Abstract
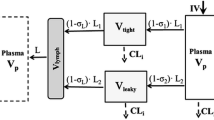
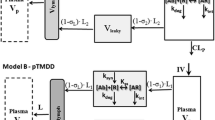
Minimal physiologically-based pharmacokinetic (mPBPK) models provide a sensible modeling approach when fitting only plasma (or blood) data yielding physiologically-relevant PK parameters that may provide more practical value than parameters of mammillary models. We propose a second-generation mPBPK model specifically for monoclonal antibodies (mAb) by including (lumping) several essential components of mAb PK used in full PBPK models. These components include convection as the primary mechanism of antibody movement from plasma into tissues and return to plasma with interstitial fluid as the major extravascular distribution space. The model divides tissue spaces into two groups according to their vascular endothelial structure, leaky and tight, which consequently allows discernment of two types and general sites of distribution. This mPBPK model was applied to two mAbs in mice and ten mAbs with linear kinetics in humans. The model captured their plasma PK profiles well with predictions of concentrations in interstitial fluid for two types of tissues. Predictions of tissue concentrations for mAb 7E3 and 8C2 were consistent with actual measurements in mice, indicating the feasibility of this model in assessing extravascular distribution in the two categories of tissues. The vascular reflection coefficients (σ 1) of tight tissues (V tight ) ranged 0.883–0.987 and coefficients (σ 2) for leaky tissues (V leaky ) ranged 0.311 to 0.837. The plasma clearance (CL p ) varied among the mAbs in humans from 0.0054 to 0.03 L/h. In addition, applying this model generates parameters for mAb transcapillary escape rates and assesses major sites of elimination. Four of ten mAbs exhibited better fitting statistics premised on elimination from interstitial fluid than from plasma. This approach allows comparisons of mAb PK when only plasma data are available, provides more realistic parameters and predictions than mammillary models, and may provide an intermediate step towards utilizing full PBPK models for mAbs.





Similar content being viewed by others
References
Rowland M, Peck C, Tucker G (2011) Physiologically-based pharmacokinetics in drug development and regulatory science. Annu Rev Pharmacol Toxicol 51:45–73
Cao Y, Jusko WJ (2012) Applications of minimal physiologically-based pharmacokinetic models. J Pharmacokinet Pharmacodyn 39:711–723
Nelson AL, Dhimolea E, Reichert JM (2010) Development trends for human monoclonal antibody therapeutics. Nat Rev Drug Discov 9:767–774
Wang W, Wang EQ, Balthasar JP (2008) Monoclonal antibody pharmacokinetics and pharmacodynamics. Clin Pharmacol Ther 84:548–558
Mould DR, Green B (2010) Pharmacokinetics and pharmacodynamics of monoclonal antibodies: concepts and lessons for drug development. BioDrugs 24:23–39
Weisman MH, Moreland LW, Furst DE, Weinblatt ME, Keystone EC et al (2003) Efficacy, pharmacokinetic, and safety assessment of adalimumab, a fully human anti-tumor necrosis factor-alpha monoclonal antibody, in adults with rheumatoid arthritis receiving concomitant methotrexate: a pilot study. Clin Ther 25:1700–1721
Xu Z, Bouman-Thio E, Comisar C, Frederick B, Van Hartingsveldt B, Marini JC, Davis HM, Zhou H (2011) Pharmacokinetics, pharmacodynamics and safety of a human anti-IL-6 monoclonal antibody (sirukumab) in healthy subjects in a first-in-human study. Br J Clin Pharmacol 72:270–281
Baxter LT, Zhu H, Mackensen DG, Jain RK (1994) Physiologically based pharmacokinetic model for specific and nonspecific monoclonal antibodies and fragments in normal tissues and human tumor xenografts in nude mice. Cancer Res 54:1517–1528
Lammerts van Bueren JJ, Bleeker WK, Bogh HO, Houtkamp M, Schuurman J, van de Winkel JG, Parren PW (2006) Effect of target dynamics on pharmacokinetics of a novel therapeutic antibody against the epidermal growth factor receptor: implications for the mechanisms of action. Cancer Res 66:7630–7638
Garg A, Balthasar JP (2007) Physiologically-based pharmacokinetic (PBPK) model to predict IgG tissue kinetics in wild-type and FcRn-knockout mice. J Pharmacokinet Pharmacodyn 34:687–709
Shah DK, Betts AM (2012) Towards a platform PBPK model to characterize the plasma and tissue disposition of monoclonal antibodies in preclinical species and human. J Pharmacokinet Pharmacodyn 39:67–86
Rossing N (1978) Intra- and extravascular distribution of albumin and immunoglobulin in man. Lymphology 11:138–142
Flessner MF, Lofthouse J, Zakariael R (1997) In vivo diffusion of immunoglobulin G in muscle: effects of binding, solute exclusion, and lymphatic removal. Am J Physiol 273:H2783–H2793
Baxter LT, Jain RK (1989) Transport of fluid and macromolecules in tumors. I. Role of interstitial pressure and convection. Microvasc Res 37:77–104
Ferl GZ, Wu AM, Distefano JJ 3rd (2005) A predictive model of therapeutic monoclonal antibody dynamics and regulation by the neonatal Fc receptor (FcRn). Ann Biomed Eng 33:1640–1652
Augustin HG, Kozian DH, Johnson RC (1994) Differentiation of endothelial cells: analysis of the constitutive and activated endothelial cell phenotypes. BioEssays 16:901–906
Parton RG, Schrotz P, Bucci C, Gruenberg J (1992) Plasticity of early endosomes. J Cell Sci 103(Pt 2):335–348
Griffiths G, Back R, Marsh M (1989) A quantitative analysis of the endocytic pathway in baby hamster kidney cells. J Cell Biol 109:2703–2720
Chen Y, Balthasar JP (2012) Evaluation of a catenary PBPK model for predicting the in vivo disposition of mAbs engineered for high-affinity binding to FcRn. AAPS J 14:850–859
Sarin H (2010) Physiologic upper limits of pore size of different blood capillary types and another perspective on the dual pore theory of microvascular permeability. J Angiogenes Res 2:14
Warren MF (1940) The lymphatic system. Annul Rev Physiol 2:109–124
Wiig H, Tenstad O (2001) Interstitial exclusion of positively and negatively charged IgG in rat skin and muscle. Am J Physiol Heart Circ Physiol 280:H1505–H1512
Wiig H, Kaysen GA, Al-Bander HA, De Carlo M, Sibley L, Renkin EM (1994) Interstitial exclusion of IgG in rat tissues estimated by continuous infusion. Am J Physiol 266:H212–H219
Lindena J, Kupper W, Trautschold I (1986) Catalytic enzyme activity concentration in thoracic duct, liver, and intestinal lymph of the dog, the rabbit, the rat and the mouse. Approach to a quantitative diagnostic enzymology, II. Communication. J Clin Chem Clin Biochem 24:19–33
Stucker O, Pons-himbert C, Laemmel E (2008) Towards a better understanding of lymph circulation. Phlebolymphology 15:31–36
Abuqayyas L, Balthasar JP (2012) Application of knockout mouse models to investigate the influence of FcgammaR on the tissue distribution and elimination of 8C2, a murine IgG1 monoclonal antibody. Int J Pharm 439:8–16
Oberneder R, Weckermann D, Ebner B, Quadt C, Kirchinger P, Raum T, Locher M, Prang N, Baeuerle PA, Leo E (2006) A phase I study with adecatumumab, a human antibody directed against epithelial cell adhesion molecule, in hormone refractory prostate cancer patients. Eur J Cancer 42:2530–2538
Smith DA, Minthorn EA, Beerahee M (2011) Pharmacokinetics and pharmacodynamics of mepolizumab, an anti-interleukin-5 monoclonal antibody. Clin Pharmacokinet 50:215–227
Cavelti-Weder C, Babians-Brunner A, Keller C, Stahel MA, Kurz-Levin M, Zayed H, Solinger AM, Mandrup-Poulsen T, Dinarello CA, Donath MY (2012) Effects of gevokizumab on glycemia and inflammatory markers in type 2 diabetes. Diabetes Care 35:1654–1662
Curtin F, Lang AB, Perron H, Laumonier M, Vidal V, Porchet HC, Hartung HP (2012) GNbAC1, a humanized monoclonal antibody against the envelope protein of multiple sclerosis-associated endogenous retrovirus: a first-in-humans randomized clinical study. Clin Ther 34:2268–2278
White B, Leon F, White W, Robbie G (2009) Two first-in-human, open-label, phase I dose-escalation safety trials of MEDI-528, a monoclonal antibody against interleukin-9, in healthy adult volunteers. Clin Ther 31:728–740
Hetherington S, Texter M, Wenzel E, Patti JM, Reynolds L, Shamp T, Swan S (2006) Phase I dose escalation study to evaluate the safety and pharmacokinetic profile of tefibazumab in subjects with end-stage renal disease requiring hemodialysis. Antimicrob Agents Chemother 50:3499–3500
Subramanian GM, Cronin PW, Poley G, Weinstein A, Stoughton SM, Zhong J, Ou Y, Zmuda JF, Osborn BL, Freimuth WW (2005) A phase 1 study of PAmAb, a fully human monoclonal antibody against Bacillus anthracis protective antigen, in healthy volunteers. Clin Infect Dis 41:12–20
Camidge DR, Herbst RS, Gordon MS, Eckhardt SG, Kurzrock R, Durbin B, Ing J, Tohnya TM, Sager J, Ashkenazi A et al (2010) A phase I safety and pharmacokinetic study of the death receptor 5 agonistic antibody PRO95780 in patients with advanced malignancies. Clin Cancer Res 16:1256–1263
Puchalski T, Prabhakar U, Jiao Q, Berns B, Davis HM (2010) Pharmacokinetic and pharmacodynamic modeling of an anti-interleukin-6 chimeric monoclonal antibody (siltuximab) in patients with metastatic renal cell carcinoma. Clin Cancer Res 16:1652–1661
Carpenter PA, Appelbaum FR, Corey L, Deeg HJ, Doney K, Gooley T, Krueger J, Martin P, Pavlovic S, Sanders J et al (2002) A humanized non-FcR-binding anti-CD3 antibody, visilizumab, for treatment of steroid-refractory acute graft-versus-host disease. Blood 99:2712–2719
D’Argenio DZ, Schumitzky A (2009) ADAPT V user’s guide: pharmacokinetic/pharmacodynamic system analysis software. Biomedical Simulations Resource, Los Angeles
ICRP Publication 89 (2002) Basic anatomical and physiological data for use in radiological protection: reference values: a report of age- and gender-related differences in the anatomical and physiological characteristics of reference individuals. ICRP Publication 89 Ann ICRP 32(3–4): 5–265
Urva SR, Yang VC, Balthasar JP (2010) Physiologically based pharmacokinetic model for T84.66: a monoclonal anti-CEA antibody. J Pharm Sci 99:1582–1600
Abuqayyas L, Balthasar JP (2012) Application of PBPK modeling to predict monoclonal antibody disposition in plasma and tissues in mouse models of human colorectal cancer. J Pharmacokinet Pharmacodyn 39:683–710
Hart TK, Cook RM, Zia-Amirhosseini P, Minthorn E, Sellers TS, Maleeff BE, Eustis S, Schwartz LW, Tsui P, Appelbaum ER et al (2001) Preclinical efficacy and safety of mepolizumab (SB-240563), a humanized monoclonal antibody to IL-5, in cynomolgus monkeys. J Allergy Clin Immunol 108:250–257
Choy EH, Connolly DJ, Rapson N, Jeal S, Brown JC, Kingsley GH, Panayi GS, Johnston JM (2000) Pharmacokinetic, pharmacodynamic and clinical effects of a humanized IgG1 anti-CD4 monoclonal antibody in the peripheral blood and synovial fluid of rheumatoid arthritis patients. Rheumatol (Oxf) 39:1139–1146
Tabrizi M, Bornstein GG, Suria H (2010) Biodistribution mechanisms of therapeutic monoclonal antibodies in health and disease. AAPS J 12:33–43
Wiig H, Swartz MA (2012) Interstitial fluid and lymph formation and transport: physiological regulation and roles in inflammation and cancer. Physiol Rev 92:1005–1060
Boswell CA, Tesar DB, Mukhyala K, Theil FP, Fielder PJ, Khawli LA (2010) Effects of charge on antibody tissue distribution and pharmacokinetics. Bioconjug Chem 21:2153–2163
Tabrizi MA, Tseng CM, Roskos LK (2006) Elimination mechanisms of therapeutic monoclonal antibodies. Drug Discov Today 11:81–88
Davies PF, Ross R (1978) Mediation of pinocytosis in cultured arterial smooth muscle and endothelial cells by platelet-derived growth factor. J Cell Biol 79:663–671
Bordon Y (2011) Antibody responses: FcRn-not just for the kids. Nat Rev Immunol 11:234–235
Xiao JJ (2012) Pharmacokinetic models for FcRn-mediated IgG disposition. J Biomed Biotechnol 2012:282989
Jin F, Balthasar JP (2005) Mechanisms of intravenous immunoglobulin action in immune thrombocytopenic purpura. Hum Immunol 66:403–410
Havas E, Parviainen T, Vuorela J, Toivanen J, Nikula T, Vihko V (1997) Lymph flow dynamics in exercising human skeletal muscle as detected by scintography. J Physiol 504(Pt 1):233–239
Aukland K (1984) Distribution of body fluids: local mechanisms guarding interstitial fluid volume. J Physiol (Paris) 79:395–400
Acknowledgments
The authors acknowledge Drs. Lubna Abuqayyas and Amit Garg for kindly sharing the original datasets of mAb 7E3 and 8C2. This research was supported by the National Institutes of Health Grant GM57980, the University at Buffalo—Pfizer Strategic Alliance, and the UB Center for Protein Therapeutics.
Author information
Authors and Affiliations
Corresponding author
Additional information
The supplementary file provides the Adapt 5 and the Phoenix software code for application of the mPBPK model for data from human subjects.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Cao, Y., Balthasar, J.P. & Jusko, W.J. Second-generation minimal physiologically-based pharmacokinetic model for monoclonal antibodies. J Pharmacokinet Pharmacodyn 40, 597–607 (2013). https://doi.org/10.1007/s10928-013-9332-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10928-013-9332-2