated transformation of Brassica juncea with a cyanobacterial (Synechocystis PCC6803) delta-6 desaturase gene leads to production of gamma-linolenic acid. Plant Cell Tissue Org Cult 86:219–231
De Buck S, Windels P, De Loose M, Depicker A (2004) Single-copy T-DNAs integrated at different positions in the Arabidopsis genome display uniform and comparable beta-glucuronidase accumulation levels. Cell Mol Life Sci 61:2632–2645
de las Mercedes Dana M, Pintor-Toro JA, Cubero B (2006) Transgenic tobacco plants overexpressing chitinases of fungal origin show enhanced resistance to biotic and abiotic stress agents. Plant Physiol 142:722–730
Demeke T, Hucl P, Båga M, Caswell K, Leung N, Chibbar RN (1999) Transgene inheritance and silencing in hexaploid spring wheat. Theor Appl Genet 99:947–953
Finnegan J, McElroy D (1994) Transgene inactivation: plants fight back! Nat Biotechnol 12:883–888
Ganesan M, Bhanumathi P, Ganesh Kumari K, Lakshmi Prabha A, Song PS, Jayabalan N (2009) Transgenic Indian cotton (Gossypium hirsutum) harboring rice chitinase gene (Chi II) confers resistance to two fungal pathogens. Am J Biochem Biotechnol 5:63–74
Graham LS, Sticklen MB (1994) Plant chitinases. Can J Bot 72:1057–1083
Hardt M, Laine RA (2004) Mutation of active site residues in the chitin-binding domain ChBDChiA1 from chitinase A1 of Bacillus circulans alters substrate specificity: use of a green fluorescent protein binding assay. Arch Biochem Biophys 426:286–297
Huang X, Wang J, Du Z, Zhang C, Li L, Xu Z (2013) Enhanced resistance to stripe rust disease in transgenic wheat expressing the rice chitinase gene RC24. Transgenic Res 22:939–947
Jha S, Chattoo BB (2010) Expression of a plant defensin in rice confers resistance to fungal phytopathogens. Transgenic Res 19:373–384
Jha S, Tank HG, Prasad BD, Chattoo BB (2009) Expression of Dm-AMP1 in rice confers resistance to Magnaporthe oryzae and Rhizoctonia solani. Transgenic Res 18:59–69
Khan RS, Sjahril R, Nakamura I, Mii M (2008) Production of transgenic potato exhibiting enhanced resistance to fungal infections and herbicide applications. Plant Biotechnol Rep 2:13–20
Khan RS, Darwish NA, Khattak B, Ntui VO, Kong K, Shimomae K, Nakamura I, Mii M (2014) Retransformation of marker-free potato for enhanced resistance against fungal pathogens by pyramiding chitinase and wasabi defensin genes. Mol Biotechnol 56:814–823
Kim JK, Jang IC, Wu R, Zuo WN, Boston RS, Lee YH, Ahn IP, Nahm BH (2003) Co-expression of a modified maize ribosome-inactivating protein and a rice basic chitinase gene in transgenic rice plants confers enhanced resistance to sheath blight. Transgenic Res 12:475–484
Kirkegaard JA, Robertson MJ, Hamblin P, Sprague SJ (2006) Effect of blackleg and sclerotinia stem rot on canola yield in the high rainfall zone of southern New South Wales, Australia. Aust J Agric Res 57:201–212
Kramer KJ, Muthukrishnan S (1997) Insect chitinases: molecular biology and potential use as biopesticides. Insect Biochem Mol Biol 27:887–900
Kumar V, Parkhi V, Kenerley CM, Rathore KS (2009) Defense-related gene expression and enzyme activities in transgenic cotton plants expressing an endochitinase gene from Trichoderma virens in response to interaction with Rhizoctonia solani. Planta 230:277–291
Li Z, Zhou M, Zhang Z, Ren L, Du L, Zhang B, Xu H, Xin Z (2011) Expression of a radish defensin in transgenic wheat confers increased resistance to Fusarium graminearum and Rhizoctonia cerealis. Funct Integr Genomics 11:63–70
Limón MC, Lora JM, García I, de la Cruz J, Llobell A, Benítez T, Pintor-Toro JA (1995) Primary structure and expression pattern of the 33-kDa chitinase gene from the mycoparasitic fungus Trichoderma harzianum. Curr Genet 28:478–483
Limón MC, Chacón MR, Mejías R, Delgado-Jarana J, Rincón AM, Codón AC, Benítez T (2004) Increased antifungal and chitinase specific activities of Trichoderma harzianum CECT 2413 by addition of a cellulose binding domain. Appl Microbiol Biotechnol 64:675–685
Liu M, Sun ZX, Zhu J, Xu T, Harman GE, Lorito M (2004) Enhancing rice resistance to fungal pathogens by transformation with cell wall degrading enzyme genes from Trichoderma atroviride. J Zhejiang Univ Sci 5:133–136
Liu H, Guo X, Naeem MS, Liu D, Xu L, Zhang W, Tang G, Zhou W (2011) Transgenic Brassica napus L. lines carrying a two gene construct demonstrate enhanced resistance against Plutella xylostella and Sclerotinia sclerotiorum. Plant Cell Tissue Organ Cult 106:143–151
Lorito M, Woo SL, Garcia I, Colucci G, Harman GE, Pintor-Toro JA, Filippone E, Muccifora S, Lawrence CB, Zoina A, Tuzun S, Scala F (1998) Genes from mycoparasitic fungi as a source for improving plant resistance to fungal pathogens. Proc Natl Acad Sci U S A 95:7860–7865
Matsuzaki K, Yoneyama S, Fujii N, Miyajima K, Yamada K, Kirino Y, Anzai K (1997) Membrane permeabilization mechanisms of a cyclic antimicrobial peptide, tachyplesin I, and its linear analog. Biochemistry 36:9799–9806
Murashige T, Skoog F (1962) A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol Plant 15:473–497
Ntui VO, Azadi P, Thirukkumaran G, Khan RS, Chin DP, Nakamura I, Mii M (2011) Increased resistance to fusarium wilt in transgenic tobacco lines co-expressing chitinase and wasabi defensin genes. Plant Pathol 60:221–231
Olli S, Kirti PB (2006) Cloning, characterization and antifungal activity of defensin Tfgd1 from Trigonella foenum-graecum L. J Biochem Mol Biol 39:278–283
Picart P, Pirttilä AM, Raventos D, Kristensen HH, Sahl HG (2012) Identification of defensin-encoding genes of Picea glauca: characterization of PgD5, a conserved spruce defensin with strong antifungal activity. BMC Plant Biol 12:180
Portieles R, Ayra C, Gonzalez E, Gallo A, Rodriguez R, Chacón O, López Y, Rodriguez M, Castillo J, Pujol M, Enriquez G, Borroto C, Trujillo L, Thomma BP, Borrás-Hidalgo O (2010) NmDef02, a novel antimicrobial gene isolated from Nicotiana megalosiphon confers high-level pathogen resistance under greenhouse and field conditions. Plant Biotechnol J 8:678–690
Sagaram US, Pandurangi R, Kaur J, Smith TJ, Shah DM (2011) Structure–activity determinants in antifungal plant defensins MsDef1 and MtDef4 with different modes of action against Fusarium graminearum. PLoS One 6(4):e18550
Saitoh H, Kiba A, Nishihara M, Yamamura S, Suzuki K, Terauchi R (2001) Production of antimicrobial defensin in Nicotiana benthamiana with a potato virus X vector. Mol Plant Microbe Interact 14:111–115
Schaefer SC, Gasic K, Cammue B, Broekaert W, van Damme EJ, Peumans WJ, Korban SS (2005) Enhanced resistance to early blight in transgenic tomato lines expressing heterologous plant defense genes. Planta 222:858–866
Srivastava V, Vasil V, Vasil IK (1996) Molecular characterization of the fate of transgenes in transformed wheat (Triticum aestivum L.). Theor Appl Genet 92:1031–1037
Terras FRG, Eggermont K, Kovaleva V, Raikhel NV, Osborn RW, Kester A, Rees SB, Torrekens S, Van Leuven F, Vanderleyden J (1995) Small cysteine-rich antifungal proteins from radish: their role in host defense. Plant Cell 7:573–588
Thomma BPJ, Cammue BPA, Thevissen K (2002) Plant defensins. Planta 216:193–202
Tohidfar M, Mohammadi M, Ghareyazie B (2005) Agrobacterium-mediated transformation of cotton (Gossypium hirsutum) using a heterologous bean chitinase gene. Plant Cell Tissue Organ Cult 83:83–96
van der Biezen EA (2001) Quest for antimicrobial genes to engineer disease-resistant crops. Trends Plant Sci 6:89–91
van der Weerden NL, Lay FT, Anderson MA (2008) The plant defensin, NaD1, enters the cytoplasm of Fusarium oxysporum hyphae. J Biol Chem 283:14445–14452
Wang J, Chen Z, Du J, Sun Y, Liang A (2005) Novel insect resistance in Brassica napus developed by transformation of chitinase and scorpion toxin genes. Plant Cell Rep 24:549–555
Wong JH, Ng TB (2005) Sesquin, a potent defensin-like antimicrobial peptide from ground beans with inhibitory activities toward tumor cells and HIV-1 reverse transcriptase. Peptides 26:1120–1126
Zeilinger S, Galhaup C, Payer K, Woo SL, Mach RL, Fekete C, Lorito M, Kubicek CP (1999) Chitinase gene expression during mycoparasitic interaction of Trichoderma harzianum with its host. Fungal Genet Biol 26:131–140
Acknowledgments
This research was financially supported by the National Institute of Genetic Engineering and Biotechnology (NIGEB) of I. R. Iran.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors have no conflict of interest.
Additional information
Communicated by: Andrzej Górny
Rights and permissions
About this article
Cite this article
Zarinpanjeh, N., Motallebi, M., Zamani, M.R. et al. Enhanced resistance to Sclerotinia sclerotiorum in Brassica napus by co-expression of defensin and chimeric chitinase genes. J Appl Genetics 57, 417–425 (2016). https://doi.org/10.1007/s13353-016-0340-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13353-016-0340-y