Abstract
Introduction
The use of insulin analogs for the treatment of type 1 diabetes mellitus (T1DM) is widespread; however, the therapeutic benefits still require further evaluation given their higher costs. The objective of this study was to evaluate the effectiveness and safety of analog insulin glargine compared to recombinant DNA (rDNA) insulin in patients with T1DM in observational studies, building on previous reviews of randomized controlled trials comparing neutral protamine Hagedorn insulin and insulin glargine.
Methods
A systematic review with a meta-analysis was performed. The review included cohort studies and registries available on PubMed, LILACS, and the Cochrane Central Register of Controlled Trials (CENTRAL), as well as manual and gray literature searches. The meta-analysis was conducted in Review Manager 5.3 software. The primary outcomes were glycated hemoglobin (Hb1Ac), weight gain, and hypoglycemia. Methodological quality was assessed using the Newcastle-Ottawa scale.
Results
Out of 796 publications, 11 studies were finally included. The meta-analysis favored insulin glargine in HbA1c outcomes (adult patients) and hypoglycemic episodes (P < 0.05), but without reaching glycemic control (Hb1Ac to approximately 7%). The methodological quality of the studies was moderate, noting that 45% of studies were funded by pharmaceutical companies.
Conclusion
Given the high heterogeneity of the studies, the discrete value presented by the estimated effect on effectiveness and safety, potential conflicts of interest of the studies, and the appreciable higher cost of insulin glargine, there is still no support for recommending first-line therapy with analogs. The role of analogs in the treatment of T1DM could be better determined by further observational studies of good methodological quality to assess their long-term effectiveness and safety, as well as their cost-effectiveness.
Similar content being viewed by others
Introduction
Diabetes mellitus (DM) is a chronic metabolic disease characterized by hyperglycemia due to changes in insulin secretion or altered action of insulin, or both. Type 1 DM (T1DM) results from the destruction of pancreatic beta cells mediated by cellular autoimmune responses [1].
The treatment of patients with T1DM consists of repositioning of insulin that is not produced endogenously. This involves administering either rapid-acting insulin, more intermediate or long-acting insulin. Recombinant DNA (rDNA) insulin and neutral protamine Hagedorn (NPH), which has an intermediate-acting time, are typically first-line choices among the insulins used for basal glycemic control [2, 3]. Glargine, a long-acting insulin analog, is used as an alternative to rDNA insulin. It is a molecule structurally similar to human insulin and is developed by modification of the amino acid sequence, with the aim of prolonging the duration of the effect and decreasing intra-individual variability [4].
Metabolic control through active management of patients with T1DM is based on three fundamental principles: adequate food, weight, and glycemic control. These provide benefits to patients and decrease the risk of complications [5, 6]. The glycated hemoglobin (HbA1c) level is the average glycemic level of an individual over a period of 2–3 months prior to the test day. Consequently, it can be used to evaluate glycemic control and the effectiveness of current treatments. Glycemia tests indicate blood glucose levels during testing. Both these methods are important, since the information they provide is complementary and helps to obtain a more global evaluation of glycemic control. When used together, they provide safer and more accurate results, thereby minimizing possible interferences due to the different technical methodologies used [7].
T1DM may cause acute and chronic complications, with hypoglycemia one of the most important acute complications that can occur. Microvascular (causing retinopathy, nephropathy, and neuropathy) and macrovascular (causing peripheral arterial disease, carotid disease, and coronary artery diseases) are the most prevalent chronic complications [1].
The studies and systematic reviews performed to date to compare rDNA insulin with long-acting analogs, including the authors’ own systematic review of randomized controlled trials (RCTs), did not show any significant differences in the clinical benefits obtained between the different formulations of insulin although there can be considerable differences in costs [3, 8–10]. Published studies though, including observational studies, have reported better effectiveness of insulin analogs compared with human insulin [11–13]. However, the published studies that have evaluated the performance of different insulins in non-controlled situations do appear inconclusive when combined. It is important to address this confusion given, as mentioned, the considerable differences in costs that can occur between the different formulations, for example, in Brazil, the cost of treating a patient with insulin glargine is 536% that of treatment with NPH insulin [3].
Consequently, the aim of this study was to evaluate the clinical effectiveness of insulin glargine through a systematic review of observational studies, which was not addressed in the authors’ original systematic review [3], and as a result, help to determine the performance of long-acting insulins versus NPH and other insulins in the real-world in non-controlled situations to provide future guidance. It is not about assessing the effectiveness of different interventions to encourage the prescribing of particular insulin formulations.
Methods
This review was conducted in accordance with the recommendations of the Meta-Analyses and Systematic Reviews of Observational Studies (MOOSE) guidelines [14]. This article is based on previously conducted studies and does not involve any new studies of human or animal subjects performed by any of the authors.
Study Search
Electronic searches of relevant articles published until June 2015 in MEDLINE (PubMed), Latin American and Caribbean Health Sciences (LILACS), and Cochrane Library were performed. Various combinations of terms were used, including terms related to the disease and type of intervention study (Table 1).
Hand searching was conducted in the references of all included studies and the electronic journal Diabetes Care from 2003 until March 2015. Diabetes Care was chosen as this is a reputable publication for studies involving patients with diabetes. The search for studies in the grey literature was also made among the theses and dissertations database of the Coordination for the Improvement of Higher Education Personnel (CAPES), the Digital Library of Theses and Dissertations of the Universidade Federal de Minas Gerais (UFMG), and ProQuest Dissertations and database thesis to ensure that the authors did not miss out important observational studies. These included lectures, publications and academic theses, government, congress, books, and reports.
Eligibility Criteria
Prospective and retrospective cohort studies and database records of patients with T1DM were selected. Studies that evaluated the insulin glargine preparations in comparison with rDNA insulin to assess the effectiveness and safety outcomes were included.
Studies that assessed the dosage, intervention methods, pregnant patients, clinical protocols, reviews, case reports, animal studies, in vitro studies, pharmacodynamics and/or pharmacokinetics studies, studies that included patients with T2DM, studies that evaluated concomitant oral therapies with insulin therapy for patients with T1DM, studies that evaluated less than or equal to 30 participants as deemed as too small for meaningful comparisons, or studies that had a follow-up time of less than 4 weeks were excluded.
Data Collection and Assessment of Methodological Quality
The studies found in the electronic databases were brought together in a single database for deleting duplicates. The selection was carried out in three stages by two independent reviewers and included the analysis of titles, abstracts, and full texts. Disagreements were resolved by a third reviewer. Data including methodological quality, information of participants, duration of treatment, efficacy, and safety data were extracted and collected in duplicate in an Excel form developed for this purpose and previously tested.
For the assessment of methodological quality, the authors used the Newcastle-Ottawa scale for observational studies [15]. On this scale, each study was measured in three dimensions: selection of study groups, comparability of groups, and determination of the results of interest. The total score is nine, with studies considered of high methodological quality if above six. In addition, funding sources were identified to verify potential sources of bias. The possibility of publication bias was assessed by analysis of the funnel plot [16]. It was felt there was conflict of interest in the study when somewhere in the text there was commentary on conflict of interest, it referred to sources of industry funding, or when there was some link of the study authors with the pharmaceutical industry.
Summary of the Findings and Statistical Analysis
Assessed outcomes included the concentration of HbA1c, or capillary blood glucose plasma fasting and episodes of severe hypoglycemia. Secondary outcomes included the impact on body mass index (BMI), weight gain, and the occurrence of adverse reactions.
Data from the studies were combined using random effects model the Review Manager (RevMan) software version 5.3. The authors chose RevMan as this is a typical software program used for preparing and maintaining Cochrane Reviews. It was developed through a continuous process of consultation with its users and Cochrane methodologists, to support standards and guidelines for Cochrane Reviews, and provide analytic methods, access to ‘online’ help, and validation mechanisms. RevMan is free to use for authors preparing a Cochrane Review or for purely academic use. The results are presented as mean difference (MD) for continuous variables with a 95% confidence interval (CI). Analysis with an I 2 > 40% and a P value of Chi square test <0.10 were considered significant heterogeneity. Sensitivity analyses were conducted to investigate the causes of any heterogeneity, excluding a study each time and recorded the changes in I 2 and P values.
Results
Study Inclusion
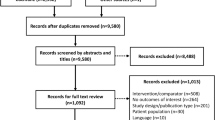
Seven-hundred and ninety-six publications were found in the electronic database. After excluding duplicates, 626 articles were selected for title assessment, 40 for abstract assessment, and 18 to be read in their entirety. After assessing the entire papers, 7 studies were included and another 4 were added from the manual check; therefore, a total of 11 studies were included in the meta-analysis (Fig. 1).
Study Characteristics
From the 11 included observational studies, 1 comprised a database record and 10 were cohort studies, with 8 being retrospective design studies and 3 prospective studies. The follow-up time varied from 6 to 54 months. Only one study did not have any conflict of interest, while five stated conflicts of interest. Three studies did not report any financial sources, and the remaining four were supported by pharmaceutical companies (Table 2). To evaluate the clinical effectiveness and safety of insulin glargine compared with rDNA insulin, 11,426 participants were evaluated from the 11 included studies.
Concerning the patients’ characteristics, the average age varied between 11 and 57 years. Four studies assessed adult patients [12, 13, 17, 18], five pediatric patients [19–23], and two studies assessed both adults and children [24, 25]. The total sample included an average of 55% males. The average time duration of the disease varied between 2 and 19 years. The sample size varied between 43 and 10,469 participants (Table 2).
Methodological Quality
The methodological quality assessment of the studies using the Newcastle-Ottawa scale indicated that none of the studies obtained the maximum score corresponding to nine stars, while four studies scored eight, four scored seven, and three had a score of six (Table 2). Overall, the studies were of moderate quality. There was no asymmetry in the funnel chart for the HbA1c outcome, suggesting an absence of publication bias (Fig. 2).
Date Synthesis
To evaluate the effectiveness and safety of the outcomes of the HbA1c values, the insulin total dosage, severe hypoglycemia, patient weight gain, and BMI were evaluated. Concerning the adverse effects night hypoglycemia events and fasting capillary glycemia, only the results presented in each study were described since the data discussed in the studies could not be combined in the meta-analysis.
Primary Outcomes
The outcome of HbA1c was assessed in two subgroups: with pediatric patients [19–24] and with adult patients [12, 13, 17, 18, 24]. The meta-analysis of the pediatric subgroup of patients did not show significant differences between the groups (MD = −0.38; 95% CI −0.79, 0.04; P = 0.07; I 2 = 86%), and the adult patients subgroup favored insulin glargine (MD = −0.26; 95% CI −0.48, −0.04; P = 0.02; I 2 = 53%). In the total combination of subgroups, the estimate of the effect favored insulin glargine and the heterogeneity was high and significant (MD = −0.33; 95% CI −0.54, −0.12; P = 0.002; I 2 = 81%; Table 3; Fig. 3). In the sensitivity analysis, exclusion of three studies [17, 20, 24] reduced the heterogeneity, but did not change the outcome.
Only two studies [19, 21] evaluated the fasting capillary glycemia, and their results did not reveal significant differences between the groups (Table 3).
For the meta-analysis of severe hypoglycemic episode occurrence, four studies [17, 19, 24, 25] were included. Data revealed an estimated difference in the means of −0.58 (95% CI −0.99, −0.16; P < 0.007; I 2 = 95%), favoring analog glargine. In the sensitivity analysis, the exclusion of Colino et al. [19] decreased the heterogeneity, without changing the direction of the outcome (Table 3; Fig. 4).
Analysis of the Subgroup: Follow-up Time of the Study
The impact on HbA1c levels was assessed according to the follow-up time of the studies. Studies considered intermediate [19, 20] revealed an insignificant difference in the mean values between the insulin formulations (MD = −0.05; 95% CI −0.92, 0.82; P = 0.91; I 2 = 95%). In studies of a longer duration [12, 13, 17, 18, 21–24], the difference in the means was estimated at −0.37 (CI −0.61, −0.13; P = 0.003; I 2 = 70%), thereby favoring insulin glargine. The consolidation of the above-mentioned groups revealed an estimated difference in the means of −0.29, favoring insulin glargine (95% CI −0.51, −0.08; P = 0.008; I 2 = 80%) with a high heterogeneity pattern (Table 3; Fig. 5). In the sensitivity analyses, the individual exclusion of the studies affected neither the direction of the outcomes nor the significance of the heterogeneity.
Subgroup Analysis: Conflict of Interest
The impact on HbA1c levels was evaluated in the subgroups to determine the presence of conflicts of interest on the findings. The subgroup without any conflict of interest [18–23] revealed an insignificant difference in means (MD = −0.31; 95% CI −0.70, 0.07; P = 0.11; I 2 = 85%). In the subgroup with conflicts of interest [12, 13, 17, 24], the difference in the means was estimated at −0.30 (95% CI −0.59, −0.01; P = 0.05; I 2 = 41%), favoring insulin glargine. The total result revealed an estimated difference in means of −0.31, favoring insulin glargine (95% CI −0.56, −0.05; P = 0.02; I 2 = 76%) with a high heterogeneity pattern (Table 3; Fig. 6). In the sensitivity analyses, the exclusion of the two studies [20, 24] affected the direction of the outcome (Table 3; Fig. 6).
Secondary Outcomes
The meta-analysis that evaluated the BMI (in kg/m2) [10, 11, 13, 15] revealed an insignificant difference in the means (MD = −0.15; 95% CI −0.71, 0.40; P = 0.59; I 2 = 74%; Table 3). The sensitivity analyses excluding the study conducted by Dixon et al. [20] resulted in a statistical heterogeneity equal to zero, without changing the direction of the outcome.
Concerning the impact on body weight gain (in kg), the study results [12, 13, 17, 18, 22, 23] revealed that there was no significant difference between the different insulins (MD = −1.38; 95% CI −4.86, 2.10; P = 0.44; I 2 = 91%). In the sensitivity analyses that excluded the study conducted by Garg et al. [17], a statistical heterogeneity equal to zero was observed, without any change in the direction of the outcome (Table 3).
For the total dosage of the analog or insulin (in U/kg/day), the subgroups of pediatric [19–24] and adult patients [12, 13, 17, 18, 24] were evaluated. Data revealed that there was no significant difference in any of the groups (MD = −0.01; 95% CI −0.12, 0.09; P = 0.83; I 2 = 88%; and MD = −0.06; 95% CI −0.14, 0.02; P = 0.16; I 2 = 72%, respectively). The total result of the meta-analysis also did not show a significant difference (MD = −0.03; 95% CI −0.09, 0.04; P = 0.37; I 2 = 84%; Table 3). In the sensitivity analyses of the adult patient subgroup, exclusion of Yamamoto-Honda et al. [18] changed the heterogeneity and the direction of the outcome, favoring insulin glargine (MD = −0.09; 95% CI −0.12, −0.06; P < 0.00001; I 2 = 0%).
Night-time hypoglycemic events were not evaluated in this meta-analysis, since the final studies did not present data that could be combined statistically. Only three studies described this outcome [18, 20, 22]. In the study by Dixon et al. [20], the night hypoglycemic events in the insulin glargine group decreased from 12 to 1 during the study period. However, in the studies by Päivärinta et al. [22] and Yamamoto-Honda et al. [18], the results revealed that there was no significant difference between the different insulins.
Asymptomatic hypoglycemic episodes were assessed in two studies [20, 21]. In the study by Dixon et al. [20], the asymptomatic hypoglycemic events did not present significant differences between the insulin glargine and rDNA insulin groups (2.3 ± 1.3 and 2.3 ± 1.5, respectively; P > 0.05). In the study by Hathout et al. [21], the average frequency of hypoglycemia decreased from 10.6% to 9.2% after 9 months’ treatment with insulin glargine; however, it was not statistically significant for all the groups under study (P = 0.3). The decrease was more evident in very small children with pre- and post-glargine hypoglycemic events of 20% and 15%, respectively.
Adverse reactions were assessed in four studies [13, 18, 19, 24]. In the studies by Colino et al. [19] and Herwig et al. [24], there was no significant difference between the insulin groups. Several patients reported that they felt more pain during the insulin glargine injection, but this did not result in the discontinuation of the treatment [19]. In the study by Johansen et al. [13], a patient developed edema and pain in the articulations immediately after the beginning treatment with analog glargine, but this did not result in the suspension of the treatment. The results by Yamamoto-Honda et al. [18] revealed that insulin glargine was well tolerated by all the patients, except for five episodes of failure in the injection system.
Discussion
The introduction of analogs as therapeutic options to treat T1DM presented hope to millions of patients to obtain greater glycemic control and prevent both microvascular and macrovascular complications associated with hyperglycemia as well as potential injury caused by hypoglycemic episodes. In this systematic review with meta-analysis, the authors aimed to assess evidence of the improved effectiveness and safety of analog glargine compared to rDNA insulin for the treatment of patients with T1DM obtained through observational studies, that is, the real word [26], thus building on their previous systematic review of RCTs [3]. It is important to emphasize that, despite the fact that they provide robust evidence regarding the efficacy of interventions, RCTs can have low external validity, that is, extrapolation of the results to the community at large including patients with greater co-morbidities can be limited [27, 28].
The HbA1c evaluation was performed on 1422 participants, comparing insulin glargine with rDNA insulin, and the result of the meta-analysis favored insulin glargine; however, this was without HbA1c control. Furthermore, for the pediatric subgroup of patients, the results of the difference in means did not show any significance. It should be emphasized that the discrete value of this result, which involved not achieving ideal control of HbA1c by the patients, established by the Brazilian Diabetes Society guidelines, was lower than 7.5% [29].
In the study by Warren et al. [30], a systematic review of the efficacy of analog glargine showed it to be more effective than rDNA insulin in decreasing fasting blood glucose, but not for reducing the HbA1c level. Another outcome assessed in the study involving 10,967 participants was the reduction of severe hypoglycemic episodes, with the findings favoring rDNA insulin [30]. The study by Siebenhofer et al. [31] showed similar results.
The follow-up period in the reported studies were divided into short duration (up to 3 months), intermediate (more than 3 months and up to 6 months), and long (more than 6 months) duration. Short-duration studies were not included since this parameter reflects the average glycemic control obtained in the period from three to 4 months, based on the red blood cell life cycle [32]. Very short studies of one-month duration, for example, detected only 50% of the estimated variation in the glycemic control [33], a fact that could introduce bias in the results. The intermediate duration studies [19, 20] did not demonstrate significant statistical differences. Most studies included in this systematic review were of long duration [12, 13, 17, 18, 21–24] and showed significant results favoring insulin glargine.
In this systematic review, it was observed that insulin glargine showed better effectiveness results compared with rDNA insulin. Vardi et al. [34] showed similar results in their systematic review, but the analysis suggested only a modest clinical benefit using long-acting analogs instead of intermediate acting insulin preparations for patients with T1DM. Its effect was more prominent for the control of night hypoglycemia [34].
Only one study reported the reason why insulin glargine treatment was discontinued [22], which was observed in 9% of the patients who discontinued treatment before completing 1 year of follow-up. The reasons for interrupting therapy were night hypoglycemia (n = 2), failure to reach good glycemic control (n = 3), patients considered the multiple injection therapy too laborious (n = 2), and pain associated with the application (n = 1). After interruption, two of the patients continued their treatment with an insulin pump and five with rDNA insulin.
The authors believe it is worth highlighting the conflict of interest associated with research, especially in regards to its ethical and bioethical aspects. According to Thompson [35], conflict of interest is a group of conditions in which professional judgment could be improperly influenced by interests such as financial gain. Conflicts of interest include, for instance, omission of sponsorship or financial involvement when publishing a scientific paper or presenting results at congresses, avoiding disclosing negative results or delaying this disclosure with the aim of protecting a potential market [36]. Publications confirm that financial relations between the industry, scientific researchers, and academic institutions can be persuasive affecting studies and utilization patterns [37, 38] and may influence important aspects of biomedical research [39]. In this systematic review when assessing the results of HbA1c, the subgroup of studies in which there was no conflict of interest did not demonstrate significant statistical difference between the findings from either insulin glargine or rDNA insulin. On the other hand, in the subgroup that reported conflicts of interest, the findings were favorable for insulin glargine (Fig. 6).
The studies selected in this systematic review and meta-analysis may have been influenced by publication bias, which is the tendency of the results published being systematically different from reality. For example, examination of clinical trials with a registered protocol in the registry database ClinicalTrials.gov revealed that <70% of the studies are eventually published [40], which may be due to a variety of reasons [41]. For instance, in a review of published studies comparing different atypical antipsychotics, in 90% of the studies supported by pharmaceutical companies the reported overall outcome was in favor of the sponsor’s drug [42]. However, in this systematic review, the analysis of the funnel chart did not show asymmetry, suggesting the absence of publication bias. Having said this, there were differences in results between the reviewed studies with and without conflicts of interest (Fig. 6). In addition, the majority of studies that showed little precision were generally performed with small samples and distributed symmetrically in the largest part of the funnel. Only the study by Johansen et al. [13] showed greater precision and was situated in the narrowest part of the funnel.
This systematic review included only cohort and patient record studies, which is one of the limitations of systematic reviews of observational studies, that is, referring to selection bias inherent to this type of study design and to non-controlled confounding factors. Some studies did not present complete and accurate information to be included in the quantitative analysis, thereby affecting the explanation of the high heterogeneity found in some comparisons. Differences in the number of participants between the groups were also observed as well as during the follow-up period. Despite this fact, observational studies have the advantage of potentially large patient groups and represent real-world conditions since they are performed in non-controlled conditions without the strict confines of RCTs [27].
Another limitation in the interpretation of the results was the statistical heterogeneity among the studies found in the meta-analysis. The small number of studies included in the comparisons, in addition to the lack of complete and accurate information in these studies, hindered the explanation of the sources of heterogeneity. In the sensitivity analysis, the inclusion and exclusion of studies in each comparison did not change the direction of a majority of the outcomes, with alterations in heterogeneity. It should be highlighted that the studies that significantly changed heterogeneity [17–20, 24, 36] were all sponsored by the pharmaceutical industry, with the exception of Yamamoto-Honda et al. [18].
The absence of other published systematic reviews on the effectiveness and safety of the different insulins in ‘real-world’ conditions hinders any comparison with the results from this review. Typically, systematic reviews evaluate efficacy studies, that is, patients enrolled into RCTs, as seen by ours and other published reviews [3, 8, 31, 34, 43], rather than including real-world studies. Overall, any recommendation of the insulin analogs as first-line therapy should still be considered with caution, considering the small difference between the outcomes in the meta-analyses that have been performed including this study, potential conflict of interests, and the appreciable differences in treatment costs in comparison with therapeutic alternatives that are available.
Conclusions
Taking into account the high heterogeneity of the published studies, the discrete value shown by assessing the effectiveness and safety outcomes, the potential conflict of interest of the included studies, and treatment costs in contrast to the therapeutic alternatives available, there is evidence of improved effectiveness with the analogs. However, these results need to be treated with caution as there were differences in findings between studies where conflicts of interest were reported and those without conflict of interest. The role of the analogs in T1DM treatment should be better determined through more studies with good methodologies to assess their effectiveness and safety profile over a long duration as well as well-conducted economic evaluations focusing on available therapies. This is particularly important where there are considerable acquisition cost differences between available insulin formulations. In view of these controversies, the authors are currently performing their own analysis of the effectiveness of insulin glargine in real life amongst a Brazilian population. They hope to report on this shortly.
References
Pasqualotto KR, Alberton D, Frigeri HR. Diabetes mellitus and complications. J Biotechnol Biodivers. 2012;3(4):134–45.
Lepore M, Pampanelli S, Fanelli C, et al. Pharmacokinetica and pharmacodynamics of subcutaneous injection of long-acting human insulin and continuous subcutaneous infusion of insulin lispro. Diabetes. 2000;49:2142–8.
Caires de Souza AL, de Assis Acurcio F, Guerra Júnior AA, Rezende Macedodo Nascimento RC, Godman B, Diniz LM. Insulin glargine in a Brazilian state: should the government disinvest? An assessment based on a systematic review. Appl Health Econ Health Policy. 2014;12(1):19–32.
Micromedex® Healthcare Series. Drugdex® Evaluations. Available from: http://www.periodicos.capes.gov.br. Accessed Nov 10, 2010.
American Diabetes Association. Standards of medical care in diabetes. Position statement. Diabetes Care. 2007;30(Suppl. 1):S4–41.
Heinemann L, Linkeschova R, Rave K, Hompesch B, Sedlak M, Heise T. Time-action profile of the long-acting insulin analog insulin glargine (HOE901) in comparison with those of NPH insulin and placebo. Diabetes Care. 2000;23(5):644–9.
Rohlfing CL, Wiedmeyer HM, Little RR, England JD, Tennill A, Goldstein DE. Defining the relationship between plasma glucose and HbA1c analysis of glucose profiles and HbA1c in the Diabetes Control and Complications Trial. Diabetes Care. 2002;25(2):275–8.
Sanches ACC, Correr CJ, Venson R, et al. Insulin analogues versus human insulin in type 1 diabetes: direct and indirect meta-analyses of efficacy and safety. Braz J Pharm Sci. 2013;49(3):501–9.
Institutfürqualität und WirtschaftlichkeitimGesundheitswesen [Institute of Quality and Efficiency in Health Care]. Long-acting insulin analogues in the treatment of diabetes mellitus type 1 (2010). Available from: http://www.iqwig.de/download/A05-01_Executive-Summary_Long-acting_insulin_analogues_in_diabetes_mellitus_type_1.pdf. Accessed May 2015.
Institute for Quality and Efficiency in Health Care—IQWIG Long acting insulins for the treatment of diabetes mellitus type 1—Documentation. 2008. Available from: https://www.iqwig.de/download/A05-01_Dokumentation_und_Wuerdigung_der_Stellungnahmen_zum_Berichtsplan_V_1_0.pdf. Accessed May 2015.
Szypowska A, Golicki D, Groele L, Pańkowska E. Long-acting insulin analogue detemir compared with NPH insulin in type 1 diabetes. Pol Arch Med Wewn. 2011;121:237–46.
Schreiber SA, Russmann A. Long-term efficacy of insulin glargine therapy with an educational programme in type 1 diabetes patients in clinical practice. Curr Med Res Opin. 2007;23(12):3131–6.
Johansen OE. VanbergPJ, Kilhovd BK, Jørgensen AP. Changing basal insulin from NPH to detemir or glargine in patients with type 1 diabetes and a history of severe hypoglycemia. Vasc Health Risk Manag. 2009;5(1):121–8.
Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283(15):2008–12.
Hartling L, Hamm M, Milne A, et al. Validity and inter rater reliability testing of quality assessment instruments [internet]. Appendix E, decision rules for application of the Newcastle-Ottawa Scale. Agency for Healthcare Research and Quality (US), Rockville (MD). Available from: http://www.ncbi.nlm.nih.gov/books/NBK92291/. 2012.
Higgins JPT, et al., editors. Cochrane handbook for systematic reviews of interventions. Chichester: Wiley-Blackwell; 2008.
Garg SK, Paul JM, Karsten JI, Menditto L, Gottlieb PA. Reduced severe hypoglycemia with insulin glargine in intensively treated adults with type 1 diabetes. Diabetes Technol Ther. 2004;6(5):589–95.
Yamamoto-Honda R, Takahashi Y, Yoshida Y, et al. Use of insulin glargine in japanese patients with type 1 diabetes. Intern Med. 2007;46(13):937–43.
Colino E, López-Capapé M, Golmayo L, Alvarez MA, Alonso M, Barrio R. Therapy with insulin glargine (Lantus1) in toddlers, children and adolescents with type 1 diabetes. Diabetes Res Clin Pract. 2005;70(1):1–7.
Dixon B, Chase HP, Burdick J, et al. Use of insulin glargine in children under age 6 with type 1 diabetes. Pediatr Diabetes. 2005;6(3):150–4.
Hathout EH, Fujishige L, Geach J, Ischandar M, Maruo S, Mace JW. Effect of therapy with insulin glargine (Lantus®) on glycemic control in toddlers, children, and adolescents with diabetes. Diabetes Technol Ther. 2003;5(5):801–6.
Päivärinta M, Tapanainen P, Veijola R. Basal insulin switch from NPH to glargine in children and adolescents with type 1 diabetes. Pediatr Diabetes. 2008;9:83–90.
Salemyr J, Bangand P, Örtqvist E. Lower HbA1c after 1 year, in children withtype1 diabetes treated with insulin glargine vs. NPH insulin from diagnosis: a retrospective study. Pediatr Diabetes. 2011;12(5):501–5.
Herwig J, Schöll-Schilling G, Böhles H. Glycaemic control and hypoglycaemia in children, adolescents and young adults with unstable type 1 diabetes mellitus treated with insulin glargine or intermediate-acting insulin. J Pediatr Endocrinol Metab. 2007;20(4):517–25.
Haukka J, Hoti F, Erästö P, Saukkonen T, Mäkimattila S, Korhonen P. Evaluation of the incidence and risk of hypoglycemic coma associated with selection of basal insulin in the treatment of diabetes: a Finnish register linkage study. Pharmacoepidemiol Drug Saf. 2013;22(12):1326–35.
Marley J. Efficacy, effectiveness, efficiency. Aust Prescr. 2000;23:114–5.
Pereira C, Veiga N. Educação Para a Saúde Baseada em Evidências. 2014.
Malmström RE, Godman BB, Diogene E, et al. Dabigatran - a case history demonstrating the need for comprehensive approaches to optimize the use of new drugs. Front Pharmacol. 2013;14(4):39.
Oliveira JEPD, Vencio S. Diretrizes da Sociedade Brasileira de Diabetes: 2014-2015. Sa˜o Paulo: AC Farmacêutica, 2015, pp. 1–360.
Warren E, Weatherley-Jones E, Chilcott J, Beverley C. Systematic review and economic evaluation of a long-acting insulin analogue, insulin glargine. Health Technol Assess. 2004;8(45):iii, 1–57.
Siebenhofer A, Plank J, Berghold A, et al. Short acting insulin analogues versus regular human insulin in patients with diabetes mellitus. Cochrane Database Syst Rev. 2006;2:CD003287.
Netto AP, Andriolo A, Filho FF, et al. Atualização sobre hemoglobina glicada (HbA1C) para avaliação do controle glicêmico e para o diagnóstico do diabetes: aspectos clínicos e laboratoriais. J Bras Patol Med Lab. 2009;45(1):31–48.
Sacks DB. Hemoglobin variants and hemoglobin A1c analysis: problem solved? Clin Chem. 2003;49:1245–7.
Vardi M, Jacobson E, Nini A, Bitterman H. Intermediate acting versus long acting insulin for type 1 diabetes mellitus. Cochrane Database Syst Rev. 2008;16(3):CD006297.
Thompson DF. Understanding financial conflicts of interest. N Engl J Med. 1993;329:573–6.
Ggoldim JR. Conflito de interesses na área da saúde. 2007.
Davis C, Abraham J. Is there a cure for corporate crime in the drug industry? BMJ. 2013;346:f755.
Civaner M. Sale strategies of pharmaceutical companies in a “pharmerging” country: the problems will not improve if the gaps remain. Health Policy. 2012;106:225–32.
Bekelman JE, Li Y, Gross CP. Scope and impact of financial conflicts of interest in biomedical research: a systematic review. JAMA. 2003;289(4):454–65.
Ross JS, Tse T, Zarin DA, Xu H, Zhou L, Krumholz HM. Publication of NIH funded trials registered in ClinicalTrials.gov: cross sectional analysis. BMJ. 2012;344(d7292):1–10.
Pereira MG, Galvão TF. Heterogeneidade e viés de publicação em revisões sistemáticas. Epidemiologia e Serviços de Saúde. 2014;23(4):775–8.
Heres S, Davis J, Maino K, Jetzinger E, Kissling W, Leucht S. Why olanzapine beats risperidone, risperidone beats quetiapine, and quetiapine beats olanzapine: an exploratory analysis of head-to-head comparison studies of second-generation antipsychotics. Am J Psychiatry. 2006;163(2):185–94.
Wang F, Carabino JM, Vergara CM. Insulin glargine: a systematic review of a long-acting insulin analogue. Clin Ther. 2003;25(6):1541–77.
Acknowledgments
The research was supported by the Research Group in Pharmacoepidemiology UFMG. This systematic review is an integral part of the research project ‘Comparative Clinical Effectiveness and cost-effectiveness of Glargine insulin analog for the treatment of patients suffering from Diabetes Mellitus’ with financial support from the National Scientific and Technological Development Council (CNPq). The write-up was in part supported by a Newton Advanced Fellowship awarded to Professor Augusto Afonso Guerra Junior by the Academy of Medical Sciences, through the UK Government’s Newton Fund programme. All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this manuscript, take responsibility for the integrity of the work as a whole, and have given final approval for the version to be published.
Disclosures
Lays P. Marra, Vania E. Araújo, Thales B. C. Silva, Leonardo M. Diniz, Augusto A. Guerra Junior, Francisco A. Acurcio, Brian Godman, and Juliana Álvares declare they have no conflicts of interest.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not involve any new studies of human or animal subjects performed by any of the authors.
Open Access
This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Author information
Authors and Affiliations
Corresponding author
Additional information
Enhanced content To view enhanced content for this article go to http://www.medengine.com/Redeem/6B84F0607D1C1574.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0), which permits use, duplication, adaptation, distribution, and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Marra, L.P., Araújo, V.E., Silva, T.B.C. et al. Clinical Effectiveness and Safety of Analog Glargine in Type 1 Diabetes: A Systematic Review and Meta-Analysis. Diabetes Ther 7, 241–258 (2016). https://doi.org/10.1007/s13300-016-0166-y
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13300-016-0166-y