Abstract
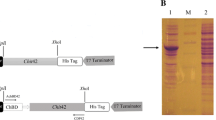
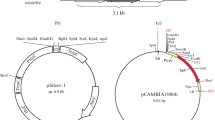
Trichoderma harzianum is a widely distributed soil fungus that antagonizes numerous fungal phytopathogens. The antagonism of T. harzianum usually correlates with the production of antifungal activities including the secretion of fungal cell walls that degrade enzymes such as chitinases. Chitinases Chit42 and Chit33 from T. harzianum CECT 2413, which lack a chitin-binding domain, are considered to play an important role in the biocontrol activity of this strain against plant pathogens. By adding a cellulose-binding domain (CBD) from cellobiohydrolase II of Trichoderma reesei to these enzymes, hybrid chitinases Chit33-CBD and Chit42-CBD with stronger chitin-binding capacity than the native chitinases have been engineered. Transformants that overexpressed the native chitinases displayed higher levels of chitinase specific activity and were more effective at inhibiting the growth of Rhizoctonia solani, Botrytis cinerea and Phytophthora citrophthora than the wild type. Transformants that overexpressed the chimeric chitinases possessed the highest specific chitinase and antifungal activities. The results confirm the importance of these endochitinases in the antagonistic activity of T. harzianum strains, and demonstrate the effectiveness of adding a CBD to increase hydrolytic activity towards insoluble substrates such as chitin-rich fungal cell walls.








Similar content being viewed by others
References
Abuja PM, Pilz I, Claeyssens M, Tomme P (1988) Domain structure of cellobiohydrolase II as studied by small angle X-ray scattering: close resemblance to cellobiohydrolase I. Biochem Biophys Res Commun 156:180–185
Ait-Lahsen H, Soler A, Rey M, de La Cruz J, Monte E, Llobell A (2001) An antifungal exo-alpha-1,3-glucanase (AGN13.1) from the biocontrol fungus Trichoderma harzianum. Appl Environ Microbiol 67:5833–5839
Alfthan K, Takkinen K, Sizmann D, Söderlund H, Teeri T (1995) Properties of a single-chain antibody containing different linker peptides. Protein Eng 8:725–731
Baek JM, Howell CR, Kenerley CM (1999) The role of an extracellular chitinase from Trichoderma virens Gv29-8 in the biocontrol of Rhizoctonia solani. Curr Genet 35:41–50
Bradford M (1976) A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:249–254
Belloch C, Lopez L, Esteve B, Martinez PV, Gª-Lopez MD, Uruburu F (eds) (1998) Spanish Type Culture Collection. Universitat de Valencia, Valencia, Spain
Blackwell J (1988) Physical methods for the determination of chitin structure and conformation. Methods Enzymol 161:435–442
Boller T, Mauch F (1988) Colorimetric assay for chitinase. Methods Enzymol 161:430–435
Boysen M, Borja M, del Moral C, Salazar O, Rubio V (1996) Identification at strain level of Rhizoctonia solani AG4 isolates by direct sequence of asymmetric PCR products of the ITS regions. Curr Genet 29:174–181
Carsolio C, Benhamou N, Haran S, Cortes C, Gutierrez A, Chet I, Herrera-Estrella A (1999) Role of the Trichoderma harzianum endochitinase gene, ech42, in mycoparasitism. Appl Environ Microbiol 65:929–935
Chet I, Benhamou N, Haran S (1998) Mycoparasitism and lytic enzymes. In: Kubicek Geha CP (ed) Trichoderma and Gliocladium, vol 2. Taylor & Francis, London, pp 153–172
De la Cruz J, Pintor-Toro JA, Benítez T, Llobell A (1995) Purification and characterization of an endo-β1,6-glucanase from Trichoderma harzianum that is related to its mycoparasitism. J Bacteriol 177:1864–1871
Delgado-Jarana J, Pintor-Toro JA, Benitez T (2000) Overproduction of β-1,6-glucanase in Trichoderma harzianum is controlled by extracellular acidic proteases and pH. Biochim Biophys Acta 1481:289–296
Delgado-Jarana J, Rincon AM, Benitez T (2002) Aspartyl protease from Trichoderma harzianum CECT 2413: cloning and characterization. Microbiology 148:1305–1315
Flores A, Chet I, Herrera-Estrella A (1997) Improved biocontrol activity of Trichoderma harzianum by over-expression of the proteinase-encoding gene prb1. Curr Genet 31:30–37
Garcia I (1997) Caracterización del gen de la quitinasa CHIT42 del hongo antagonista Trichoderma harzianum y análisis de su expresión en plantas de tabaco. Doctoral thesis, Universidad de Sevilla, Seville, Spain
Garcia I, Lora JM, de la Cruz J, Benitez T, Llobell A, Pintor-Toro JA (1994) Cloning and characterization of a chitinase (chit42) cDNA from the mycoparasitic fungus Trichoderma harzianum. Curr Genet 28:83–89
Goldstein MA, Takagi M, Hashida S, Shoseyov O, Doi RH, Segel IH (1993) Characterization of the cellulose-binding domain of the Clostridium cellulovorans cellulose-binding protein A. J Bacteriol 175:5762–5768
Holwerda BC, Rogers JC (1992) Purification and characterization of aleurain. A plant thiol protease functionally homologous to mammalian cathepsin. Plant Physiol 99:848–855
Hynes MJ, Corrick CM, King JA (1983) Isolation of genomic clones containing the amdS gene of Aspergillus nidulans and their use in the analysis of structural and regulatory mutations. Mol Cell Biol 3:1430–1439
Kim DJ, Baek JM, Uribe P, Kenerley CM, Cook DR (2002) Cloning and characterization of multiple glycosyl hydrolase genes from Trichoderma virens. Curr Genet 40:374–384
Kurek I, Kawagoe Y, Jacob-Wilk D, Doblin M, Delmer D (2002) Dimerization of cotton fiber cellulose synthase catalytic subunits occurs via oxidation of the zinc-binding domains. Proc Natl Acad Sci USA 99:11109–11114
Langsford ML, Gilkes NR, Singh B, Moser B, Miller RC Jr, Warren RA, Kilburn DG (1987) Glycosylation of bacterial cellulases prevents proteolytic cleavage between functional domains. FEBS Lett 225:163–167
Limon MC, Lora JM, Garcia I, de La Cruz J, Llobell A, Benitez T, Pintor-Toro JA (1995) Primary structure and expression pattern of the 33 kDa chitinase gene from the mycoparasitic fungus Trichoderma harzianum. Curr Genet 28:478–483
Limon MC, Pintor-Toro JA, Benitez T (1999) Increased antifungal activity of Trichoderma harzianum transformants that overexpress a 33-kDa chitinase. Phytopathology 89:254–261
Limon MC, Margolles-Clark E, Benitez T, Penttila M (2001) Addition of substrate-binding domains increases substrate-binding capacity and specific activity of a chitinase from Trichoderma harzianum. FEMS Microbiol Lett 198:57–63
Linder M, Teeri T (1996) The cellulose-binding domain of the major cellobiohydrolase of Trichoderma reesei exhibits true reversibility and a high exchange rate on crystalline cellulose. Proc Natl Acad Sci USA 93:12251–12255
Linder M, Salovuori I, Ruohonen L, Teeri TT (1996) Characterization of a double cellulose-binding domain. Synergistic high affinity binding to crystalline cellulose. J Biol Chem 271:21268–21272
Lorito M, Woo SL, Garcia I, Colucci G, Harman GE, Pintor-Toro JA, Filippone E, Muccifora S, Lawrence CB, Zoina A, Tuzun S, Scala F, Fernandez IG (1998) Genes from mycoparasitic fungi as a source for improving plant resistance to fungal pathogens. Proc Natl Acad Sci USA 95:7860–7865
Mach RL, Schindler M, Kubicek CP (1994) Transformation of Trichoderma reesei based on hygromycin B resistance using homologous expression signals. Curr Genet 25:567–570
Margolles-Clark E, Hayes CK, Harman GE, Penttila M (1996) Improved production of Trichoderma harzianum endochitinase by expression in Trichoderma reesei. Appl Environ Microbiol 62:2145–2151
Morimoto K, Karita S, Kimura T, Sakka K, Ohmiya K (1997) Cloning, sequencing, and expression of the gene encoding Clostridium paraputrificum chitinase ChiB and analysis of the functions of novel cadherin-like domains and a chitin-binding domain. J Bacteriol 179:7306–7314
Ong E, Gilkes NR, Miller RC, Warren RA, Kilburn DG (1993) The cellulose-binding domain (CBDCex) of an exoglucanase from Cellulomonas fimi: production in Escherichia coli and characterization of the polypeptide. Biotechnol Bioeng 42:401–409
Pembroke JT (1998) Function and biotechnology of cellulose binding domains in bacterial and fungal cellulose and xylan hydrolysing enzymes. In: Pandalau SG (ed) Recent research developments in biotechnology and bioengineering. Trivandrum, India, pp 177–190
Penttila M, Nevalainen H, Ratto M, Salminen E, Knowles J (1987) A versatile transformation system for the cellulolytic filamentous fungus Trichoderma reesei. Gene 61:155–164
Reissig JL, Strominger JL, Leloir LF (1955) A modified colorimetric method for the estimation of N-acetylamino sugars. J Biol Chem 217:959–966
Sambrook J, Fritsch EF, Maniatis T (1989) Molecular cloning: a laboratory manual, 2nd edn. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
Srisodsuk M, Reinikainen T, Penttila M, Teeri TT (1993) Role of the interdomain linker peptide of Trichoderma reesei cellobiohydrolase I in its interaction with crystalline cellulose. J Biol Chem 268:20756–20761
Tjoelker LW, Gosting L, Frey S, Hunter CL, Trong HL, Steiner B, Brammer H, Gray PW (2000) Structural and functional definition of the human chitinase chitin-binding domain. J Biol Chem 275:514–520
Viterbo A, Montero M, Ramot O, Friesem D, Monte E, Llobell A, Chet I (2002) Expression regulation of the endochitinase chit36 from Trichoderma asperellum (T. harzianum T-203). Curr Genet 42:114–122
Walter S, Schrempf H (2003) Oligomerization, membrane anchoring, and cellulose-binding characteristics of AbpS, a receptor-like Streptomyces protein. J Biol Chem 278:26639–26647
Yanai K, Takaya N, Kojima N, Horiuchi H, Ohta A, Takagi M (1992) Purification of two chitinases from Rhizopus oligosporus and isolation and sequencing of the encoding genes. J Bacteriol 174:7398–7406
Acknowledgements
We wish to thank Dr. I. Garcia for construction of plasmid pLMRS3-chit42. This research was supported by the CICYT, project numbers FD97-0820 and AGL2000-0524, and Junta de Andalucía PAI CVI-107.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Limón, M.C., Chacón, M.R., Mejías, R. et al. Increased antifungal and chitinase specific activities of Trichoderma harzianum CECT 2413 by addition of a cellulose binding domain. Appl Microbiol Biotechnol 64, 675–685 (2004). https://doi.org/10.1007/s00253-003-1538-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-003-1538-6