Abstract
Background and Objectives
The prediction of pharmacokinetics of monoclonal antibodies (mAbs) exhibiting non-linear pharmacokinetics in preclinical species to human is challenging, and very limited scientific work has been published in this field of research. Therefore, we have conducted an elaborate comparative assessment to determine the most reliable preclinical to clinical scaling strategy for mAbs with non-linear pharmacokinetics.
Methods
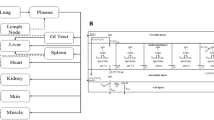
We have compared three different scaling approaches to predict human pharmacokinetics from cynomolgus monkey. In the first approach, cynomolgus monkey pharmacokinetic parameters estimated using a two-compartment model with parallel linear and non-linear elimination were allometrically scaled to simulate human pharmacokinetics. In the second approach, allometric exponents were integrated with a minimal physiologically based pharmacokinetic (mPBPK) model to translate human pharmacokinetics. In the third approach, we have employed a species time-invariant method, wherein a two-compartment model with parallel linear and non-linear elimination was used as a framework model for simulation of the human profile.
Results
Human exposure parameters projected by an integrated allometric method were only within two fold for approximately 45–70% of predictions at different doses of five mAbs evaluated, while approximately 70–80% of Cmax and AUC predictions by integrated mPBPK modelling as well as the species time-invariant method were within two-fold error. The average fold error for clearance predictions by the integrated mPBPK method was 1.10–1.45 fold, whilst for the species time-variant and integrated allometric methods, the average fold error was between 1.04 and 1.37 fold and 1.24 and 2.13 fold, respectively.
Conclusions
Our findings suggest that the species time-variant method and mPBPK proposed by us can be employed to reliably translate non-linear pharmacokinetics of mAbs from cynomolgus monkey to human.








Similar content being viewed by others
References
Suh HY, Peck CC, Yu K-S, Lee H. Determination of the starting dose in the first-in-human clinical trials with monoclonal antibodies: a systematic review of papers published between 1990 and 2013. Drug Des Devel Ther. 2016;10:4005–16.
Tibbitts J, Cavagnaro JA, Haller CA, Marafino B, Andrews PA, Sullivan JT. Practical approaches to dose selection for first-in-human clinical trials with novel biopharmaceuticals. Regul Toxicol Pharmacol. 2010;58:243–51.
Reigner BG, Blesch KS. Estimating the starting dose for entry into humans: principles and practice. Eur J Clin Pharmacol. 2002;57:835–45.
Mahmood I, Balian JD. The pharmacokinetic principles behind scaling from preclinical results to phase I protocols. Clin Pharmacokinet. 1999;36:1–11.
Chappell WR, Mordenti J. Extrapolation of toxicological and pharmacological data from animals to humans. In: Testa B, editor. Advances in drug research. San Diego (CA): Academic Press; 1991. p. 1–116.
Rowland M, Benet LZ. Lead PK commentary: predicting human pharmacokinetics. J Pharm Sci. 2011;100:4047–9.
Sinha VK, Vaarties K, De Buck SS, Fenu LA, Nijsen M, Gilissen RA, Sanderson W, Van Uytsel K, Hoeben E, Van Peer A, Mackie CE, Smit JW. Towards a better prediction of peak concentration, volume of distribution and half-life after oral drug administration in man, using allometry. Clin Pharmacokinet. 2011;50:307–18.
Caldwell GW, Masucci JA, Yan Z, Hageman W. Allometric scaling of pharmacokinetic parameters in Drug Discovery: Can Human CL, Vss and t1/2 be predicted from in-vivo rat data? Eur J Drug Metab Pharmacokinet. 2004;29:133–43.
Agoram BM. Use of pharmacokinetic/pharmacodynamic modelling for starting dose selection in first-in-human trials of high-risk biologics. Br J Clin Pharmacol. 2009;67:153–60.
Mahmood I, Balian JD. Interspecies scaling: predicting clearance of drugs in humans. Three different approaches. Xenobiotica. 1996;26:887–95.
Mahmood I. Interspecies pharmacokinetic scaling. In: Mahmood I, editor. Interspecies pharmacokinetic scaling: principles and application of allometric scaling. Danvers, MA: Pine House Publishers; 2005. p. 39–85.
Tang H, Hussain A, Leal M, Mayersohn M, Fluhler E. Interspecies prediction of human drug clearance based on scaling data from one or two animal species. Drug Metab Dispos Biol Fate Chem. 2007;35:1886–93.
Dedrick RL. Animal scale-up. J Pharmacokinet Biopharm. 1973;1:435–61.
Boxenbaum H, Ronfeld R. Interspecies pharmacokinetic scaling and the dedrick plots. Am J Physiol. 1983;245:R768–75.
Suzuki H, Iwatsubo T, Sugiyama Y. Applications and prospects for physiologically based pharmacokinetic (PB-PK) models involving pharmaceutical agents. Toxicol Lett. 1995;82–83:349–55.
Rowland M, Peck C, Tucker G. Physiologically-based pharmacokinetics in drug development and regulatory science. Annu Rev Pharmacol Toxicol. 2011;51:45–73.
Cao Y, Jusko WJ. Applications of minimal physiologically-based pharmacokinetic models. J Pharmacokinet Pharmacodyn. 2012;39:711–23.
Cao Y, Balthasar JP, Jusko WJ. Second-generation minimal physiologically-based pharmacokinetic model for monoclonal antibodies. J Pharmacokinet Pharmacodyn. 2013;40:597–607.
Lobo ED, Hansen RJ, Balthasar JP. Antibody pharmacokinetics and pharmacodynamics. J Pharm Sci. 2004;93:2645–68.
Mould DR, Sweeney KR. The pharmacokinetics and pharmacodynamics of monoclonal antibodies: mechanistic modeling applied to drug development. Curr Opin Drug Discov Devel. 2007;10:84–96.
Ryman JT, Meibohm B. Pharmacokinetics of monoclonal antibodies. CPT Pharmacometrics Syst Pharmacol. 2017;6:576–88.
Mager DE. Target-mediated drug disposition and dynamics. Biochem Pharmacol. 2006;72:1–10.
Luu KT, Bergqvist S, Chen E, Hu-Lowe D, Kraynov E. A model-based approach to predicting the human pharmacokinetics of a monoclonal antibody exhibiting target-mediated drug disposition. JPET. 2012;341:702–8.
Singh AP, Krzyzanski W, Martin SW, Weber G, Betts A, Ahmad A, Abraham A, Zutshi A, Lin J, Singh P. Quantitative prediction of human pharmacokinetics for mAbs exhibiting target-mediated disposition. AAPS J. 2015;17:389–99.
Wang J, Iyer S, Fielder PJ, Davis JD, Deng R. Projecting human pharmacokinetics of monoclonal antibodies from nonclinical data: comparative evaluation of prediction approaches in early drug development. Biopharm Drug Dispos. 2016;37:51–65.
Yan X, Mager DE, Krzyzanski W. Selection between Michaelis-Menten and target-mediated drug disposition pharmacokinetic models. J Pharmacokinet Pharmacodyn. 2010;37:25–47.
Dong JQ, Salinger DH, Endres CJ, Gibbs JP, Hsu CP, Stouch BJ, Hurh E, Gibbs MA. Quantitative prediction of human pharmacokinetics for monoclonal antibodies. Clin Pharmacokinet. 2011;50:131–42.
Benincosa LJ, Chow FS, Tobia LP, Kwok DC, Davis CB, Jusko WJ. Pharmacokinetic and pharmacodynamic of an anti-human factor IX antibody (SB-249417) in cynomolgus monkeys. J Pharm Exp Ther. 2000;292:810–6.
Chow F-S, Benincosa LJ, Sheth SB, Wilson D, Davis CB, Elisabeth A, Minthorn BS, Jusko WJ. Pharmacokinetic and pharmacodynamic modeling of humanized anti-factor IX antibody (SB 249417) in humans. Clin Pharm Ther. 2002;71:235–45.
Lin YS, Nguyen C, Mendoza JL, Escandon E, Fei D, Meng YG, Modi NB. Preclinical pharmacokinetics, interspecies scaling, and tissue distribution of a humanized monoclonal antibody against vascular endothelial growth factor. J Pharmacol Exp Ther. 1999;288:371–8.
Gordon MS, Margolin K, Talpaz M, Sledge GW Jr, Holmgren E, Benjamin R, Stalter S, Shak S, Adelman D. Phase I safety and pharmacokinetic study of recombinant human anti-vascular endothelial growth factor in patients with advanced cancer. J Clin Oncol. 2001;19:843–50.
http://www.accessdata.fda.gov/drugsatfda_docs/bla/2004/125084_ERBITUX_BIOPHARMR.PDF. Accessed 8 Jun 2021.
Glassman PM, Chen Y, Balthasar JP. Scale-up of a physiologically-based pharmacokinetic model to predict the disposition of monoclonal antibodies in monkeys. J Pharmacokinet Pharmacodyn. 2015;42:527–40.
Davis B, Morris T. Physiological parameters in laboratory animals and humans. Pharm Res. 1993;10:1093–5.
Deng R, Iyer S, Theil FP, Mortensen DL, Fielder PJ, Prabhu S. Projecting human pharmacokinetics of therapeutic antibodies from nonclinical data: what have we learned? MAbs. 2011;3:61–6.
Neuber T, Frese K, Jaehrling J, Jäger S, Daubert D, Felderer K, Linnemann M, Höhne A, Kaden S, Kölln J, Tiller T, Brocks B, Ostendorp R, Pabst S. Characterization and screening of IgG binding to the neonatal Fc receptor. MAbs. 2014;6(4):928–42.
Shah DK, Betts AM. Towards a platform PBPK model to characterize the plasma and tissue disposition of monoclonal antibodies in preclinical species and human. J Pharmacokinet Pharmacodyn. 2012;39:67–86.
Ling J, Zhou H, Jiao Q, Davis HM. Interspecies scaling of therapeutic monoclonal antibodies: initial look. J Clin Pharmacol. 2009;49:1382–402.
Keizer RJ, Huitema ADR, Schellens JHM, Beijnen JH. Clinical pharmacokinetics of therapeutic monoclonal antibodies. Clin Pharmacokinet. 2010;49:493–507.
Mordenti J, Chen SA, Moore JA, Ferraiolo BL, Green JD. Interspecies scaling of clearance and volume of distribution data for five therapeutic proteins. Pharm Res. 1991;8:1351–9.
Wang W, Prueksaritanont T. Prediction of human clearance of therapeutic proteins: simple allometric scaling method revisited. Biopharm Drug Dispos. 2010;31:253–63.
Zhao J, Cao Y, Jusko WJ. Across-Species scaling of monoclonal antibody pharmacokinetics using a Minimal PBPK model. Pharm Res. 2015;32:3269–81.
Oitate M, Masubuchi N, Ito T, Yabe Y, Karibe T, Aoki T, Murayama N, Kurihara A, Okudaira N, Izumi T. Prediction of human pharmacokinetics of therapeutic monoclonal antibodies from simple allometry of monkey data. Drug Metab Pharmacokinet. 2011;26:423–30.
Betts A, Keunecke A, van Steeg TJ, van der Graaf PH, Avery LB, Jones H, Berkhout J. Linear pharmacokinetic parameters for monoclonal antibodies are similar within a species and across different pharmacological targets: a comparison between human, cynomolgus monkey and hFcRn Tg32 transgenic mouse using a population-modeling approach. MAbs. 2018;10:751–64.
van Brummelen EMJ, Ros W, Wolbink G, Beijnen JH, Schellens JHM. Antidrug antibody formation in oncology: clinical relevance and challenges. Oncologist. 2016;21(10):1260–8.
McDonald TA, Zepeda ML, Tomlinson MJ, Bee WH, Ivens IA. Subcutaneous administration of biotherapeutics: current experience in animal models. Curr Opin Mol Ther. 2010;12:461–70.
Richter WF, Jacobsen B. Subcutaneous absorption of biotherapeutics: knowns and unknowns. Drug Metab Dispos. 2014;42:1881–9.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This research work was not funded or supported by any organization.
Conflict of interest
The authors declare that they have no conflict of interest.
Ethics approval
No ethical approval was required for the research work published in the manuscript.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Code availability
Relevant mathematical code has been provided in the manuscript.
Availability of data and material
All the data and material in the manuscript is available for the reader.
Author contribution
RS: Analysed the data and wrote the manuscript. MM and DS: Reviewed the manuscript.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Singh, R., Moreno, M. & Stanimirovic, D. Comparison of Various Approaches to Translate Non-Linear Pharmacokinetics of Monoclonal Antibodies from Cynomolgus Monkey to Human. Eur J Drug Metab Pharmacokinet 46, 555–567 (2021). https://doi.org/10.1007/s13318-021-00691-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13318-021-00691-3